Welcome ICP Todays Learning Targets Leading into 2


























- Slides: 64

Welcome ICP – Today’s Learning Targets… Leading into #2, #3 and #5: We will discuss… • Chemical formulas – tell us the elements and number of them • Chemical bonds - the attractive force that holds compounds together

Combining Elements • Compounds formed when elements combine often have different properties than the elements that formed them https: //www. youtube. com/wat ch? v=Yv. Sk. Xd_VVYk

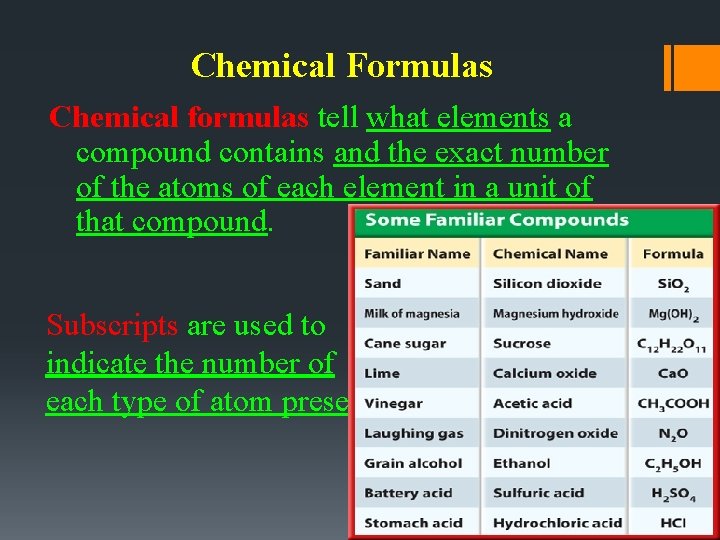
Chemical Formulas Chemical formulas tell what elements a compound contains and the exact number of the atoms of each element in a unit of that compound. Subscripts are used to indicate the number of each type of atom present

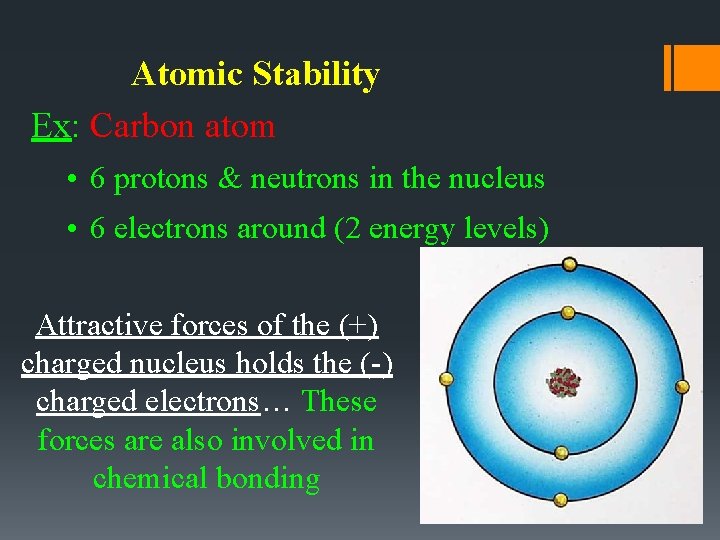
Atomic Stability Ex: Carbon atom • 6 protons & neutrons in the nucleus • 6 electrons around (2 energy levels) Attractive forces of the (+) charged nucleus holds the (-) charged electrons… These forces are also involved in chemical bonding

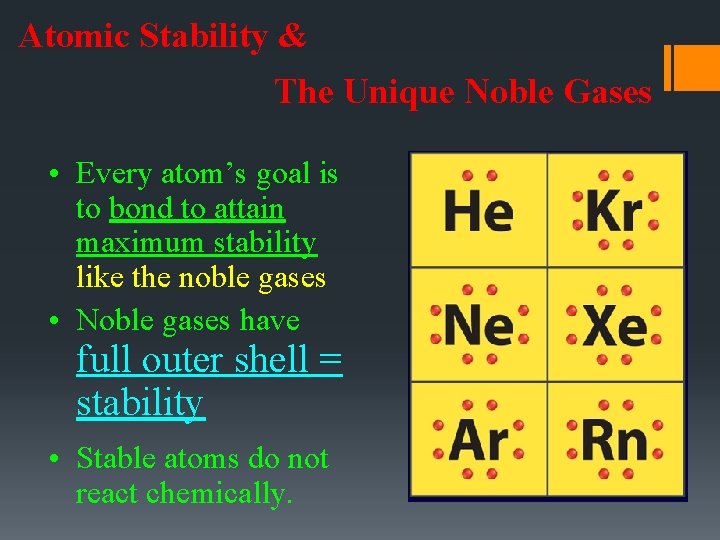
Atomic Stability & The Unique Noble Gases • Every atom’s goal is to bond to attain maximum stability like the noble gases • Noble gases have full outer shell = stability • Stable atoms do not react chemically.

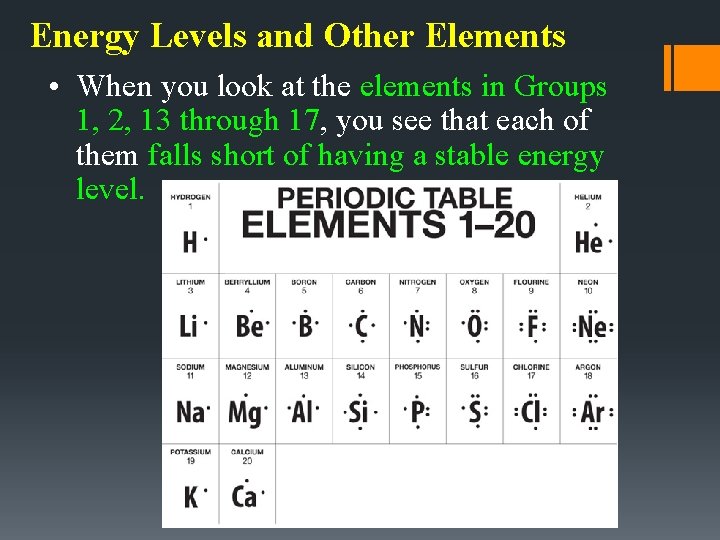
Energy Levels and Other Elements • When you look at the elements in Groups 1, 2, 13 through 17, you see that each of them falls short of having a stable energy level.

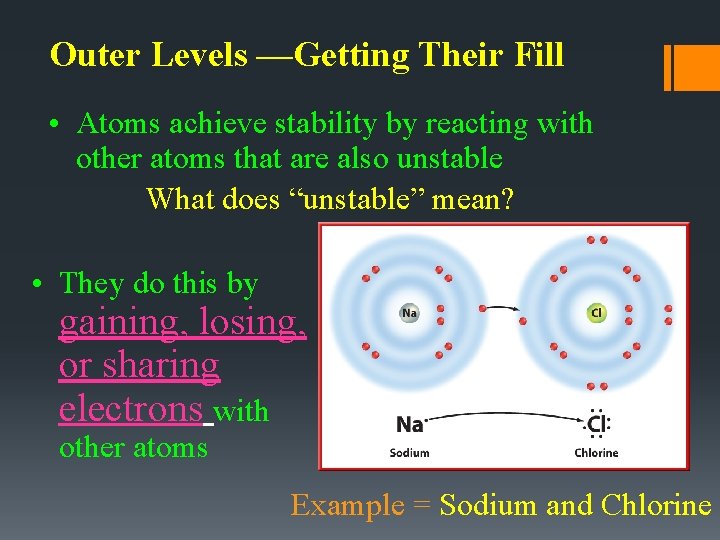
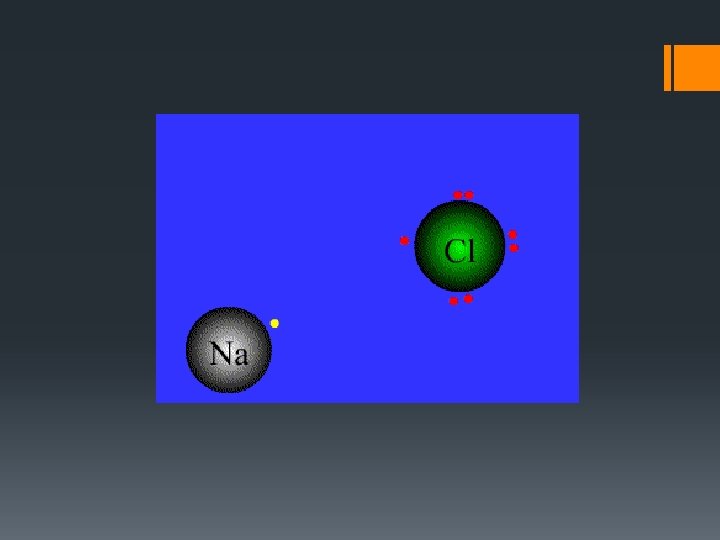
Outer Levels —Getting Their Fill • Atoms achieve stability by reacting with other atoms that are also unstable What does “unstable” mean? • They do this by gaining, losing, or sharing electrons with other atoms Example = Sodium and Chlorine

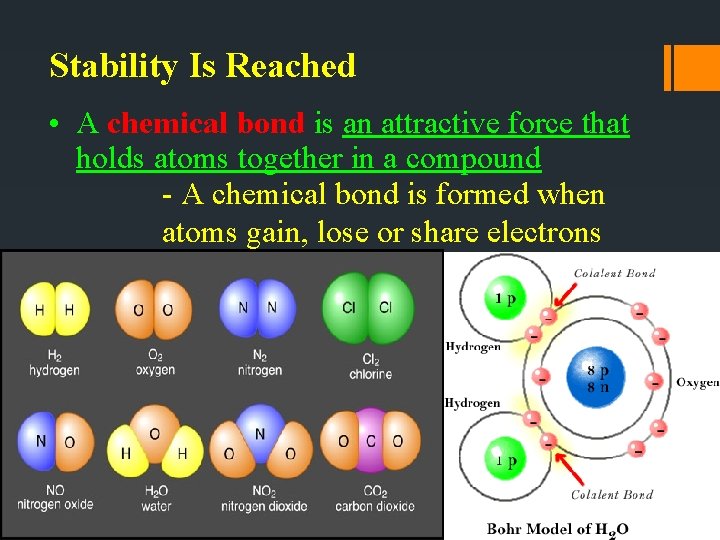
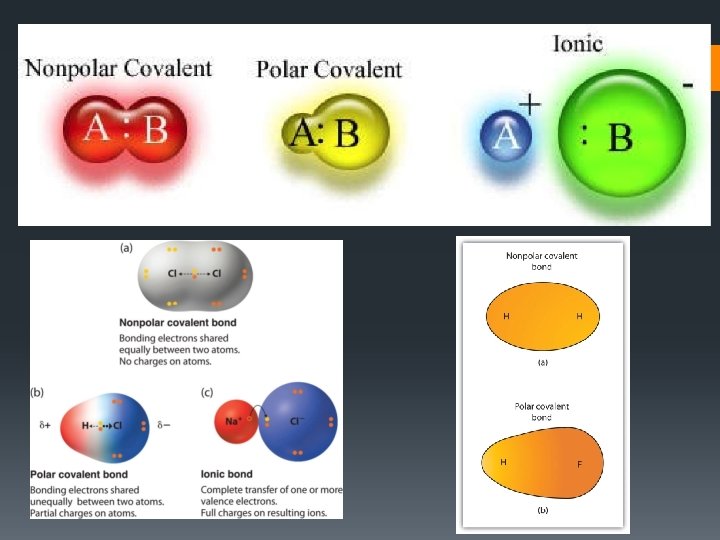
Stability Is Reached • A chemical bond is an attractive force that holds atoms together in a compound - A chemical bond is formed when atoms gain, lose or share electrons

ICP Section 18. 1 Check 1. What tells the elements a compound contains and how many atoms of each element per molecule of the compound? _______ 2. The number of _____ in each group’s outer energy level increases across the periodic table. 3. What is the force that holds atoms together in compounds? _______

Section Check Question 1 - Answer A chemical formula is a form of chemical shorthand that tells what elements and how many atoms of each are in one molecule of a compound.

Section Check Question 2 The number of _____ in each group’s outer energy level increases across the periodic table. A. B. C. D. electrons neutrons protons and neutrons

Section Check Answer The answer is A. Protons and neutrons are located in the nucleus of the atom.

Section Check Question 3 What is the force that holds atoms together in compounds? Answer The force that holds atoms together in compounds is a chemical bond.

Learning Targets: 3. Write ionic names and chemical formulas based upon ionic charges. 5. Explain that when charge is transferred from one object to another, the amount lost by one object equals the amount gained by another.

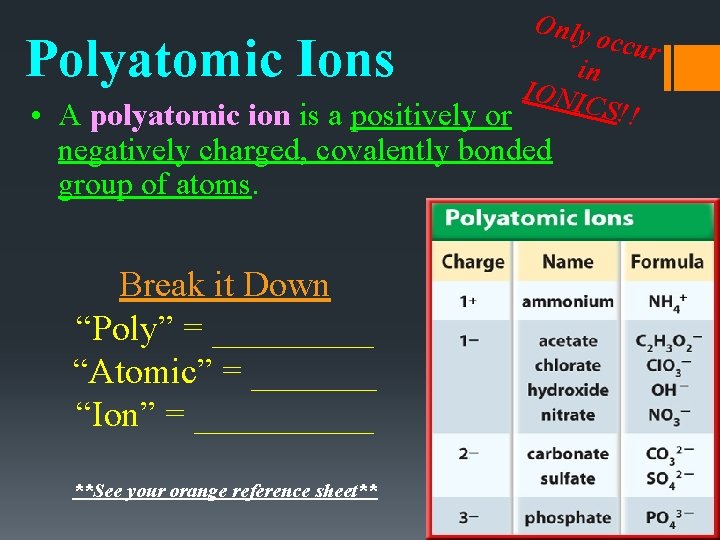
Ionic Bonding • An ion is a charged particle due to a gain/loss of electrons. Cations Positively Charge Lost Electrons Ex = Na+, K+, Fe+3 Anions Negatively Charge Gained Electrons Ex = Cl-, F-, O-2

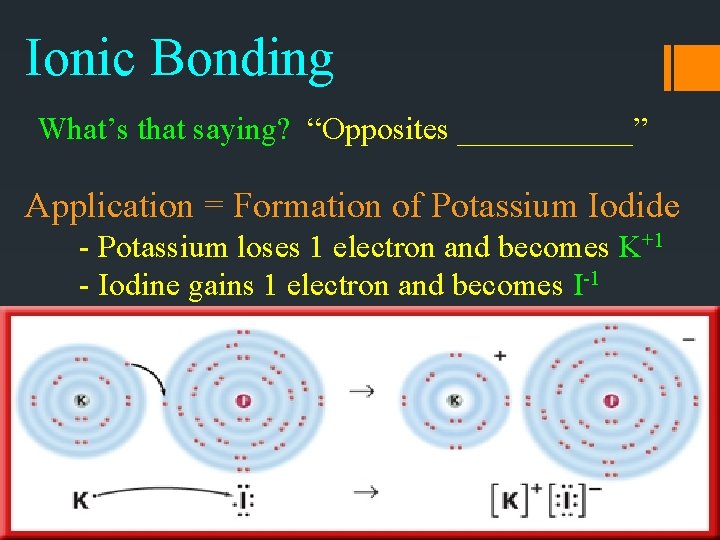
Ionic Bonding What’s that saying? “Opposites ______” Application = Formation of Potassium Iodide - Potassium loses 1 electron and becomes K+1 - Iodine gains 1 electron and becomes I-1



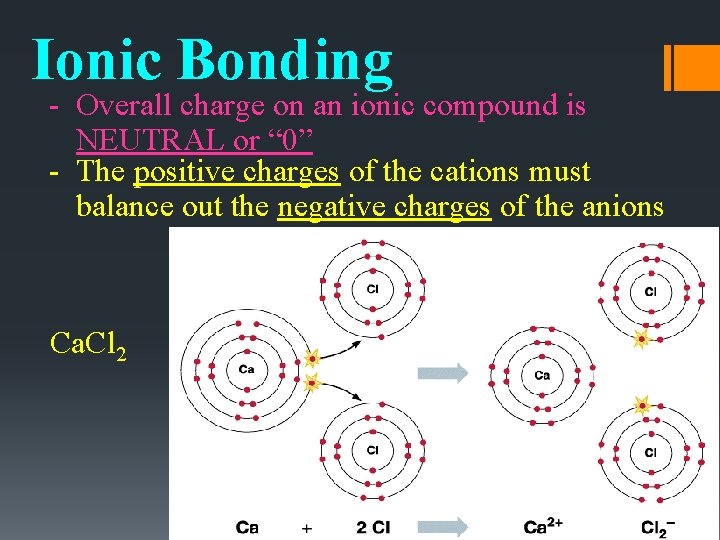
Ionic Bonding • An ionic bond is the force of attraction between the opposite charges of the ions in an ionic compound • If an element loses electrons, one or more elements must gain an equal number of electrons to maintain the neutral charge of the compound. NEUTRAL Compound!

Ionic Bonding - Overall charge on an ionic compound is NEUTRAL or “ 0” - The positive charges of the cations must balance out the negative charges of the anions Ca. Cl 2

Ionic Bonding Examples/Practice §What compound would form from Mg+2 and Cl. Mg. Cl 2 §What compound would form from K+ and O 2 K 2 O §Remember: We need NEUTRAL!!

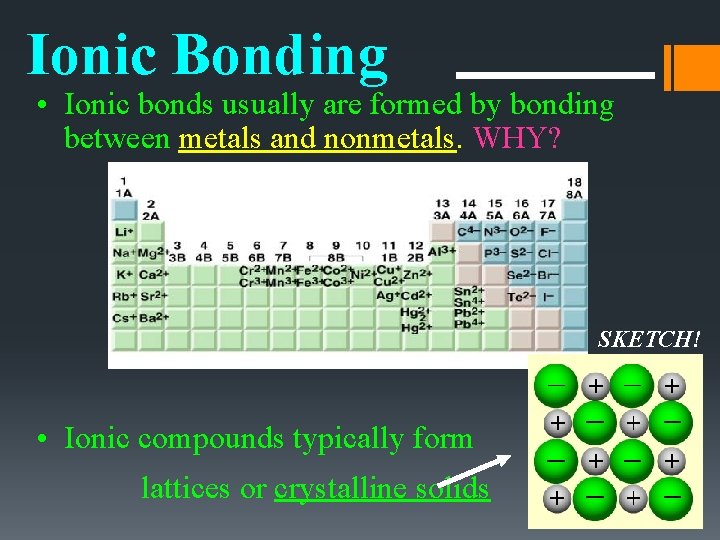
Ionic Bonding • Ionic bonds usually are formed by bonding between metals and nonmetals. WHY? SKETCH! • Ionic compounds typically form lattices or crystalline solids

Covalent Bonding - Sharing Electrons • Some atoms of nonmetals are unlikely to lose or gain electrons WHY? • TOO MANY to gain/lose it’s just not realistic. e Examples: Carbon Silicon Nitrogen

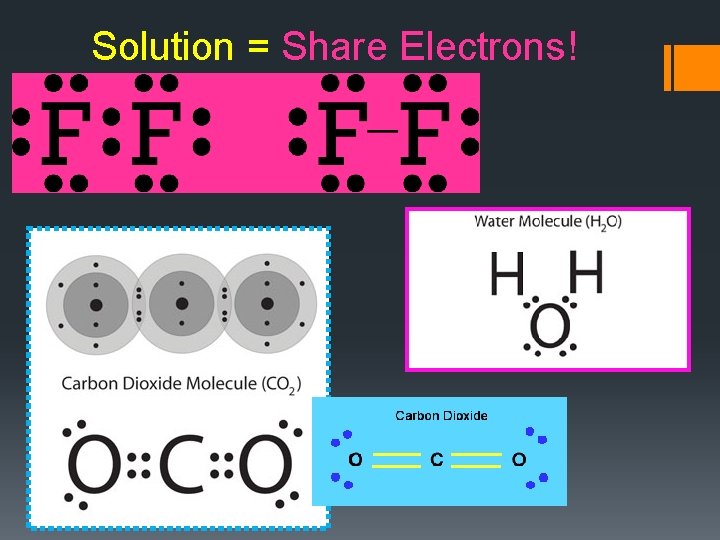
Solution = Share Electrons!

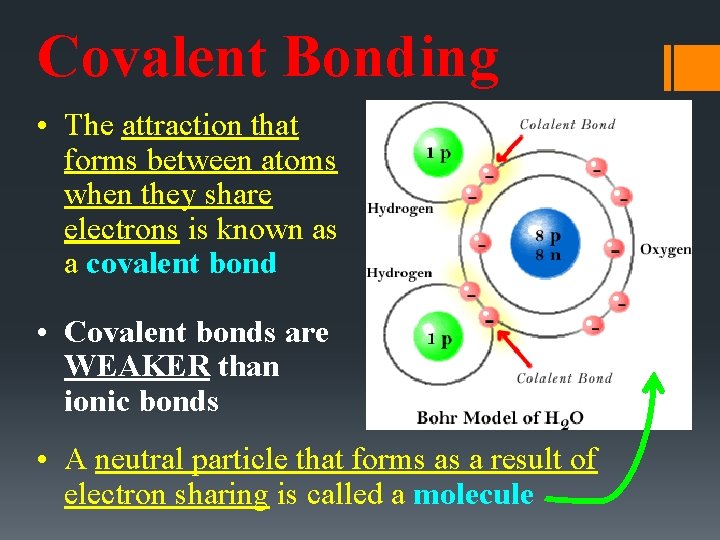
Covalent Bonding • The attraction that forms between atoms when they share electrons is known as a covalent bond • Covalent bonds are WEAKER than ionic bonds • A neutral particle that forms as a result of electron sharing is called a molecule


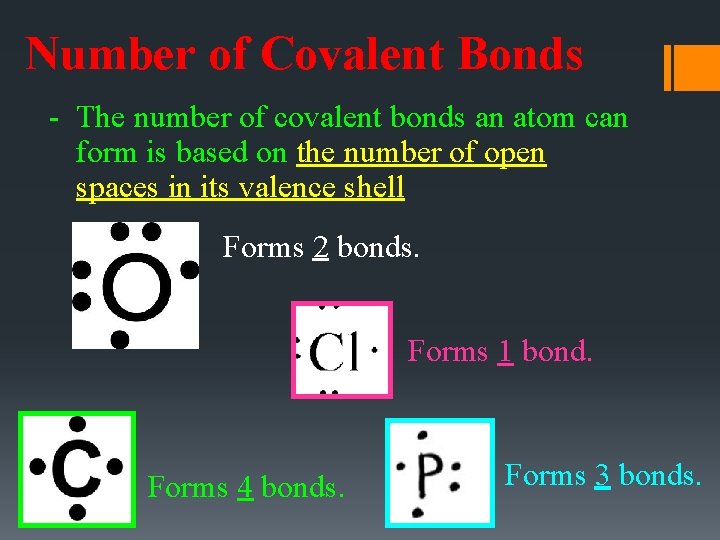
Number of Covalent Bonds - The number of covalent bonds an atom can form is based on the number of open spaces in its valence shell Forms 2 bonds. Forms 1 bond. Forms 4 bonds. Forms 3 bonds.

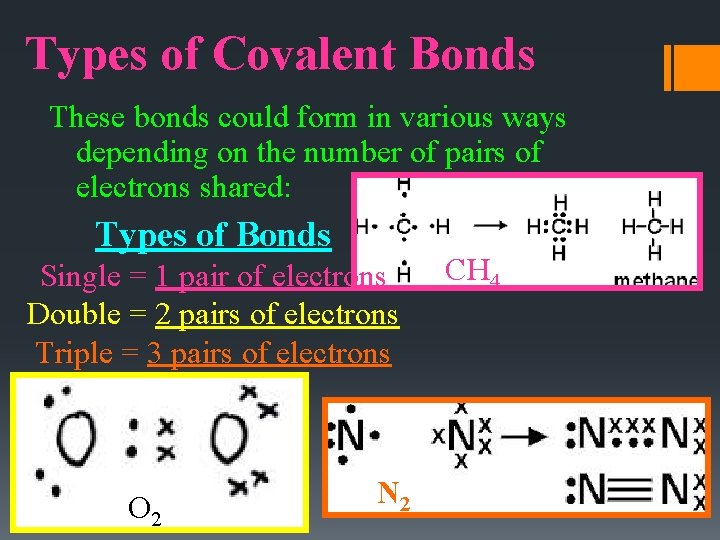
Types of Covalent Bonds These bonds could form in various ways depending on the number of pairs of electrons shared: Types of Bonds Single = 1 pair of electrons Double = 2 pairs of electrons Triple = 3 pairs of electrons O 2 N 2 CH 4

§Today’s Learning Targets: § #1 – Differentiate between covalent, ionic, hydrogen and Vander Waal's bonding. § #3 – Write ionic formulas based on ion charges. §Section Check: 1. What is the difference between ionic & covalent? 2. How do we figure out how many bonds an atom can make? a. Ex: How many bonds could sulfur form?


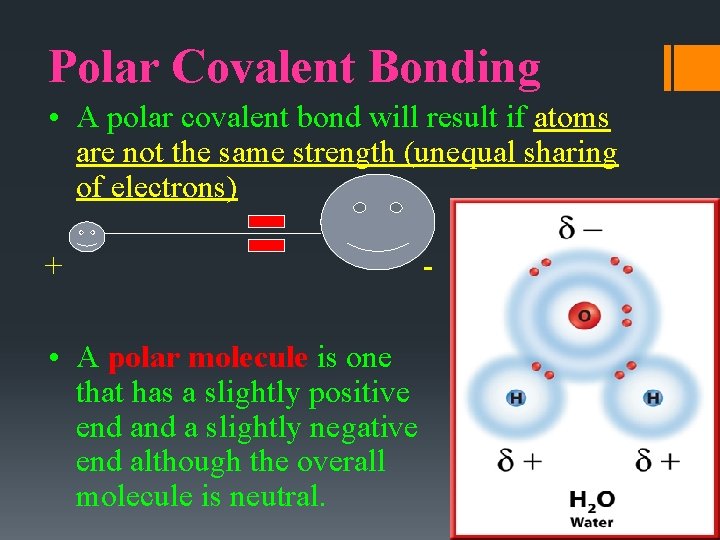
Polar Covalent Bonding • A polar covalent bond will result if atoms are not the same strength (unequal sharing of electrons) + • A polar molecule is one that has a slightly positive end a slightly negative end although the overall molecule is neutral. -

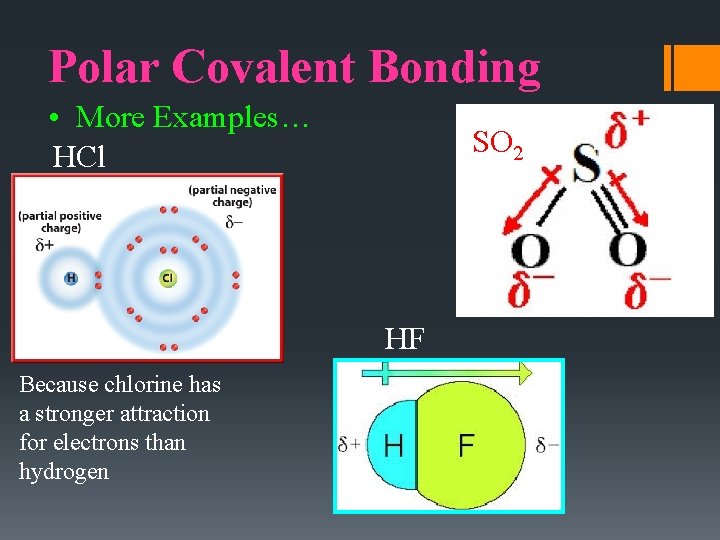
Polar Covalent Bonding • More Examples… HCl SO 2 HF Because chlorine has a stronger attraction for electrons than hydrogen

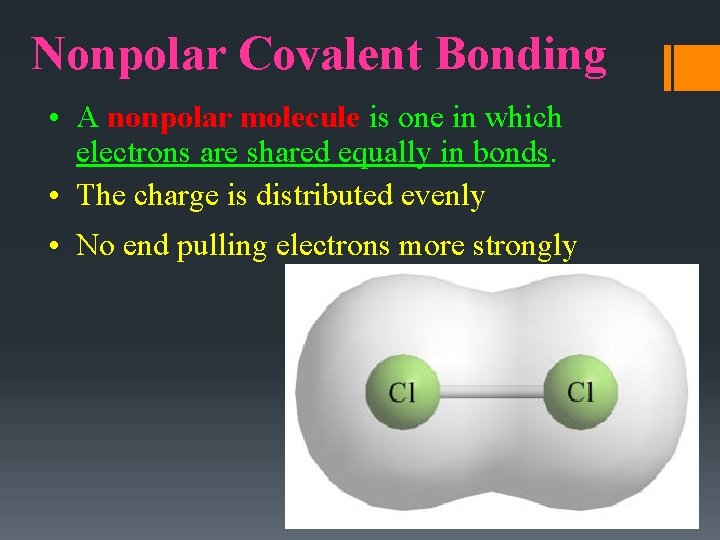
Nonpolar Covalent Bonding • A nonpolar molecule is one in which electrons are shared equally in bonds. • The charge is distributed evenly • No end pulling electrons more strongly

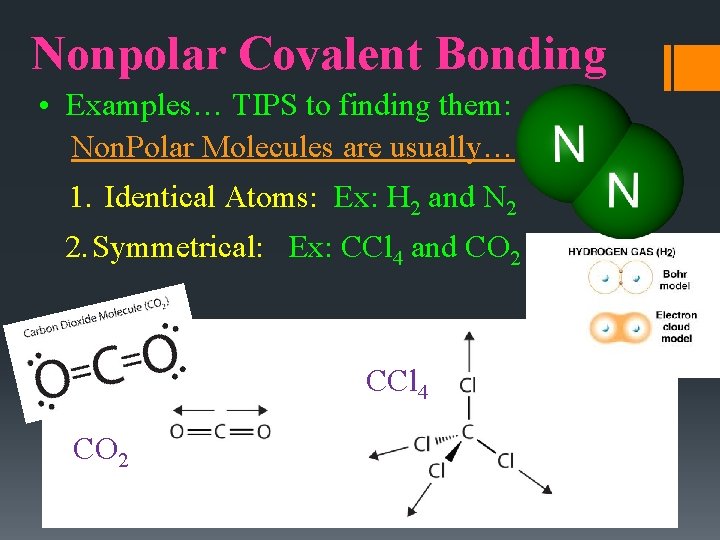
Nonpolar Covalent Bonding • Examples… TIPS to finding them: Non. Polar Molecules are usually… 1. Identical Atoms: Ex: H 2 and N 2 2. Symmetrical: Ex: CCl 4 and CO 2 CCl 4 CO 2


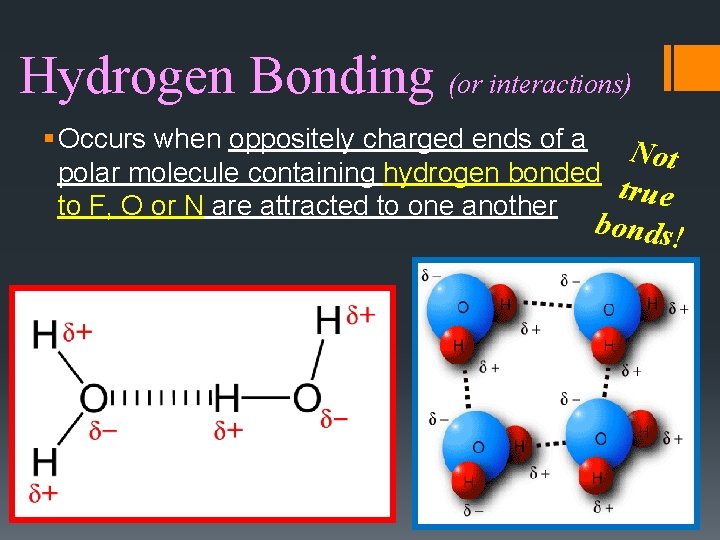
Hydrogen Bonding (or interactions) § Occurs when oppositely charged ends of a N ot polar molecule containing hydrogen bonded true to F, O or N are attracted to one another bonds!

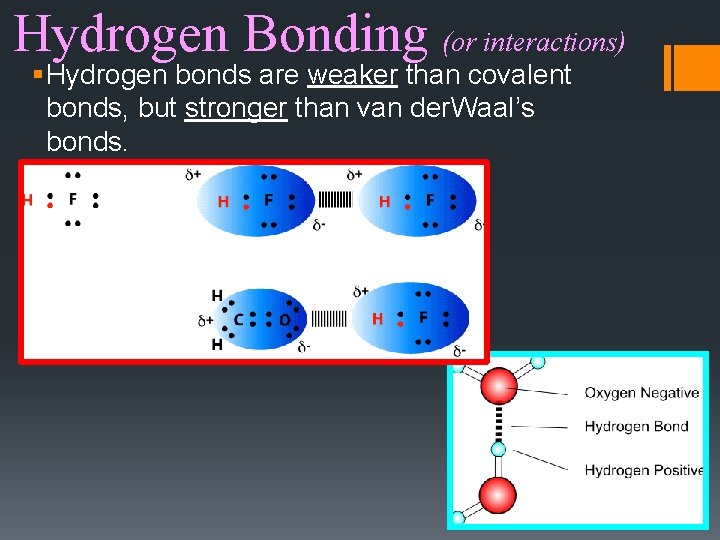
Hydrogen Bonding (or interactions) § Hydrogen bonds are weaker than covalent bonds, but stronger than van der. Waal’s bonds.

Vander. Waal’s Bonding (or force) § Occurs when oppositely charged ends of any polar molecule are attracted.

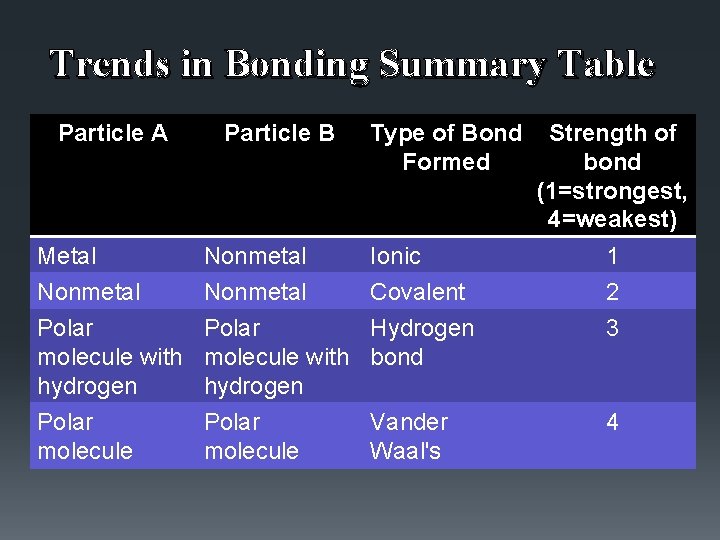
Trends in Bonding Summary Table Particle A Particle B Type of Bond Formed Strength of bond (1=strongest, 4=weakest) Metal Nonmetal Ionic 1 Nonmetal Covalent 2 Polar Hydrogen molecule with bond hydrogen 3 Polar molecule 4 Polar molecule Vander Waal's

Section Check 1. When ionic bonds form, the resulting compounds are n_____. 2. The attraction that forms between atoms when they share electrons is a c_____ b____. 3. In what type of molecule are electrons shared equally? _______ covalent molecule

Section Check Answer The answer is A. In an ionic bond, a transfer of electrons takes place and the overall neutral charge is maintained.

Section Check Question 2 The attraction that forms between atoms when they share electrons is _____. A. B. C. D. a binary compound a covalent bond an ionic bond the oxidation number

Section Check Answer The answer is B. A single covalent bond is made up of two shared electrons.

Section Check Question 3 In what type of molecule are electrons shared equally? A. B. C. D. diatomic nonpolar water

Section Check Answer The answer is B. In a nonpolar molecule, electrons are shared equally and the molecule does not have oppositely charged ends.

Bell Ringers: 1. When ionic bonds form, the resulting compounds are n_____. 2. The attraction that forms between atoms when they share electrons is a c_____ b____. 3. In what type of molecule are electrons shared equally? _______ covalent molecule

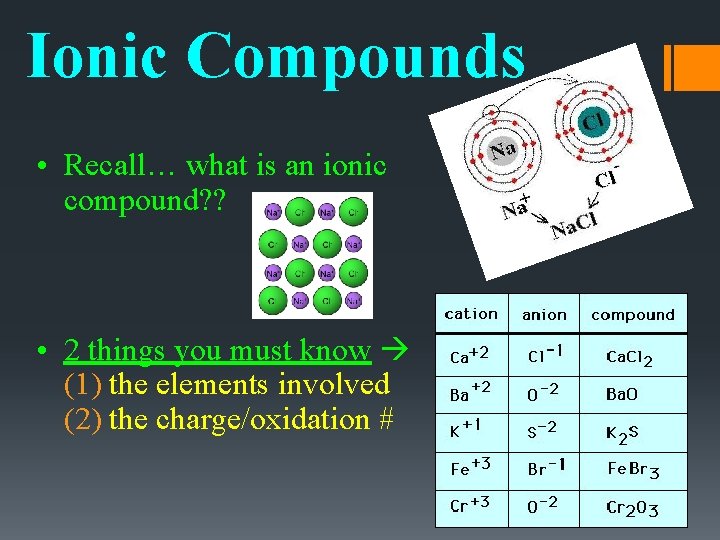
Ionic Compounds • Recall… what is an ionic compound? ? • 2 things you must know (1) the elements involved (2) the charge/oxidation #

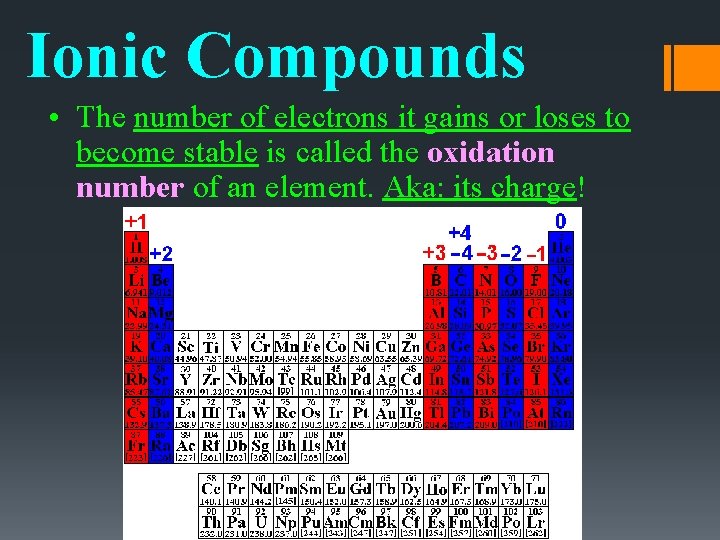
Ionic Compounds • The number of electrons it gains or loses to become stable is called the oxidation number of an element. Aka: its charge!

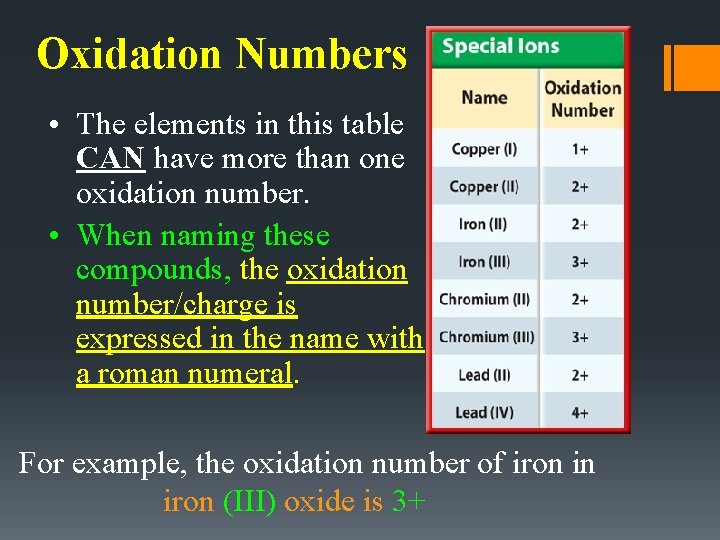
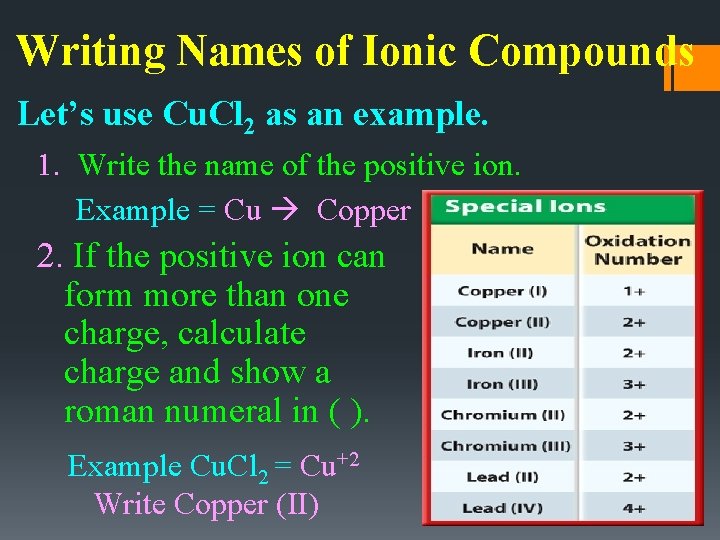
Oxidation Numbers • The elements in this table CAN have more than one oxidation number. • When naming these compounds, the oxidation number/charge is expressed in the name with a roman numeral. For example, the oxidation number of iron in iron (III) oxide is 3+

Ionic Compounds • Remember: even though the ions in a compound carry charges, the compound itself is neutral. • Charges must balance!! Charge of Cation + Charge on Anion = 0 positive charges + negative charges = 0

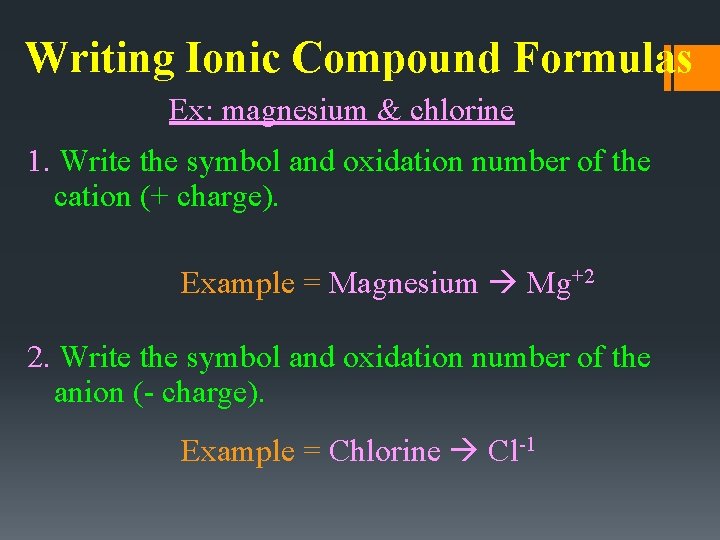
Writing Ionic Compound Formulas Ex: magnesium & chlorine 1. Write the symbol and oxidation number of the cation (+ charge). Example = Magnesium Mg+2 2. Write the symbol and oxidation number of the anion (- charge). Example = Chlorine Cl-1

Writing Ionic Compound Formulas 3. The charge (without the sign) of one ion **usually** becomes the subscript of the other ion. Charges balance at their least common multiple. Mg+2 Cl-1 Example: Mg. Cl 2 4. Check – OVERALL CHARGE ZERO? ? Charge of Cation + Charge on Anion = 0 (+2 charge)(1 atom) + (-1 charge)(2 atoms) = 0 2+-2=0

Ionic Formula Practice What is the formula for lithium phosphide? Li 3 P What is the formula for magnesium hydroxide? Mg(OH)2

Writing Names of Ionic Compounds Let’s use Cu. Cl 2 as an example. 1. Write the name of the positive ion. Example = Cu Copper 2. If the positive ion can form more than one charge, calculate charge and show a roman numeral in ( ). Example Cu. Cl 2 = Cu+2 Write Copper (II)

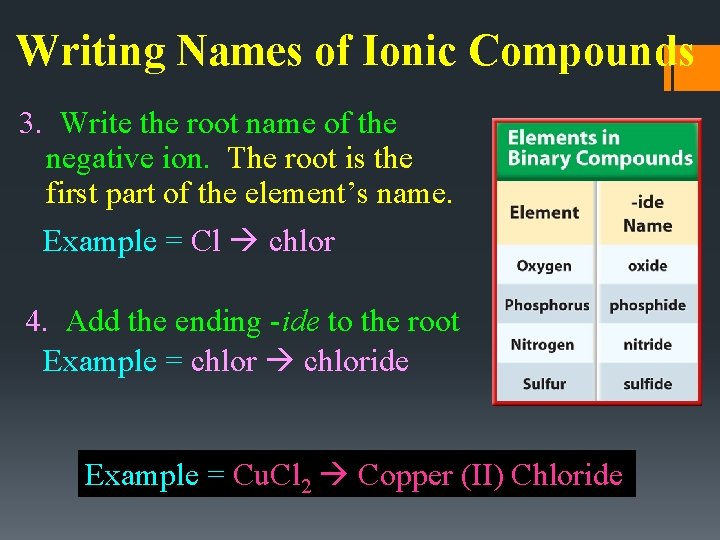
Writing Names of Ionic Compounds 3. Write the root name of the negative ion. The root is the first part of the element’s name. Example = Cl chlor 4. Add the ending -ide to the root Example = chloride Example = Cu. Cl 2 Copper (II) Chloride

Ionic Naming Practice What is the name for the compound Al. Cl 3? Aluminum Chloride What is the name for the compound Fe. O? Iron (II) Oxide

Only occur in IONI CS!! • A polyatomic ion is a positively or Polyatomic Ions negatively charged, covalently bonded group of atoms. Break it Down “Poly” = _____ “Atomic” = _______ “Ion” = _____ **See your orange reference sheet**

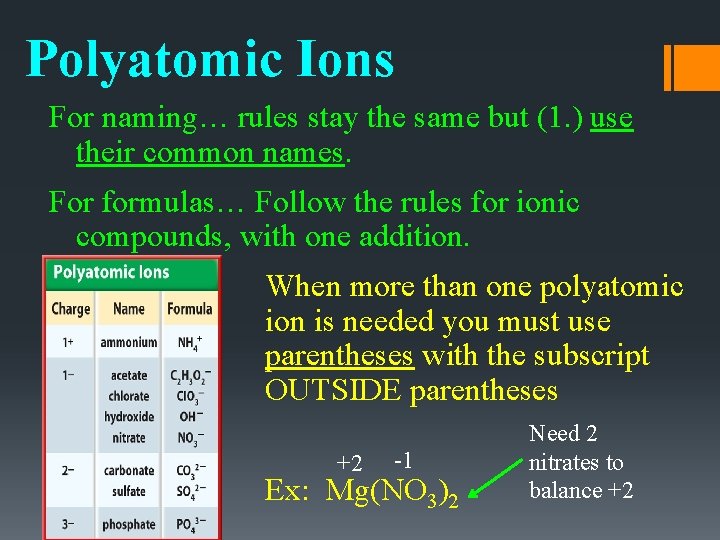
Polyatomic Ions For naming… rules stay the same but (1. ) use their common names. For formulas… Follow the rules for ionic compounds, with one addition. When more than one polyatomic ion is needed you must use parentheses with the subscript OUTSIDE parentheses +2 -1 Ex: Mg(NO 3)2 Need 2 nitrates to balance +2

LT’s § 1 – Differentiate between bond types § 4 – Write covalent names/formulas

Covalent Compounds • Covalent compounds are those formed between elements that are nonmetals. - Some pairs of nonmetals can form multiple compounds (Law of Multiple Proportions) H 2 O 2 = VS.

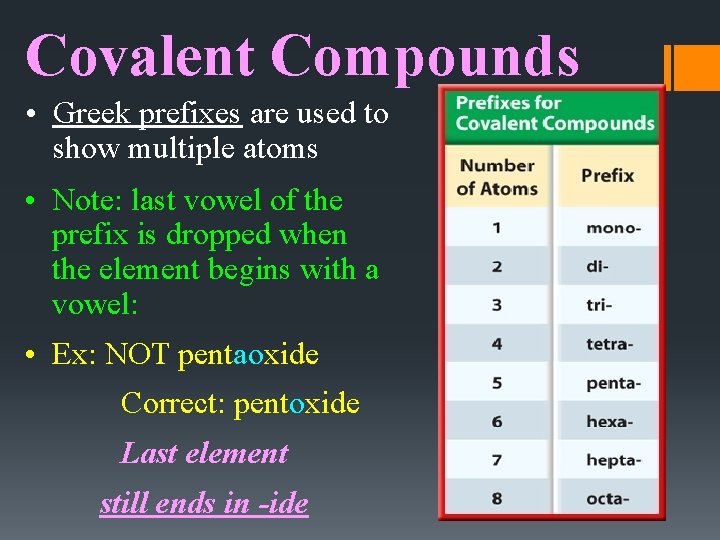
Covalent Compounds • Greek prefixes are used to show multiple atoms • Note: last vowel of the prefix is dropped when the element begins with a vowel: • Ex: NOT pentaoxide Correct: pentoxide Last element still ends in -ide

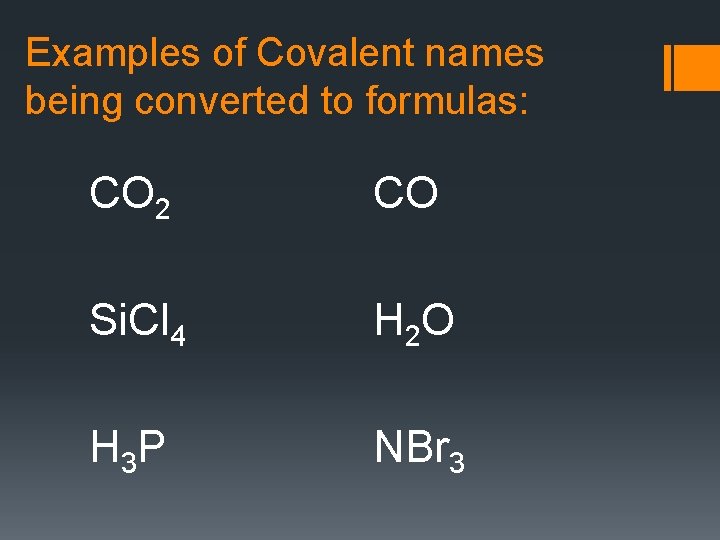
Examples of Covalent names being converted to formulas: CO 2 CO Si. Cl 4 H 2 O H 3 P NBr 3

Examples of Covalent formulas being converted to names: Nitrogen triiodide Hydrogen Monochloride Carbon disulfide Silicon tetrabromide Diphosphorus trioxide Phosphorus trifluoride

Question 1 What does the oxidation number of an element tell you? Ex: In the name copper (II) chloride, what does the (II) tell you? Answer The oxidation number indicates how many electrons an atom has gained, lost, or shared in order to become stable (THE CHARGE!!).