LECTURE 6 2 TEMPERATURE SCALES Todays Learning Targets












- Slides: 15

LECTURE 6. 2 – TEMPERATURE SCALES

Today’s Learning Targets • LT 5. 3 – I can define what STP is on an exit ticket • LT 5. 4 – I can discuss how the kinetic energy of a gas molecules is related to the temperature of the gas mixture on an exit ticket • LT 5. 5 – I can define what absolute zero and relate it to the Kelvin temperature scale on an exit ticket • LT 5. 6 – I can convert between the Kelvin and Celsius temperature scales on an exit ticket • LT 5. 7 – I can explain Boyle’s Law, how it relates pressure and volume, and I can calculate pressure and volume values for a given problem on an exit ticket

WHAT ARE THE TEMPERATURE SCALES?

I. Temperature • The temperature that we feel is created by the movement of atoms. • The movement of gas molecules in the air creates our observable temperature • The temperature is a measurement of the average energy of a sample

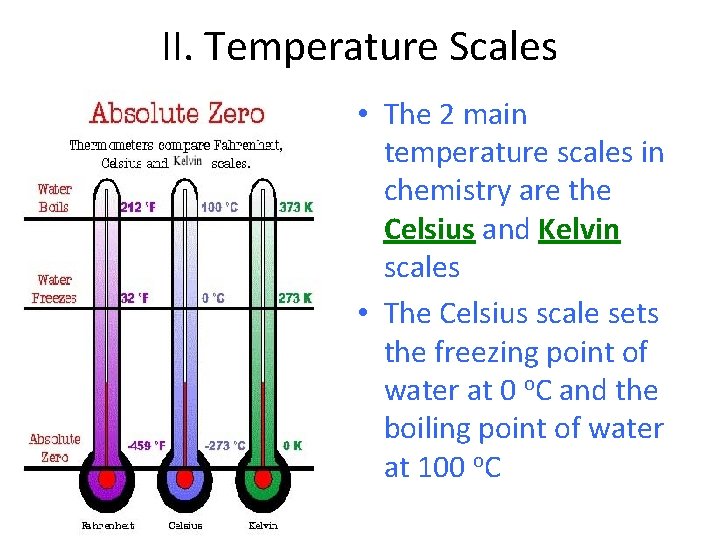
II. Temperature Scales • The 2 main temperature scales in chemistry are the Celsius and Kelvin scales • The Celsius scale sets the freezing point of water at 0 o. C and the boiling point of water at 100 o. C

WHAT IS STP?

I. STP • When working with gases, reactions are normally carried out under standard temperature and pressure or STP • STP is defined as 273 K and 1 atm

WHAT IS ABSOLUTE ZERO?

I. Kelvin Scale • The other unit of temperature used in chemistry is the Kelvin scale. • The Kelvin scale sets 0 K as being absolute zero.

II. Absolute Zero • Absolute zero is the temperature at which all molecular motion stops. • Coldest possible temperature • It is impossible to go below absolute zero (0 K) because molecular motion has stopped at this point.

HOW DO WE CONVERT BETWEEN CELSIUS AND KELVIN

I. Converting Between Kelvin and Celsius • To convert from Celsius to Kelvin: Kelvin = Co + 273 • To convert from Kelvin to Celsius: Celsius = Kelvin – 273

Class Example • It is 33 o. C outside, what is this temperature in Kelvin?

Table Talk • If I have something at 298 K, what temperature is it in Celsius?

Stop and Jot • You measure a reaction at 1250 o. C, what is the temperature in Kelvin?