Chapter 3 Physical Model vs Conceptual Model Physical





















- Slides: 72

Chapter 3

§ Physical Model vs. Conceptual Model § Physical model is a representation of a very large or small object shown at a convenient scale. § Conceptual model is an explanation that treats what is being explained as a system. In a system, it is the interactions among component parts that matter most; knowing or seeing the parts themselves does not provide the understanding we are looking for.

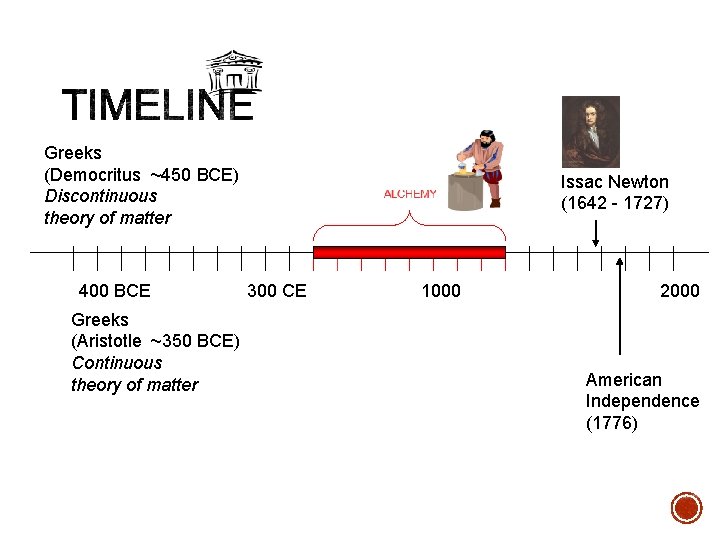
Greeks (Democritus ~450 BCE) Discontinuous theory of matter 400 BCE Greeks (Aristotle ~350 BCE) Continuous theory of matter Issac Newton (1642 - 1727) 300 CE 1000 2000 American Independence (1776)

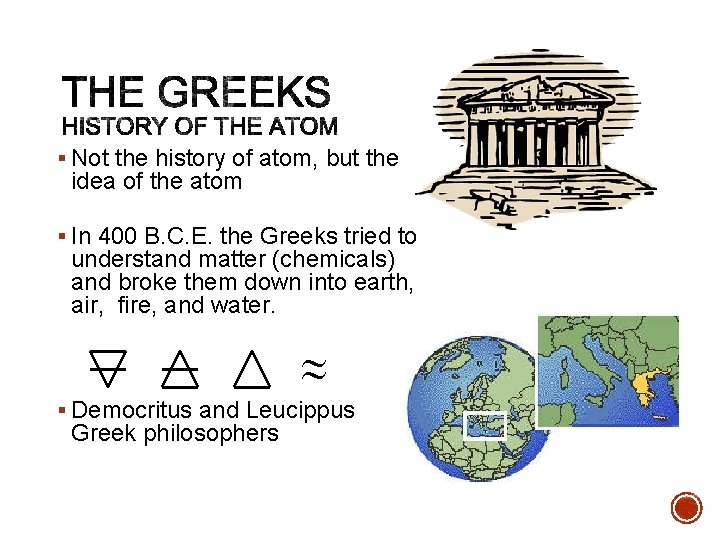
§ Not the history of atom, but the idea of the atom § In 400 B. C. E. the Greeks tried to understand matter (chemicals) and broke them down into earth, air, fire, and water. ~ ~ § Democritus and Leucippus Greek philosophers

Fire ~ ~ Water Earth Air

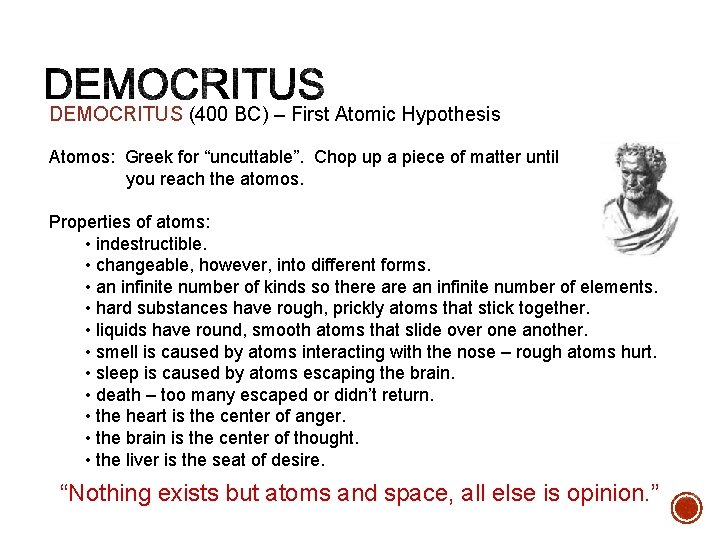
“To understand the very large, we must understand the very small. ” Democritus § Greek philosopher § Idea of ‘democracy’ § Idea of ‘atomos’ § Atomos = ‘indivisible’ § ‘Atom’ is derived § No experiments to support idea § Continuous vs. discontinuous theory of matter Democritus’s model of atom No protons, electrons, or neutrons Solid and INDESTRUCTABLE

DEMOCRITUS (400 BC) – First Atomic Hypothesis Atomos: Greek for “uncuttable”. Chop up a piece of matter until you reach the atomos. Properties of atoms: • indestructible. • changeable, however, into different forms. • an infinite number of kinds so there an infinite number of elements. • hard substances have rough, prickly atoms that stick together. • liquids have round, smooth atoms that slide over one another. • smell is caused by atoms interacting with the nose – rough atoms hurt. • sleep is caused by atoms escaping the brain. • death – too many escaped or didn’t return. • the heart is the center of anger. • the brain is the center of thought. • the liver is the seat of desire. “Nothing exists but atoms and space, all else is opinion. ”

Anaxagoras (Greek, born 500 B. C. ) –Suggested every substance had its own kind of “seeds” seeds that clustered together to make the substance, much as our atoms cluster to make molecules. Empedocles (Greek, born in Sicily, 490 B. C. ) –Suggested there were only four basic seeds – earth, air, fire, and water The elementary substances (atoms to us) combined in various ways to make everything. Democritus (Thracian, born 470 B. C. ) –Actually proposed the word atom (indivisible) because he believed that all matter consisted of such tiny units with voids between, an idea quite similar to our own beliefs. It was rejected by Aristotle and thus lost for 2000 years. Aristotle (Greek, born 384 B. C. ) –Added the idea of “qualities” – heat, cold, dryness, moisture – as basic elements which combined as shown in the diagram (previous page). Hot + dry made fire; hot + wet made air, and so on.

§ After that chemistry was ruled by alchemy. § They believed that could take any cheap metals and turn them into gold. § Alchemists were almost like magicians. § elixirs, physical immortality

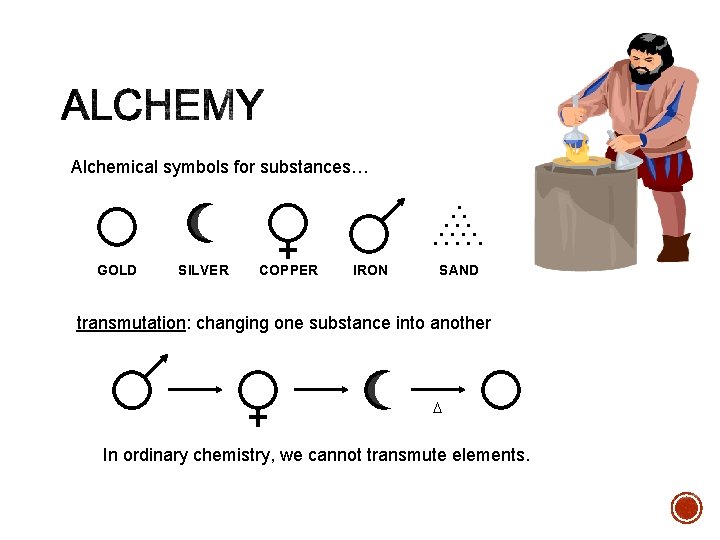
Alchemical symbols for substances… . . . . GOLD SILVER COPPER IRON SAND transmutation: changing one substance into another D In ordinary chemistry, we cannot transmute elements.


Contributions of Alchemists: Information about elements - the elements mercury, sulfur, and antimony were discovered - properties of some elements Develop lab apparatus / procedures / experimental techniques - alchemists learned how to prepare acids. - developed several alloys - new glassware

§ Late 1700’s - John Dalton- England § Teacher- summarized results of his experiments and those of others § Combined ideas of elements with that of atoms in Dalton’s Atomic Theory

THE ATOMIC THEORY OF MATTER § In 1803, Dalton proposed that elements consist of individual particles called atoms. § His atomic theory of matter contains four hypotheses: 1. All matter is composed of tiny particles called atoms, indivisible and indestructible. 2. All atoms of an element are identical in mass and fundamental chemical properties. 3. A chemical compound is a substance that always contains the same atoms in the same ratio. 4. In chemical reactions, atoms from one or more compounds or elements redistribute or rearrange in relation to other atoms to form one or more new compounds. Atoms themselves do not undergo a change of identity in chemical reactions.

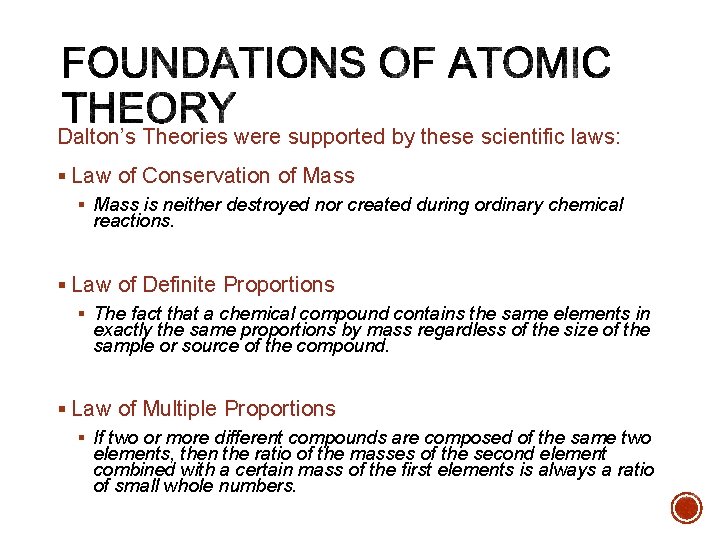
Dalton’s Theories were supported by these scientific laws: § Law of Conservation of Mass § Mass is neither destroyed nor created during ordinary chemical reactions. § Law of Definite Proportions § The fact that a chemical compound contains the same elements in exactly the same proportions by mass regardless of the size of the sample or source of the compound. § Law of Multiple Proportions § If two or more different compounds are composed of the same two elements, then the ratio of the masses of the second element combined with a certain mass of the first elements is always a ratio of small whole numbers.

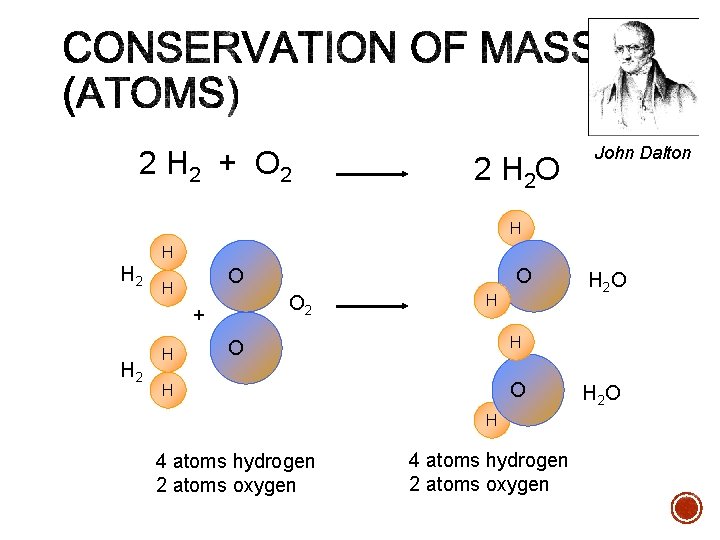
2 H 2 + O 2 2 H 2 O John Dalton H H H 2 O H O 2 + H 2 H O H H O O H H 4 atoms hydrogen 2 atoms oxygen H 2 O

§ Each compound has a specific ratio of elements § It is a ratio by mass § Water is always 8 grams of oxygen for every one gram of hydrogen § i. e. H 20, so two hydrogen for every one oxygen

THE LAW OF MULTIPLE PROPORTIONS § Dalton could not use his theory to determine the elemental compositions of chemical compounds because he had no reliable scale of atomic masses. § Dalton’s data led to a general statement known as the law of multiple proportions. § Law states that when two elements form a series of compounds, the ratios of the masses of the second element that are present per gram of the first element can almost always be expressed as the ratios of integers.

THE ATOMIC THEORY OF MATTER § Dalton’s atomic theory is essentially correct, with four minor modifications: 1. Not all atoms of an element must have precisely the same mass. 2. Atoms of one element can be transformed into another through nuclear reactions. 3. The composition of many solid compounds are somewhat variable. 4. Under certain circumstances, some atoms can be divided (split into smaller particles: i. e. nuclear fission).

John Dalton 1808

§ Scientist began to wonder what an atom was like. § Was it solid throughout with no internal structure or was it made up of smaller, subatomic particles? § It was not until the late 1800’s that evidence became available that atoms were composed of smaller parts.

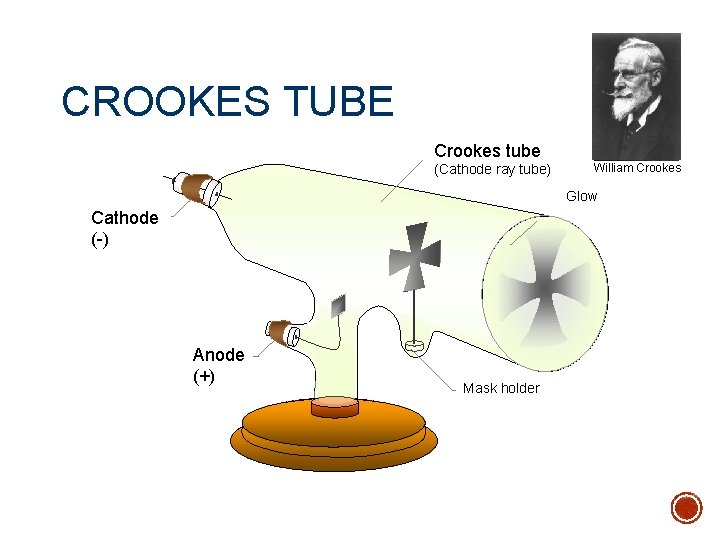
§ J. J. Thomson - English physicist 1897 § Used a piece of equipment called a cathode ray tube. § It is a vacuum tube - all the air has been pumped out. § This was made available by prior scientists including William Crookes

CROOKES TUBE Crookes tube (Cathode ray tube) William Crookes Glow Cathode (-) Anode (+) Mask holder

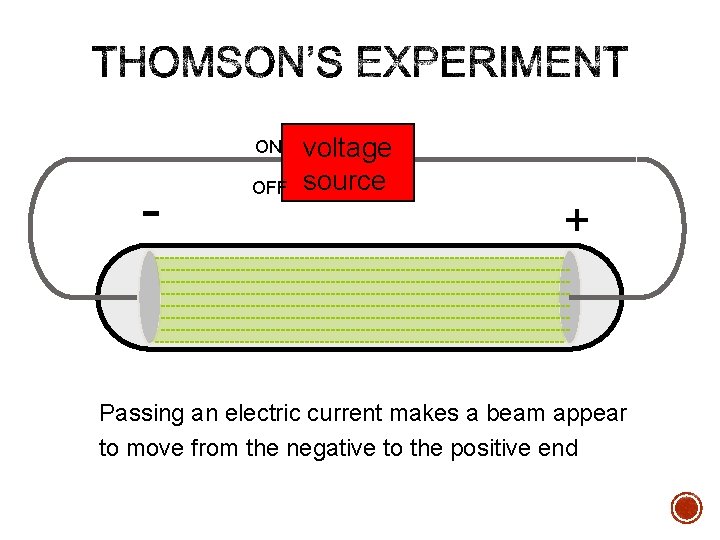
ON - OFF voltage source + Passing an electric current makes a beam appear to move from the negative to the positive end

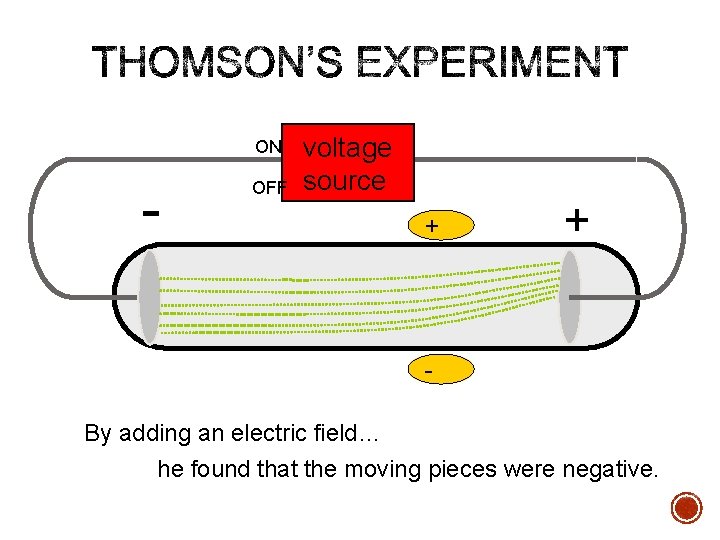
ON - OFF voltage source + + By adding an electric field… he found that the moving pieces were negative.

1897 Experimentation § Using a cathode ray tube, Thomson was able to deflect cathode rays with an electrical field. § The rays bent towards the positive pole, indicating that they are negatively charged.

§ He compared the value with the mass/ charge ratio for the lightest charged particle. § By comparison, Thomson estimated that the cathode ray particle weighed 1/1000 as much as hydrogen, the lightest atom. § He concluded that atoms do contain subatomic particles - atoms are divisible into smaller particles. § This conclusion contradicted Dalton’s postulate and was not widely accepted by fellow physicists and chemists of his day. § Since any electrode material produces an identical ray, cathode ray particles are present in all types of matter - a universal negatively charged subatomic particle later named the electron

§ He proved that atoms of any element can be made to emit tiny negative particles. § From this he concluded that ALL atoms must contain these negative particles. § He knew that atoms did not have a net negative charge and so there must be balancing of the negative charge. J. J. Thomson

§ Lord Kelvin with other scientists came up with several models in early 1900 s but most writings say… § In 1910, J. J. Thomson proposed the Plum Pudding model § Negative electrons were embedded into a positively charged spherical cloud.

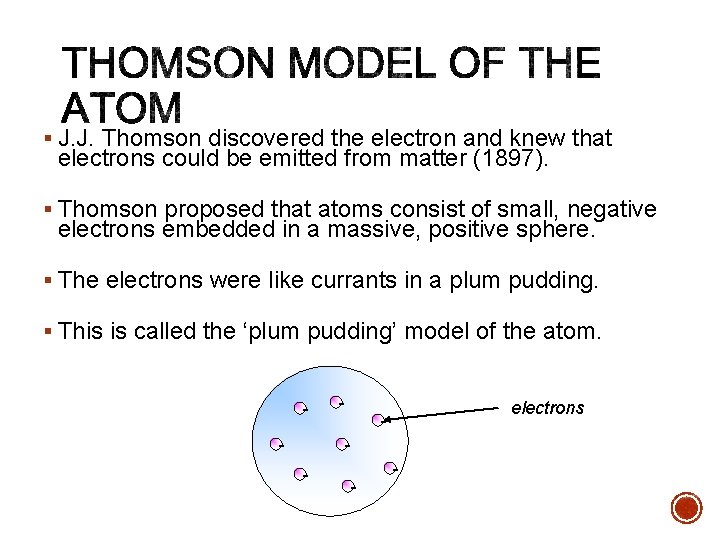
§ J. J. Thomson discovered the electron and knew that electrons could be emitted from matter (1897). § Thomson proposed that atoms consist of small, negative electrons embedded in a massive, positive sphere. § The electrons were like currants in a plum pudding. § This is called the ‘plum pudding’ model of the atom. - - electrons - -



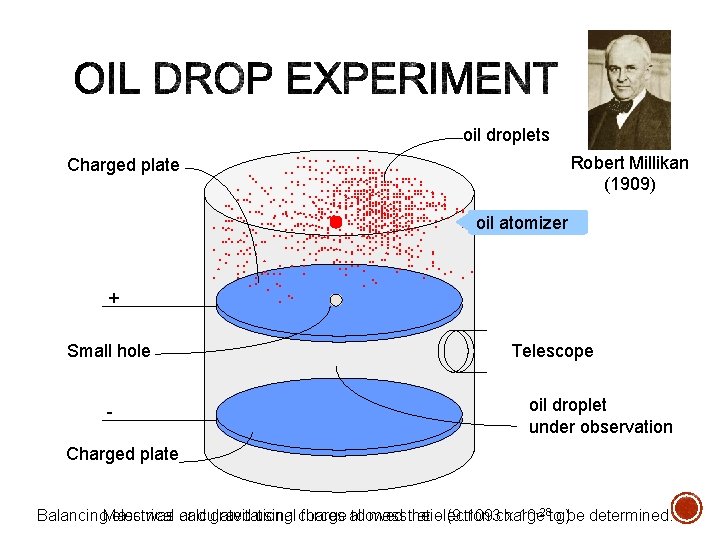
§ A fine mist of oil droplets is introduced into the chamber as the gas molecules inside the chamber are ionized (split into electrons and positive ions) by a beam of x-rays (not represented) § The electrons adhere to the oil droplets, some droplets having one electron, some two electrons, and so forth § These negatively charged oil droplets fall under the force of gravity into the region between the electrically charged plates § If you carefully adjust the voltage on the plates, the force of gravity can be exactly counterbalanced by the attractive force between the negative oil drop and upper, positively charged plate § Analysis of these forces leads to a value for the charge on thee electron § Robert Andrews Millikan (1868 – 1953) won the Nobel physics prize in 1923 for his work in isolating and weighing the electron

oil droplets Robert Millikan. . . . . (1909). . . . . . . . . . oil atomizer. . . . . . . . . . . . . . . . . . . . . . . . . . . Charged plate + Small hole - Telescope oil droplet under observation Charged plate was calculated using charge to mass the ratio (9. 1093 charge x 10 -28 tog). be determined. Balancing. Mass electrical and gravitational forces allowed electron


§ Learned physics in J. J. Thomson’s lab. § Noticed that ‘alpha’ particles were sometime deflected by something in the air. § Gold-foil experiment

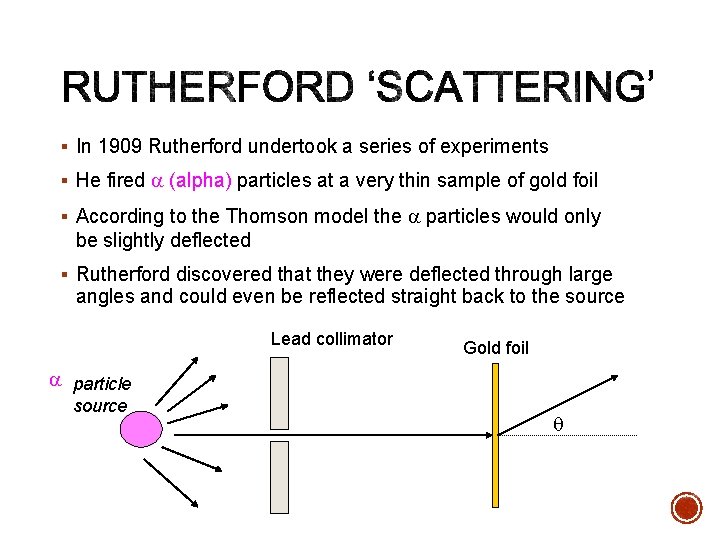
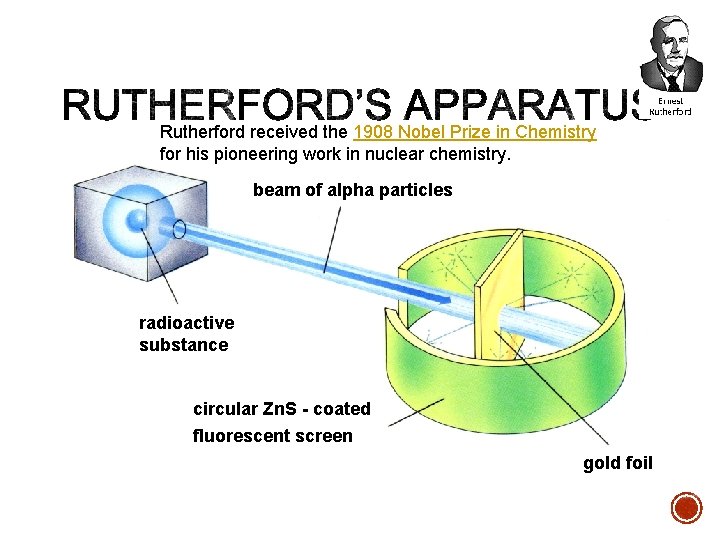
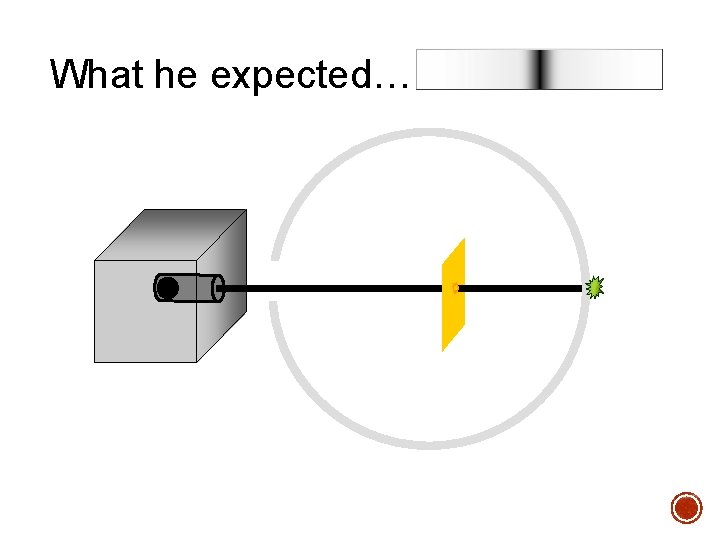
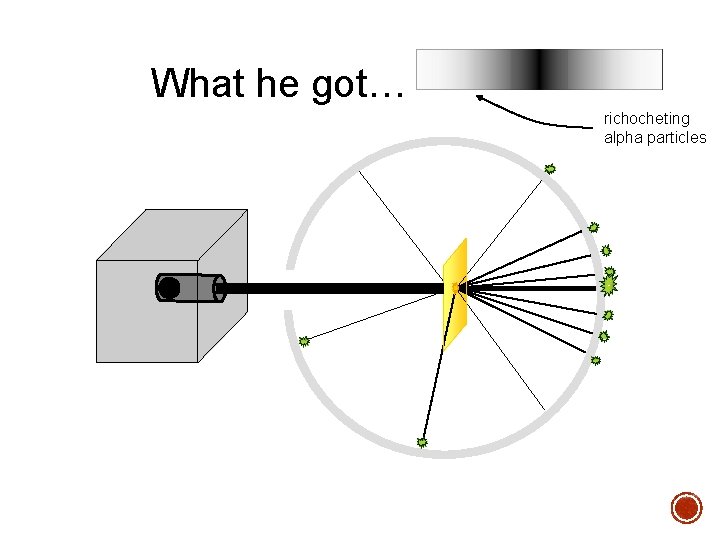
§ In 1909 Rutherford undertook a series of experiments § He fired a (alpha) particles at a very thin sample of gold foil § According to the Thomson model the a particles would only be slightly deflected § Rutherford discovered that they were deflected through large angles and could even be reflected straight back to the source Lead collimator Gold foil a particle source q

Rutherford received the 1908 Nobel Prize in Chemistry for his pioneering work in nuclear chemistry. beam of alpha particles radioactive substance circular Zn. S - coated fluorescent screen gold foil

What he expected…

What he got… richocheting alpha particles

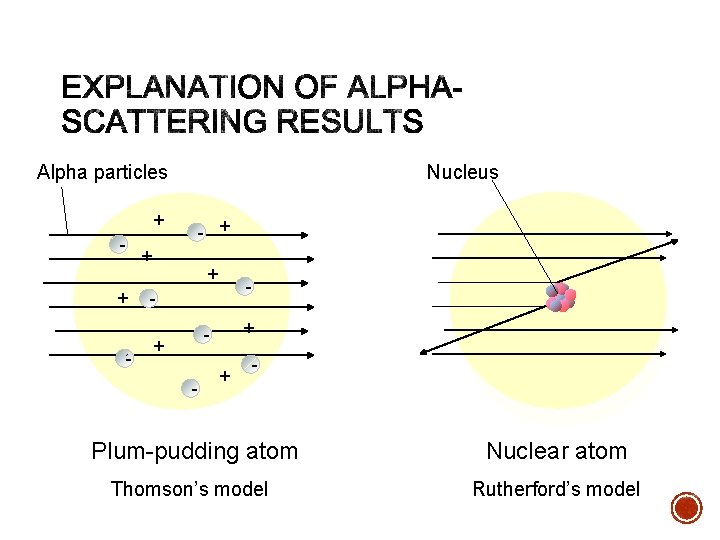
§ Since most of the particles went through, the atom was mostly empty. § Because the alpha rays were deflected so much, the positive pieces it was striking were heavy. § Small volume and big mass = big density § This small dense positive area is the nucleus

Alpha particles Nucleus + - - + + + - - + - + - Plum-pudding atom Nuclear atom Thomson’s model Rutherford’s model

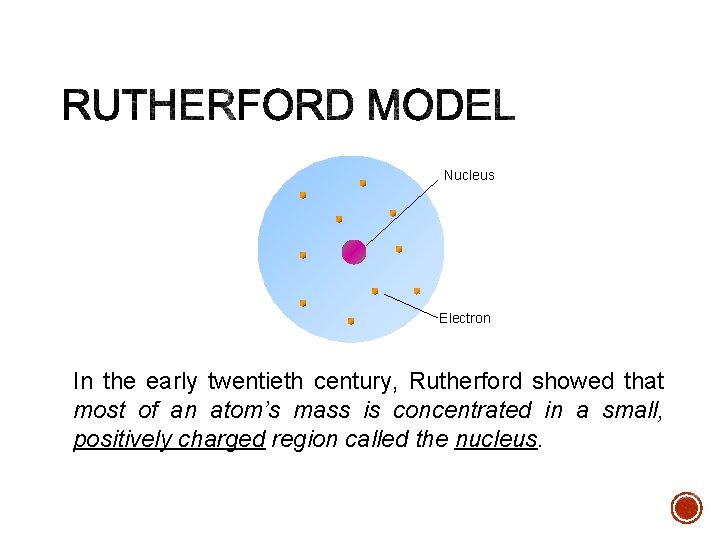
Nucleus Electron In the early twentieth century, Rutherford showed that most of an atom’s mass is concentrated in a small, positively charged region called the nucleus.

§ In the Bohr Model (1913) the neutrons and protons occupy a dense central region called the nucleus, and the electrons orbit the nucleus much like planets orbiting the Sun. § They are not confined to a planar orbit like the planets are.

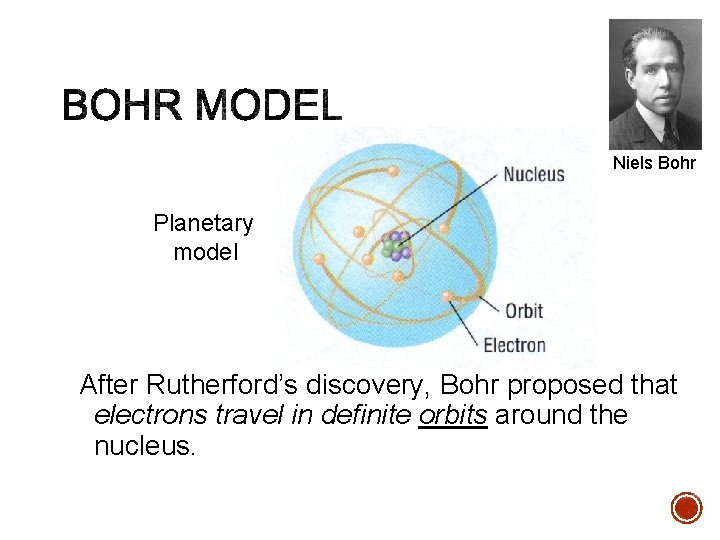
Niels Bohr Planetary model After Rutherford’s discovery, Bohr proposed that electrons travel in definite orbits around the nucleus.

§ The Bohr Model will be continued and explained in much greater detail next unit with the electron cloud § Quantum Model: § Electron Cloud – Physicists contributions to layers of suborbitals § Standard Model: § Quarks, Leptons, Higgs-Boson, etc.


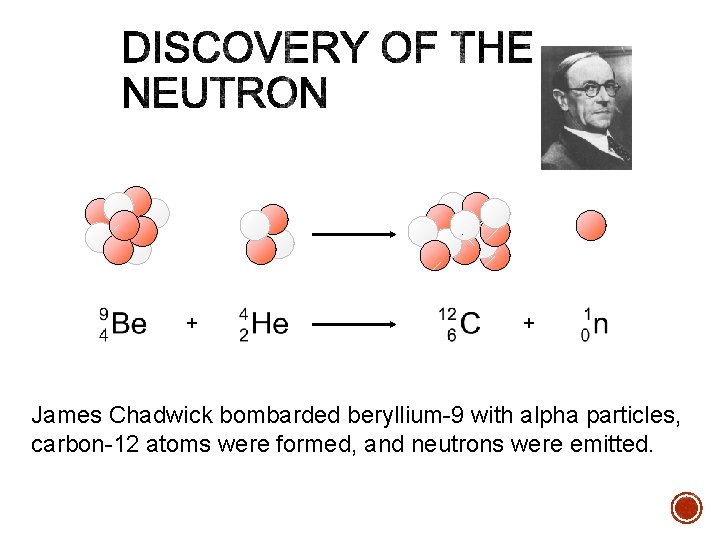
§ In 1886, Goldstein, using equipment similar to cathode ray tube, discovered particles with charge equal and opposite to that of electron, but much larger mass. § J. J. Thomson (~1897) discovered the electron § Rutherford later (1911) found “Goldstein’s particles” to be identical to hydrogen atoms minus one electron § named these particles protons § Chadwick (1932) discovered particles with similar mass to proton but zero charge. § discovered neutrons

+ + James Chadwick bombarded beryllium-9 with alpha particles, carbon-12 atoms were formed, and neutrons were emitted.

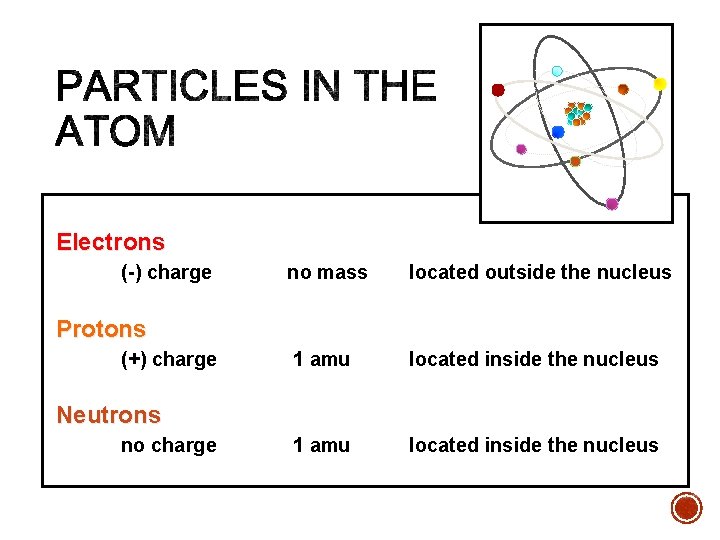
Electrons (-) charge no mass located outside the nucleus 1 amu located inside the nucleus Protons (+) charge Neutrons no charge

Atoms consist of electrons, electrons protons, protons and neutrons 1. Electrons and protons have electrical charges that are identical in magnitude but opposite in sign. Relative charges of 1 and +1 are assigned to the electron and proton, respectively. 2. Neutrons have approximately the same mass as protons but no charge—they are electrically neutral. 3. The mass of a proton or a neutron is about 1836 times greater than the mass of an electron. Protons and neutrons constitute the bulk of the mass of the atom.

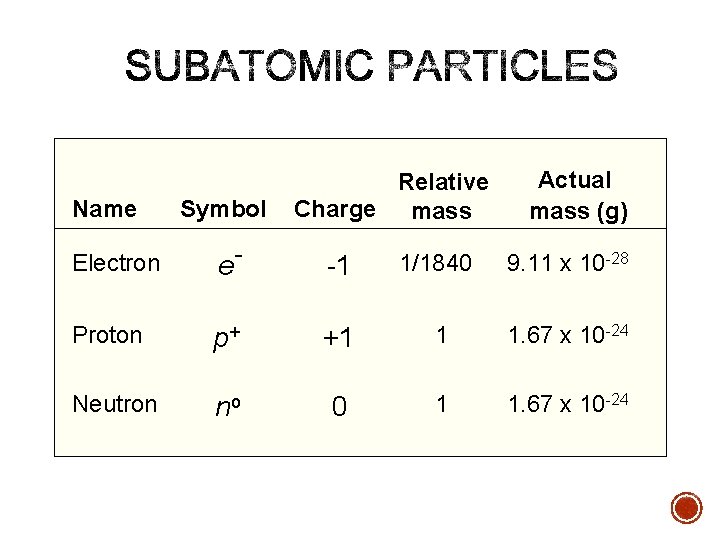
Name Symbol Relative Charge mass Actual mass (g) Electron e- -1 1/1840 9. 11 x 10 -28 Proton p+ +1 1 1. 67 x 10 -24 Neutron no 0 1 1. 67 x 10 -24

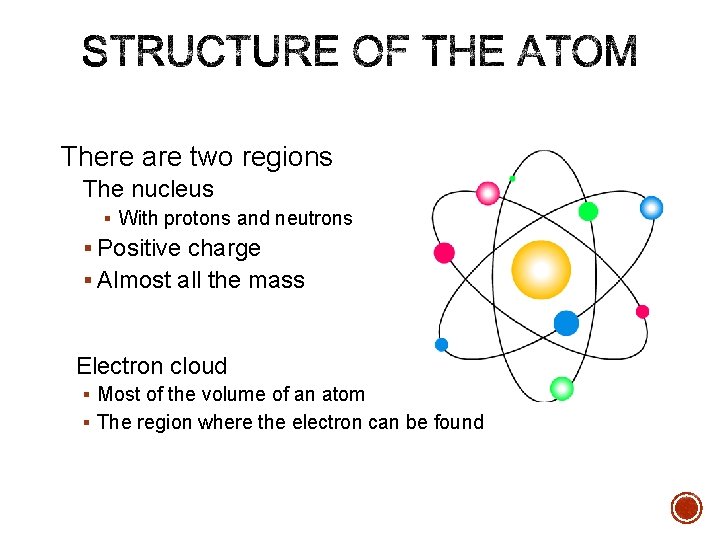
There are two regions The nucleus § With protons and neutrons § Positive charge § Almost all the mass Electron cloud § Most of the volume of an atom § The region where the electron can be found

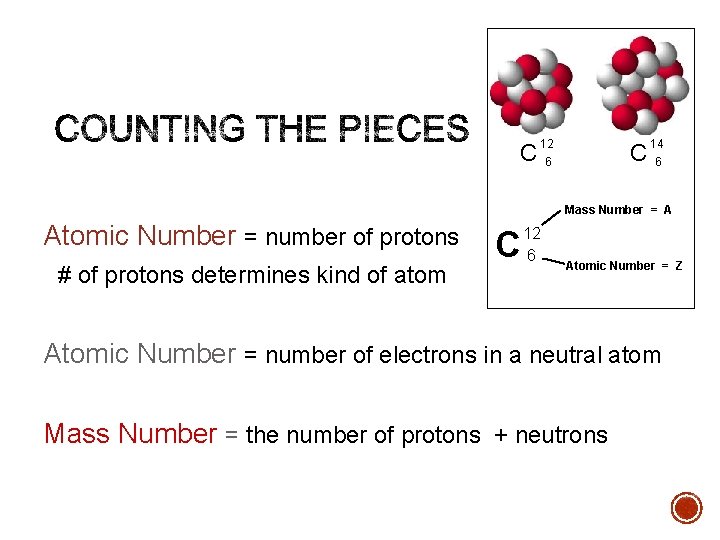
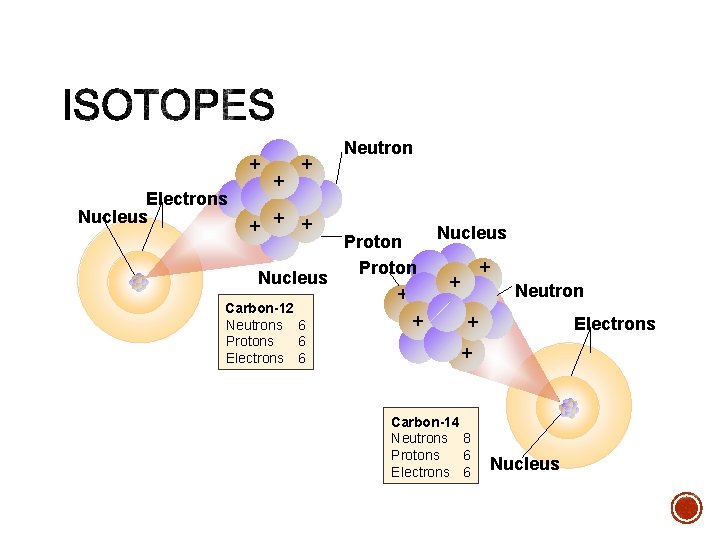
C 12 C 6 14 6 Mass Number = A Atomic Number = number of protons # of protons determines kind of atom C 12 6 Atomic Number = Z Atomic Number = number of electrons in a neutral atom Mass Number = the number of protons + neutrons

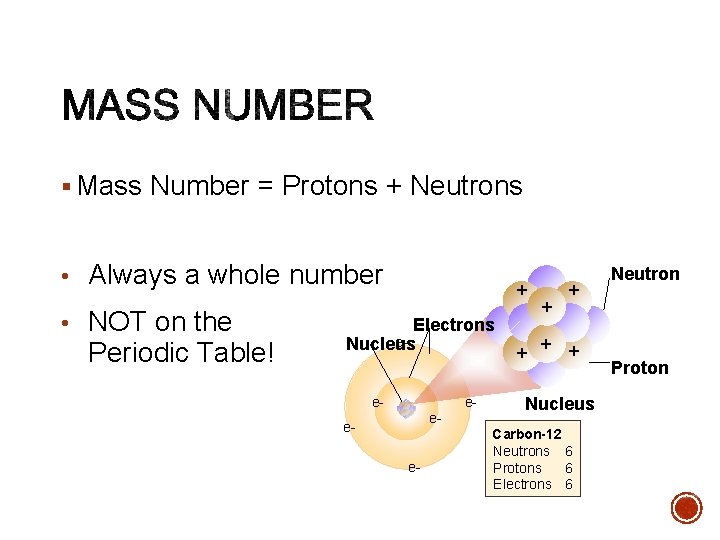
§ Mass Number = Protons + Neutrons • Always a whole number • NOT on the Periodic Table! + Electrons e. Nucleus e- ee- + + + Nucleus Carbon-12 Neutrons 6 Protons 6 Electrons 6 Neutron Proton

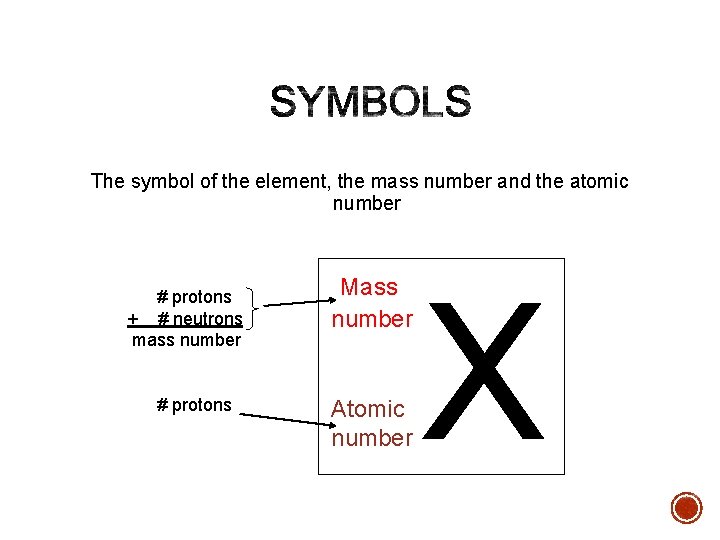
The symbol of the element, the mass number and the atomic number # protons + # neutrons mass number # protons Mass number Atomic number X

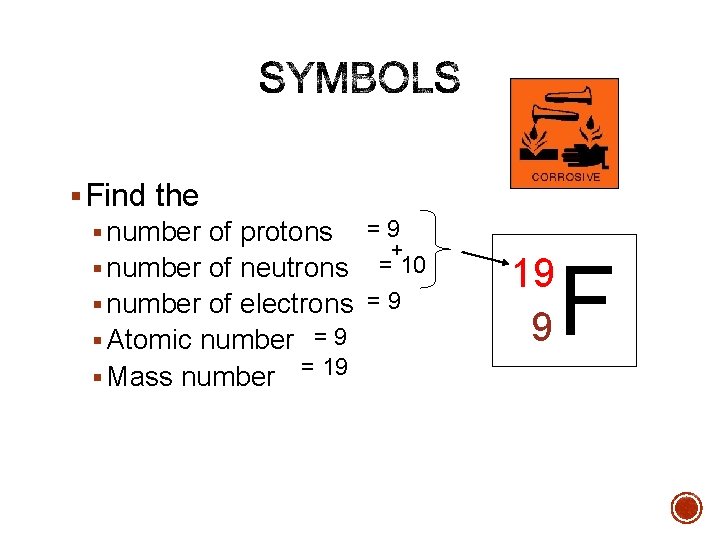
§ Find the § number of protons = 9 + § number of neutrons = 10 § number of electrons = 9 § Atomic number = 9 § Mass number = 19 19 9 F

§ If the symbol or atom is not specified as positive or negative, then it has a no overall charge and is thus neutral § This means it has equal number of protons and electrons § If they are not equal, then the atom will have either a net positive or negative charge § These are IONS § Positive ions are called Cations § Negative ions are call Anions

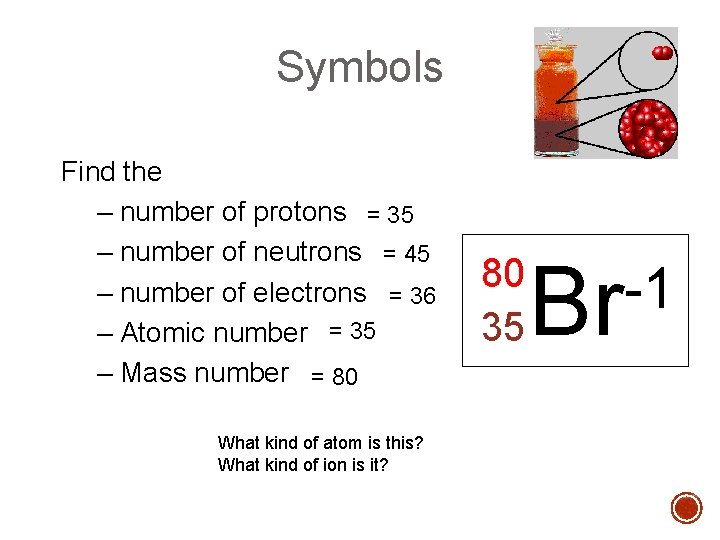
Symbols Find the – number of protons = 35 – number of neutrons = 45 – number of electrons = 36 – Atomic number = 35 – Mass number = 80 What kind of atom is this? What kind of ion is it? 80 35 Br -1

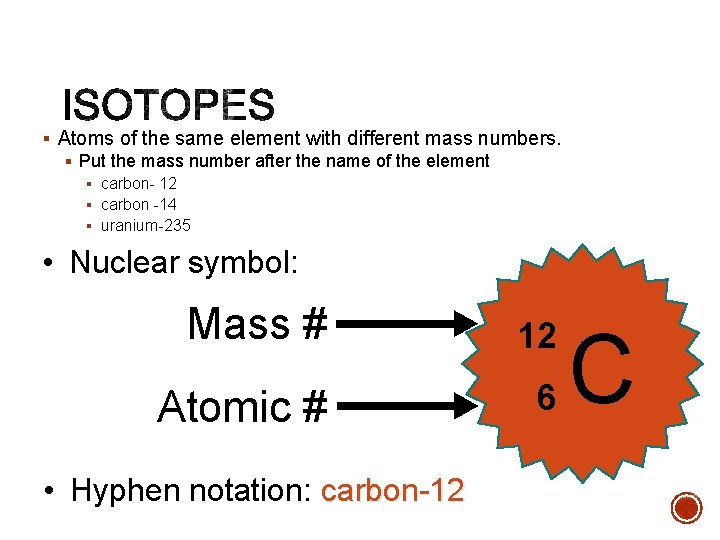
§ Atoms of the same element with different mass numbers. § Put the mass number after the name of the element § carbon- 12 § carbon -14 § uranium-235 • Nuclear symbol: Mass # 12 Atomic # 6 • Hyphen notation: carbon-12 C

+ Electrons Nucleus + + Neutron + + + Proton Nucleus + Carbon-12 Neutrons 6 Protons 6 Electrons 6 + Nucleus + + Neutron + Electrons + Carbon-14 Neutrons 8 Protons 6 Electrons 6 Nucleus

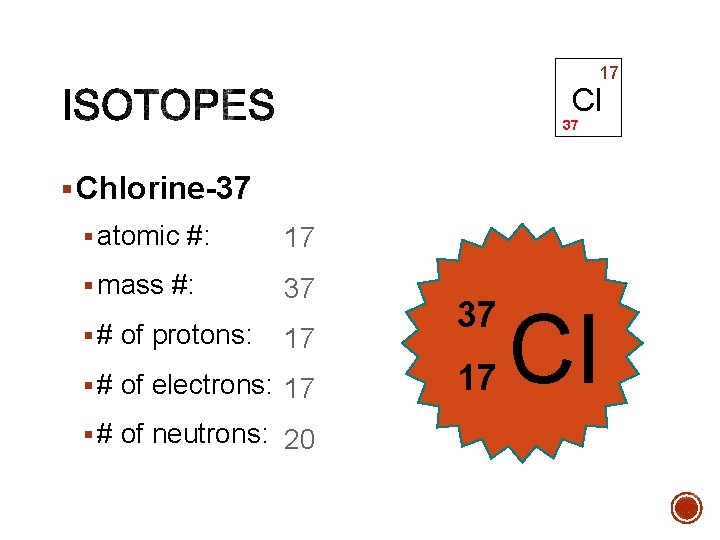
17 Cl 37 § Chlorine-37 § atomic #: 17 § mass #: 37 § # of protons: 17 § # of electrons: 17 § # of neutrons: 20 37 17 Cl

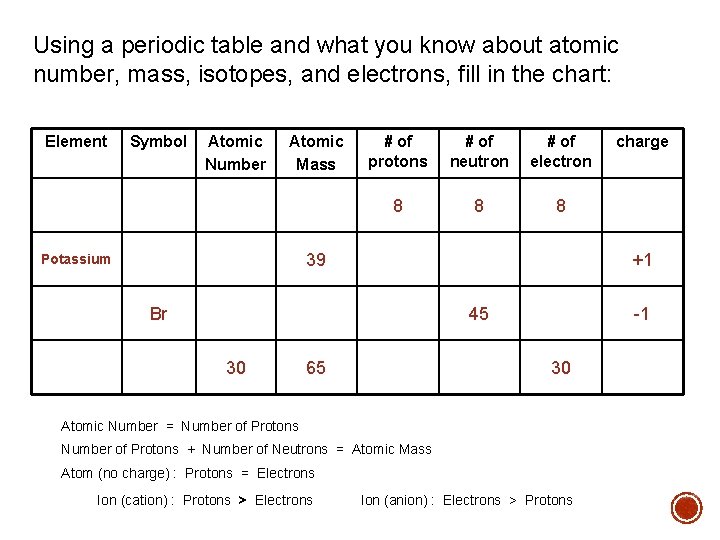
Using a periodic table and what you know about atomic number, mass, isotopes, and electrons, fill in the chart: Element Symbol Atomic Number Atomic Mass # of protons # of neutron # of electron 8 8 8 39 Potassium +1 Br 45 30 65 -1 30 Atomic Number = Number of Protons + Number of Neutrons = Atomic Mass Atom (no charge) : Protons = Electrons Ion (cation) : Protons > Electrons charge Ion (anion) : Electrons > Protons

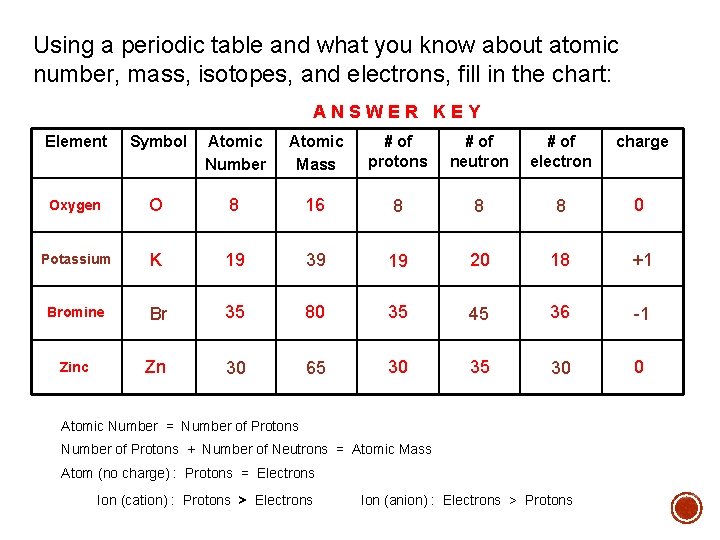
Using a periodic table and what you know about atomic number, mass, isotopes, and electrons, fill in the chart: ANSWER KEY Element Symbol Atomic Number Atomic Mass # of protons # of neutron # of electron charge Oxygen O 8 16 8 8 8 0 Potassium K 19 39 19 20 18 +1 Bromine Br 35 80 35 45 36 -1 Zinc Zn 30 65 30 35 30 0 Atomic Number = Number of Protons + Number of Neutrons = Atomic Mass Atom (no charge) : Protons = Electrons Ion (cation) : Protons > Electrons Ion (anion) : Electrons > Protons

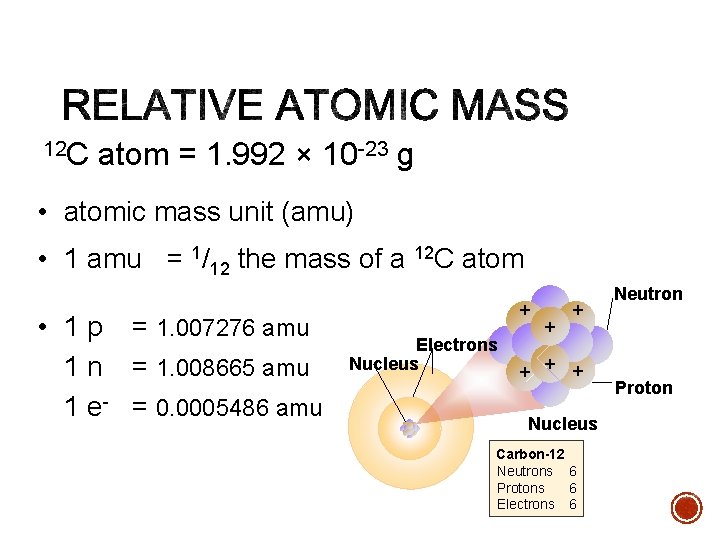
12 C atom = 1. 992 × 10 -23 g • atomic mass unit (amu) • 1 amu = 1/12 the mass of a 12 C atom • 1 p = 1. 007276 amu 1 n = 1. 008665 amu 1 e- = 0. 0005486 amu + Electrons Nucleus + + + Nucleus Carbon-12 Neutrons 6 Protons 6 Electrons 6 Neutron Proton

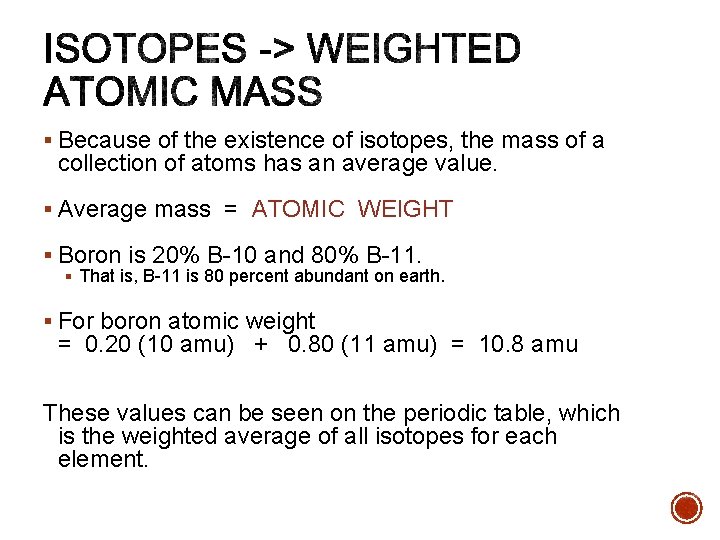
§ Because of the existence of isotopes, the mass of a collection of atoms has an average value. § Average mass = ATOMIC WEIGHT § Boron is 20% B-10 and 80% B-11. § That is, B-11 is 80 percent abundant on earth. § For boron atomic weight = 0. 20 (10 amu) + 0. 80 (11 amu) = 10. 8 amu These values can be seen on the periodic table, which is the weighted average of all isotopes for each element.

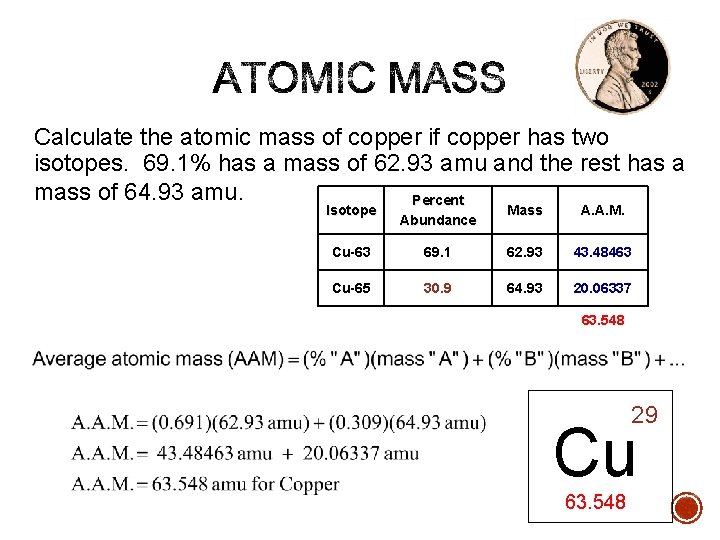
Calculate the atomic mass of copper if copper has two isotopes. 69. 1% has a mass of 62. 93 amu and the rest has a mass of 64. 93 amu. Percent Isotope Abundance Mass A. A. M. Cu-63 69. 1 62. 93 43. 48463 Cu-65 30. 9 64. 93 20. 06337 63. 548 29 Cu 63. 548

§ Assume you have only two atoms of chlorine. § One atom has a mass of 35 amu (Cl-35) § The other atom has a mass of 36 amu (Cl-36) 17 Cl 35. 453 § What is the average mass of these two isotopes? 35. 5 amu § Looking at the average atomic mass printed on the periodic table. . . approximately what percentage is Cl-35 and Cl-36? 55% Cl-35 and 45% Cl-36 is a good approximation

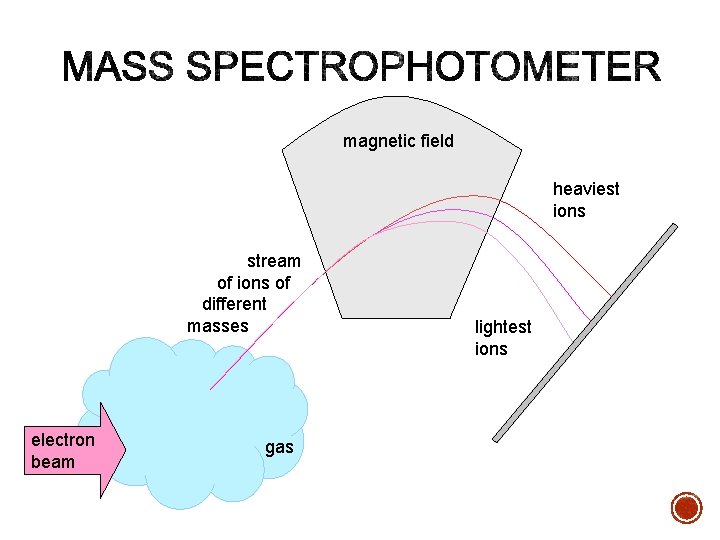
magnetic field heaviest ions stream of ions of different masses electron beam gas lightest ions

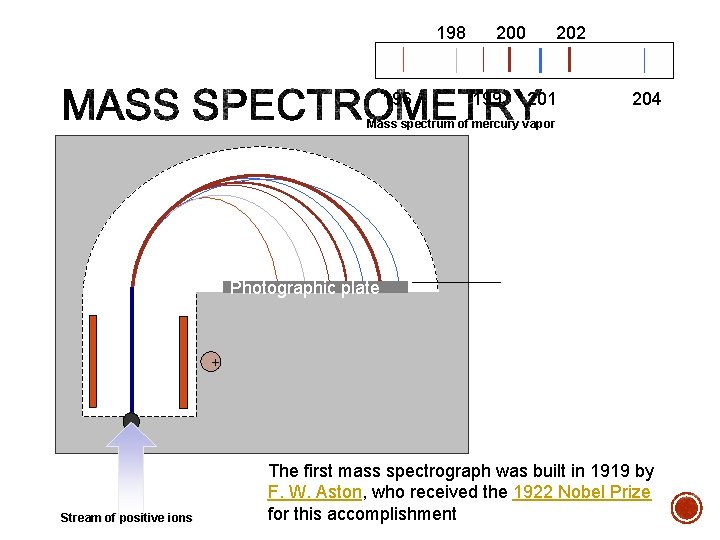
198 196 200 199 202 201 204 Mass spectrum of mercury vapor Photographic plate - Stream of positive ions + The first mass spectrograph was built in 1919 by F. W. Aston, who received the 1922 Nobel Prize for this accomplishment

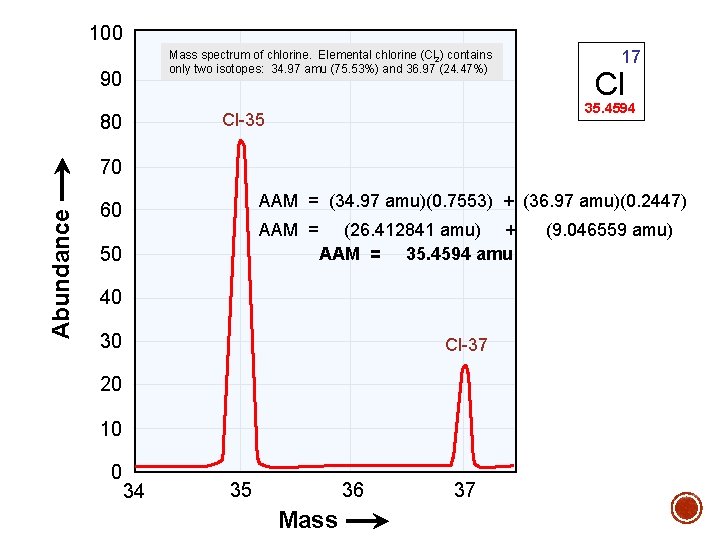
100 Mass spectrum of chlorine. Elemental chlorine (Cl 2) contains only two isotopes: 34. 97 amu (75. 53%) and 36. 97 (24. 47%) 90 Cl 35. 4594 Cl-35 80 17 Abundance 70 AAM = (34. 97 amu)(0. 7553) + (36. 97 amu)(0. 2447) 60 AAM = 50 (26. 412841 amu) + AAM = 35. 4594 amu 40 30 Cl-37 20 10 0 34 36 35 Mass 37 (9. 046559 amu)

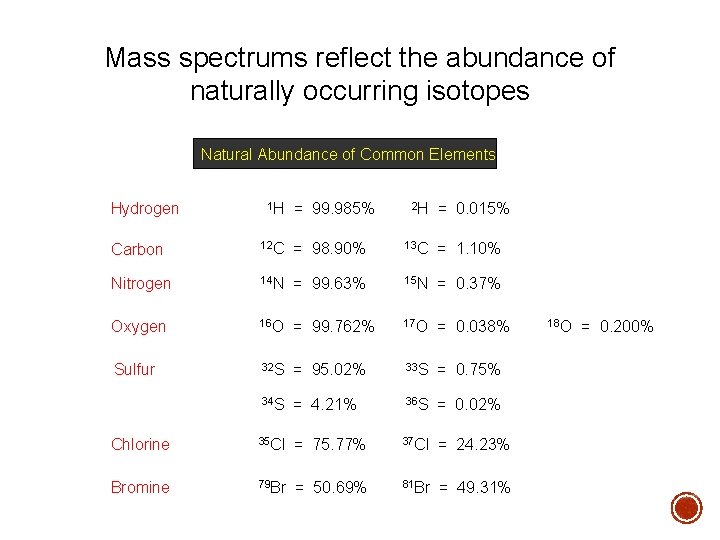
Mass spectrums reflect the abundance of naturally occurring isotopes Natural Abundance of Common Elements Hydrogen 1 H = 99. 985% 2 H = 0. 015% Carbon 12 C = 98. 90% 13 C = 1. 10% Nitrogen 14 N = 99. 63% 15 N = 0. 37% Oxygen 16 O = 99. 762% 17 O = 0. 038% Sulfur 32 S = 95. 02% 33 S = 0. 75% 34 S = 4. 21% 36 S = 0. 02% Chlorine 35 Cl = 75. 77% 37 Cl = 24. 23% Bromine 79 Br = 50. 69% 81 Br = 49. 31% 18 O = 0. 200%