Vaccination against shingles Withdrawn April 2018 Herpes Zoster








































- Slides: 48

Vaccination against shingles Withdrawn April 2018 (Herpes Zoster) An update for healthcare professionals July 2016

2 Key messages • shingles can lead to a severe painful illness in older people that can persist for several months or even years • the severity of the illness increases with age and older people aged 70 years and over are at an increased risk • over 50, 000 cases of shingles occur in people aged 70 years and over each year in England Wales with approximately 50 cases resulting in death Withdrawn April 2018 • shingles vaccine is now offered routinely to individuals aged 70 years to reduce the incidence and severity of shingles and shingles related complications in older people It is important that healthcare professionals encourage and offer vaccination to all eligible patients Vaccination against shingles (Herpes Zoster)

3 Learning outcomes After completing this training, immunisers will be able to: • describe the aetiology and epidemiology of shingles • describe the relationship between shingles and chickenpox (varicella zoster) and the severity of the disease in older people Withdrawn April 2018 • discuss the important role of vaccination against shingles with people aged 70 and 78 years • identify sources of additional information Vaccination against shingles (Herpes Zoster)

4 Contents • what is shingles? • why vaccinate adults aged 70 and 78 years against shingles? • vaccination against shingles and the use of Zostavax® Withdrawn April 2018 • the national shingles programme • resources Vaccination against shingles (Herpes Zoster)

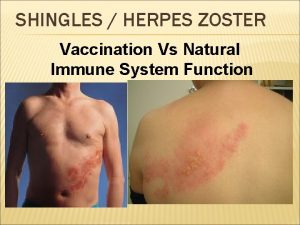
5 What is shingles? • shingles is a viral infection of the nerve cells and surrounding skin • after a person recovers from chickenpox infection (caused by the varicella zoster virus), the virus remains dormant in the nerve cells and can reactivate at a later stage when the immune system is weakened Withdrawn April 2018 • reactivation of the dormant virus leads to the clinical manifestation of shingles • reactivation can be associated with older age, malignancy, immunosuppressant therapy or HIV infection Vaccination against shingles (Herpes Zoster) Image courtesy of PHE/SPL

6 Clinical presentation of shingles Prodromal phase The first signs of shingles may include • abnormal skin sensations and pain in the affected area of skin • headache Withdrawn April 2018 • feeling generally unwell • photophobia • malaise • fever (although this is less common) A prodromal illness is experienced by 80% of individuals with shingles and can last up to 72 hours before the rash appears Vaccination against shingles (Herpes Zoster)

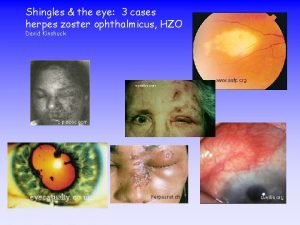
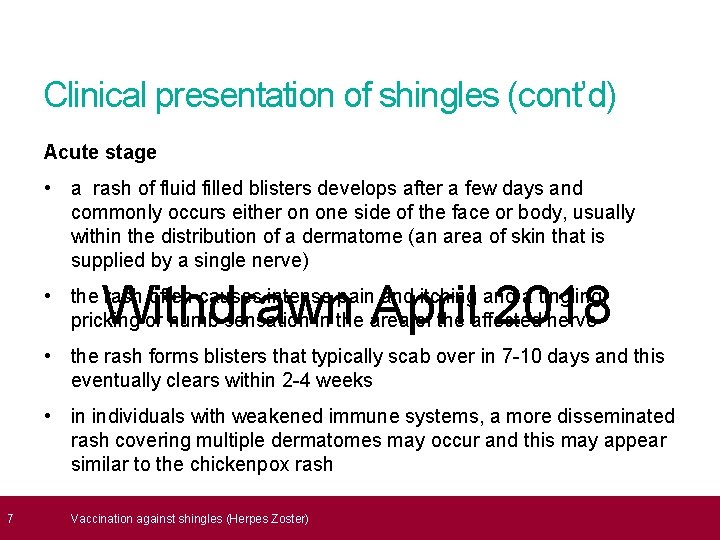
7 Clinical presentation of shingles (cont’d) Acute stage • a rash of fluid filled blisters develops after a few days and commonly occurs either on one side of the face or body, usually within the distribution of a dermatome (an area of skin that is supplied by a single nerve) Withdrawn April 2018 • the rash often causes intense pain and itching and a tingling, pricking or numb sensation in the area of the affected nerve • the rash forms blisters that typically scab over in 7 -10 days and this eventually clears within 2 -4 weeks • in individuals with weakened immune systems, a more disseminated rash covering multiple dermatomes may occur and this may appear similar to the chickenpox rash Vaccination against shingles (Herpes Zoster)

8 Possible complications of shingles Complications are more likely in adults aged over 50 years, with the severity of the illness increasing with age. The most common complications are • post herpetic neuralgia (PHN) Withdrawn April 2018 • secondary bacterial skin infections Other less common complications can include • ophthalmic zoster (leading to keratitis, corneal ulceration, conjunctivitis, retinitis, optic neuritis and/or glaucoma) • peripheral motor neuropathy In severe cases, shingles can lead to hospitalisation and death Vaccination against shingles (Herpes Zoster)

9 Possible complications of shingles (cont’d) Post herpetic neuralgia (PHN) is a common complication of shingles in older adults: • PHN is a pain at the rash site that persists for, or appears more than 90 days after the onset of the shingles rash • on average, PHN lasts from 3 to 6 months but can persist for longer • severity of pain can vary and may be constant, intermittent or triggered by stimulation of affected area, eg wind on the face • the pain may be a burning, itching, stabbing or aching pain, which is extremely sensitive to touch and is not generally relieved by common painkillers • PHN is more likely to develop, and is more severe, in people over the age of 50, with one third of sufferers over the age of 80 experiencing intense pain Withdrawn April 2018 Vaccination against shingles (Herpes Zoster)

10 Infectious period • a person with shingles is only infectious when the rash is present and fluid filled • a person is not infectious before the rash is present or when the rash has crusted Withdrawn April 2018 Shingles is less infectious than chickenpox and covering the rash will greatly reduce the risk of exposure to those non-immune to chickenpox. Vaccination against shingles (Herpes Zoster)

11 Transmission • shingles can not be transmitted from one person to another • a person exposed to shingles will not develop shingles • a person exposed to chickenpox will not develop shingles • however, a person who has not had chickenpox previously may develop chickenpox as a result of exposure to the shingles virus through direct contact with the fluid filled blisters Withdrawn April 2018 • the varicella virus that causes shingles (herpes zoster) is the same virus that causes chickenpox (varicella zoster) Shingles is not spread through coughing, sneezing or casual contact Vaccination against shingles (Herpes Zoster)

12 Why vaccinate older adults against shingles? Withdrawn April 2018 Vaccination against shingles (Herpes Zoster)

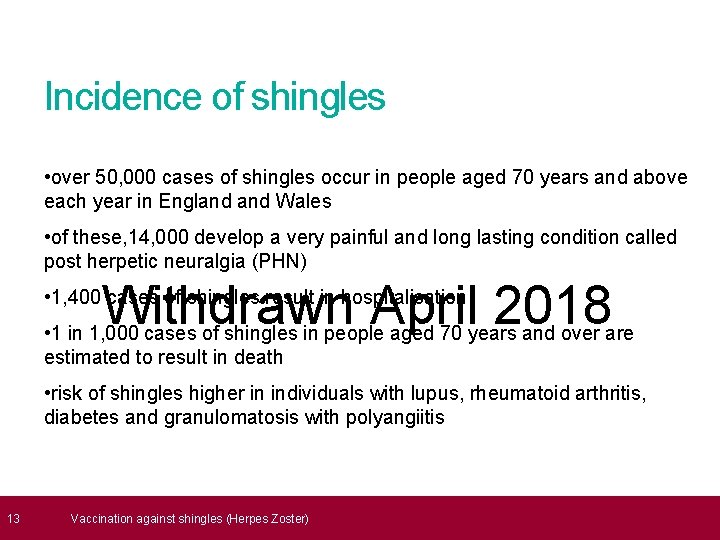
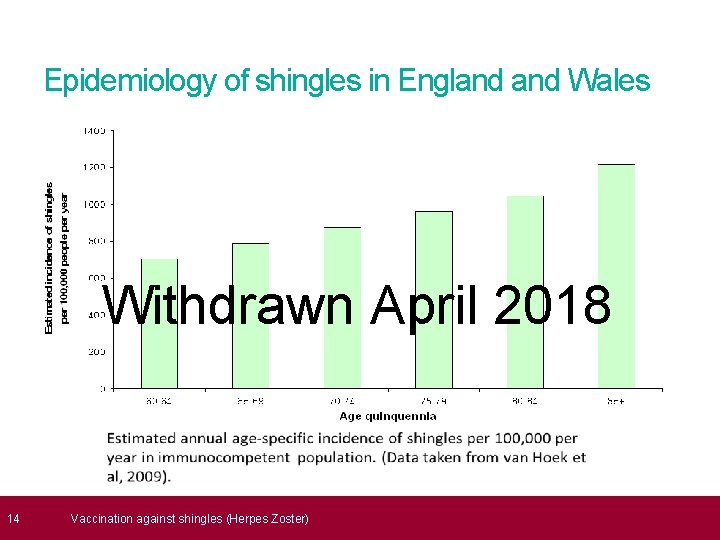
13 Incidence of shingles • over 50, 000 cases of shingles occur in people aged 70 years and above each year in England Wales • of these, 14, 000 develop a very painful and long lasting condition called post herpetic neuralgia (PHN) Withdrawn April 2018 • 1, 400 cases of shingles result in hospitalisation • 1 in 1, 000 cases of shingles in people aged 70 years and over are estimated to result in death • risk of shingles higher in individuals with lupus, rheumatoid arthritis, diabetes and granulomatosis with polyangiitis Vaccination against shingles (Herpes Zoster)

14 Epidemiology of shingles in England Wales Withdrawn April 2018 Vaccination against shingles (Herpes Zoster)

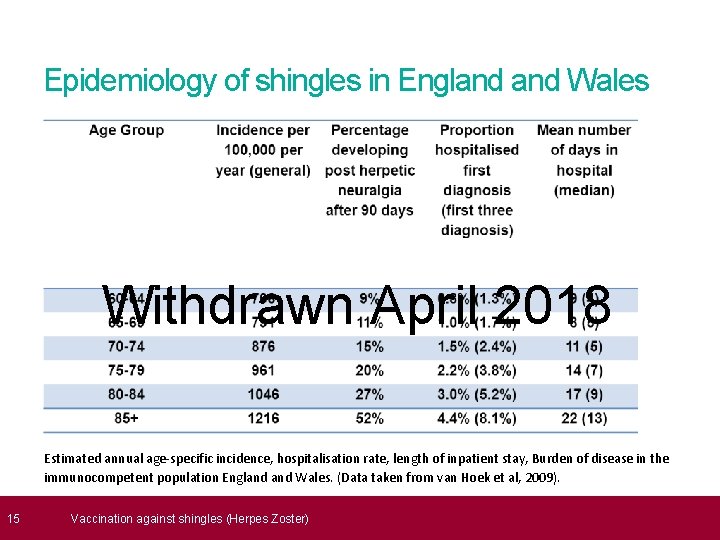
15 Epidemiology of shingles in England Wales Withdrawn April 2018 Estimated annual age-specific incidence, hospitalisation rate, length of inpatient stay, Burden of disease in the immunocompetent population England Wales. (Data taken from van Hoek et al, 2009). Vaccination against shingles (Herpes Zoster)

16 Why vaccinate older adults against shingles? The epidemiology of the disease shows that individuals over 70 years of age are not only at an increased risk of developing the disease, but they also suffer a more severe form of the illness resulting in complications such as PHN and an increase in hospital admissions. Withdrawn April 2018 Analytical studies show that the most cost-effective age for offering vaccination to prevent and/ or reduce the disease burden is for those aged 70 to 79. Vaccination for individuals over the age of 80 years is not recommended due to the decreased efficacy of the vaccine in this age group. Vaccination against shingles (Herpes Zoster)

17 Shingles vaccine coverage data: England • following the successful introduction of shingles vaccination in September 2013 in England, there has been a year on year decline in coverage in both the routine (70 -year-old) and catch-up (78 -year-old) cohorts * Withdrawn April 2018 • it is important that general practices consider how they can optimise the uptake of shingles vaccine in eligible patients in order to reduce the significant burden of disease associated with shingles among older adults * To end Feb 2016 Vaccination against shingles (Herpes Zoster)

18 Vaccination against shingles (Herpes Zoster) Withdrawn April 2018 The use of Zostavax® Vaccination against shingles (Herpes Zoster)

19 The recommended vaccine: Zostavax® Brand name: Zostavax® Generic name: Varicella Zoster Virus Marketed by Sanofi Pasteur MSD Withdrawn April 2018 • Live attenuated (ie a weakened live organism) • Licensed for use from age of 50 years and above • Recommended for adults aged 70 (with a catch-up programme for 78 and 79 -year-olds) Vaccination against shingles (Herpes Zoster)

20 The recommended vaccine: Zostavax® A one dose schedule of Zostavax® was assessed in clinical trials using 17, 775 adults aged 70 years and over The vaccine reduced the incidence of shingles by 38% and provided protection for at least 5 years Withdrawn April 2018 For those vaccinated but who later developed shingles, the vaccine • significantly reduced the burden of illness by 55% • significantly reduced the incidence of PHN by 66. 8% Vaccination against shingles (Herpes Zoster)

21 The recommended vaccine: Zostavax® • Zostavax® is the only market authorised shingles vaccine available in the UK • it is important immunisers familiarise themselves with the vaccine and its product information to avoid administration errors Withdrawn April 2018 Vaccination against shingles (Herpes Zoster) Image courtesy of Sanofi Pasteur MSD

22 Presentation of Zostavax® • the vial contains a powder as an off-white, compact crystalline plug Zostavax® contains: • the diluent in the pre-filled syringe is a clear colourless liquid • x 1 pre-filled syringe • x 1 Zostavax® vial • x 2 separate needles in secondary packaging Withdrawn April 2018 • when mixed together, Zostavax® should appear as a semi-hazy to translucent, off white to pale yellow liquid Vaccination against shingles (Herpes Zoster)

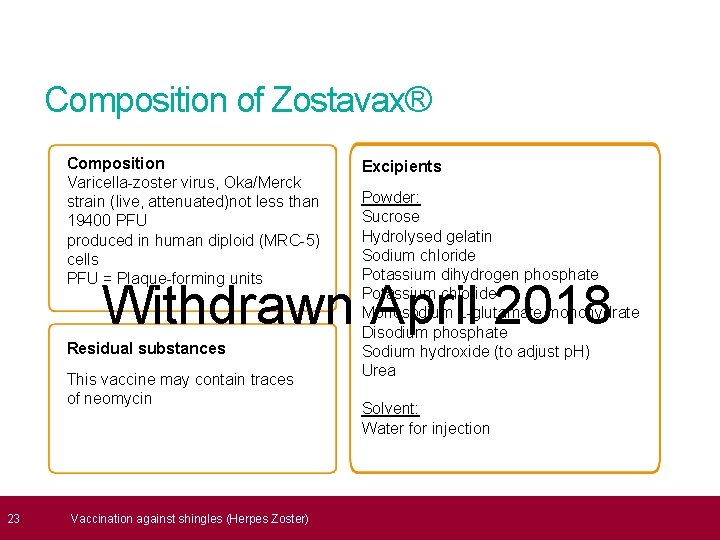
23 Composition of Zostavax® Composition Varicella-zoster virus, Oka/Merck strain (live, attenuated)not less than 19400 PFU produced in human diploid (MRC-5) cells PFU = Plaque-forming units Excipients Powder: Sucrose Hydrolysed gelatin Sodium chloride Potassium dihydrogen phosphate Potassium chloride Monosodium L-glutamate monohydrate Disodium phosphate Sodium hydroxide (to adjust p. H) Urea Withdrawn April 2018 Residual substances This vaccine may contain traces of neomycin Vaccination against shingles (Herpes Zoster) Solvent: Water for injection

24 Zostavax® reconstitution instructions • separate needles should be used for the reconstitution and administration of the vaccine • to reconstitute the vaccine, inject all the solvent in the pre-filled syringe into the vial of lyophilized vaccine and gently agitate to mix thoroughly • withdraw the entire contents of the reconstituted vaccine into a syringe for injection • change the needle and push the new needle into the extremity of the syringe and rotate a quarter of a turn (90⁰) to secure the connection • it is recommended that the vaccine be administered immediately after reconstitution • do not use the reconstituted vaccine if you notice any particulate matter or if the appearance of the solvent or of the reconstituted vaccine differs from that described in the SPC (a semi-hazy to translucent, off white to pale yellow liquid) Withdrawn April 2018 Vaccination against shingles (Herpes Zoster)

25 Administration of Zostavax® Marketing authorisation: The Green Book states: “While the vaccine is authorised for use from age 50 years and is effective in this age group, the burden of shingles disease is generally not as severe in those aged 50 -69 years when compared with older ages. Furthermore, given that the duration of protection is not known to last for more than ten years and the need for a second dose is not known, the vaccine is not recommended to be offered routinely below 70 years of age” • the Zostavax® summary of product characteristics (SPC) states that the vaccine is licensed for immunisation of individuals 50 years of age or older • while the SPC indication allows for use from 50 years of age, JCVI recommendation is that it should be used from 70 to 79 years Withdrawn April 2018 Vaccination against shingles (Herpes Zoster) • the recommendations for use of the vaccine detailed within the Green Book are based upon JCVI’s expert opinion after reviewing all the available evidence and these recommendations should be followed

26 Administration of Zostavax® • a single dose of 0. 65 ml should be given by intramuscular or subcutaneous injection, preferably in the deltoid region of the upper arm • intramuscular injection is the preferred route of administration as injection-site reactions were significantly less frequent in those who received the vaccine via this route of administration • for individuals with a bleeding disorder, Zostavax® should be given by deep subcutaneous injection to reduce the risk of bleeding • if more than one vaccine is given at the same appointment, they should be given at separate sites, preferably in different limbs. If given in the same limb, they should be given at least 2. 5 cm apart • the site at which each vaccine was given should be noted in the individual’s records Withdrawn April 2018 Vaccination against shingles (Herpes Zoster)

27 Administration of Zostavax® (cont) • Zostavax® can be administered at the same time as inactivated vaccines such as influenza and 23 -valent pneumococcal polysaccharide vaccine (PPV) • Zostavax® can also be administered at the same time as, or before or after other live vaccines except MMR and Yellow Fever • where MMR and Zostavax® are not administered at the same time, a four week minimum interval should be observed between vaccines • a four-week interval should be left between administration of Yellow Fever vaccine and Zostavax® – do not give at same appointment • Zostavax should not be administered to patients currently receiving oral or intravenous antiviral agents such as aciclovir or who are within 48 hours after cessation of treatment as therapy may reduce the response to the vaccine Withdrawn April 2018 Vaccination against shingles (Herpes Zoster)

28 Administration of Zostavax® should only be administered using a: • prescription written manually or electronically by a registered medical practitioner or other authorised prescriber • patient specific direction (PSD) Withdrawn April 2018 • patient group direction (PGD) A PGD template to support the national shingles (Zostavax®) vaccination programme for eligible adults is available on the PHE website. www. gov. uk/government/collections/immunisation-patient-group-direction-pgd NB: Local authorisation is required before PHE PGD templates can be used Vaccination against shingles (Herpes Zoster)

29 Contraindications and precautions Zostavax® is a live vaccine – it is critically important to check that the recipient has no contraindications to receiving a live vaccine • the decision to administer Zostavax® to immunosuppressed individuals should be based on a clinical risk assessment • if the individual is under highly specialist care and it is not possible to obtain full information on that individual’s treatment history, then vaccination should not proceed until the advice of the specialist or a local immunologist has been sought • if primary healthcare professionals administering Zostavax® have concerns about the nature of therapies (including biologicals) or the degree of immunosuppression, they should contact the relevant specialist for advice Withdrawn April 2018 Vaccination against shingles (Herpes Zoster)

30 Contraindications The shingles vaccine should not be given to a person who: 1. Has primary or acquired immunodeficiency states due to conditions including: • acute and chronic leukaemias, lymphoma (including Hodgkin’s lymphoma) • immunosuppression due to HIV/AIDS (see later) • cellular immune deficiencies • those remaining under follow up for a chronic lymphoproliferative disorder including haematological malignancies such as indolent lymphoma, chronic lymphoid leukaemia, myeloma and other plasma cell dyscrasias (NB: this list not exhaustive) • those who have received an allogenic stem cell transplant (cells from a donor) in the past 24 months and only then if they are demonstrated not to have ongoing immunosuppression or graft versus host disease (GVHD) • those who have received an autologous (using their own stem cells) haematopoietic stem cell transplant in the past 24 months and only then if they are in remission Withdrawn April 2018 Humoral deficiencies affecting Ig. G or Ig. A antibodies are not of themselves a contraindication unless associated with T cell deficiencies. If there is any doubt (eg common variable immune deficiency), immunological advice should be sought prior to administration. Vaccination against shingles (Herpes Zoster)

31 Contraindications (cont’d) The shingles vaccine should not be given to a person who: 2. Is on immunosuppressive or immunomodulating therapy including: • those who are receiving or have received in the past 6 months immunosuppressive chemotherapy or radiotherapy for malignant disease or non-malignant disorders • those who are receiving or have received in the past 6 months immunosuppressive therapy for a solid organ transplant (depending upon the type of transplant and the immune status of the patient) • those who are receiving or have received in the past 12 months biological therapy (eg anti -TNF therapy such as alemtuzumab, ofatumumab and rituximab) unless otherwise directed by a specialist • those who are receiving or have received in the past 3 months immunosuppressive therapy including Withdrawn April 2018 i) short term high-dose corticosteroids (>40 mg prednisolone per day for more than 1 week) ii) long term lower dose corticosteroids (>20 mg prednisolone per day for more than 14 days) iii) non-biological oral immune modulating drugs e. g. methotrexate >25 mg per week, azathioprine >3. 0 mg/kg/day or 6 -mercaptopurine >1. 5 mg/kg/day Vaccination against shingles (Herpes Zoster)

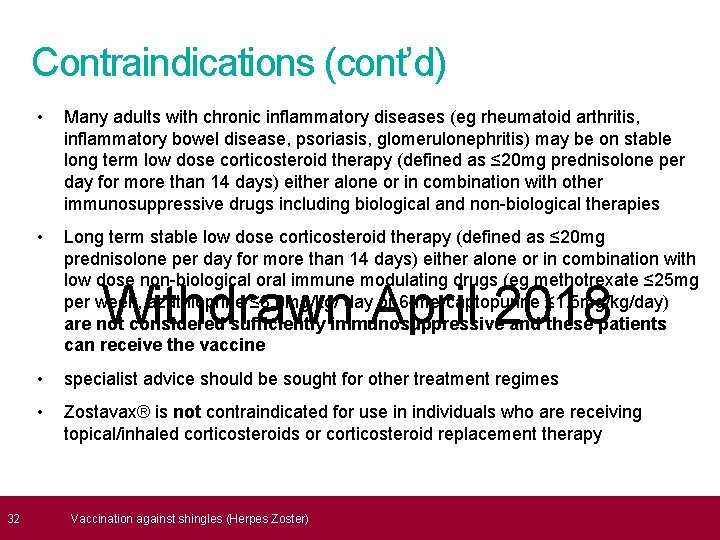
32 Contraindications (cont’d) • Many adults with chronic inflammatory diseases (eg rheumatoid arthritis, inflammatory bowel disease, psoriasis, glomerulonephritis) may be on stable long term low dose corticosteroid therapy (defined as ≤ 20 mg prednisolone per day for more than 14 days) either alone or in combination with other immunosuppressive drugs including biological and non-biological therapies • Long term stable low dose corticosteroid therapy (defined as ≤ 20 mg prednisolone per day for more than 14 days) either alone or in combination with low dose non-biological oral immune modulating drugs (eg methotrexate ≤ 25 mg per week, azathioprine ≤ 3. 0 mg/kg/ day or 6 -mercaptopurine ≤ 1. 5 mg/kg/day) are not considered sufficiently immunosuppressive and these patients can receive the vaccine Withdrawn April 2018 • specialist advice should be sought for other treatment regimes • Zostavax® is not contraindicated for use in individuals who are receiving topical/inhaled corticosteroids or corticosteroid replacement therapy Vaccination against shingles (Herpes Zoster)

33 Contraindications (cont’d) Zostavax vaccine should not be given to a person who: • is pregnant • has had a confirmed anaphylactic reaction to a previous dose of varicella-containing vaccine Withdrawn April 2018 • has had a confirmed anaphylactic reaction to any component of the vaccine, including neomycin or gelatin • is being treated with either oral or intravenous antivirals (such as aciclovir) until 48 hours after cessation of treatment (the use of topical aciclovir is not a contraindication to vaccination) Vaccination against shingles (Herpes Zoster)

34 Patients anticipating immunosuppressive therapy • the risk and severity of shingles is considerably higher among immunosuppressed individuals • therefore eligible individuals anticipating immunosuppressive therapy should ideally be assessed for vaccine eligibility before starting treatment that may contraindicate future vaccination Withdrawn April 2018 • eligible individuals who have not received Zostavax® should receive a single dose of vaccine at the earliest opportunity and at least 14 days before starting immunosuppressive therapy, although leaving one month would be preferable if a delay is possible Vaccination against shingles (Herpes Zoster)

35 HIV infection • the safety and efficacy of Zostavax® have not been conclusively established in adults who are known to be infected with HIV with or without evidence of immunosuppression • a CD 4 count of 200 cells/μl may be a suitable cut-off value below which vaccination should not be given Withdrawn April 2018 • seek advice from patient’s specialist Vaccination against shingles (Herpes Zoster)

36 Current or recent shingles infection • any acute illness – defer immunisation until fully recovered • Zostavax® not recommended for treatment of shingles or post herpetic neuralgia (PHN) • individuals who have shingles or PHN should wait until symptoms have ceased before being considered for shingles immunisation • the natural boosting that occurs following an episode of shingles makes the benefit of offering zoster vaccine immediately following recovery limited • immunocompetent individuals who develop shingles should have their shingles vaccination delayed for one year • patients who have two or more episodes of shingles in one year should have immunological investigation prior to vaccination Withdrawn April 2018 Vaccination against shingles (Herpes Zoster)

37 Transmission of vaccine virus • transmission of vaccine virus may occur rarely between recently vaccinated individuals who develop a varicella-like rash and susceptible contacts • any person who develops a vesicular rash after receiving Zostavax® should ensure the rash area is kept covered when in contact with a susceptible (chickenpox naïve) person until the rash is dry and crusted • if the person who received the vaccine is themselves immunosuppressed, they should avoid contact with susceptible people until the rash is dry and crusted, due to the higher risk of virus shedding • prophylactic aciclovir can be considered in vulnerable patients exposed to a varicella like rash in a recent vaccinee • if a varicella (widespread) or shingles-like (dermatomal) rash develops post. Zostavax®, a vesicle fluid sample should also be sent for analysis to determine whether the rash is vaccine associated or wild type Withdrawn April 2018 Vaccination against shingles (Herpes Zoster)

38 Possible adverse reactions Most commonly reported (1: 10 of people vaccinated) • erythema (redness), pain, swelling and pruritis (itching) at the injection site Less commonly reported (1: 100 of people vaccinated) Withdrawn April 2018 • haematoma, induration and warmth at the injection site • pain in arm or leg, headache Very rarely reported (1: 10, 000 of people vaccinated) • varicella (chickenpox) like-illness Vaccination against shingles (Herpes Zoster)

39 Reporting suspected adverse reactions Serious suspected adverse reactions to Zostavax® should be reported to the Medical and Healthcare products Regulatory Agency (MHRA) using the Yellow Card reporting scheme Yellow Card scheme • voluntary reporting system for suspected adverse reaction to medicines/vaccines Withdrawn April 2018 • success depends on early, complete and accurate reporting • report even if uncertain about whether vaccine caused condition • http: //mhra. gov. uk/yellowcard • see chapter 8 of Green Book for details Vaccination against shingles (Herpes Zoster)

40 The national shingles immunisation programme Withdrawn April 2018 Vaccination against shingles (Herpes Zoster)

41 The national shingles immunisation programme • the national shingles immunisation programme began on 1 September 2013 • shingles vaccine is now routinely offered to adults aged 70 years • a catch-up programme for adults aged 71 -79 years is being rolled out gradually with those aged 78 on 1 September each year being eligible for immunisation Withdrawn April 2018 • in addition, patients who have been or have become eligible for immunisation since the programme began but have not yet been vaccinated against shingles remain eligible until their 80 th birthday • any individual who reaches their 80 th birthday is no longer eligible for the vaccination due to the reducing efficacy of the vaccine as age increases Vaccination against shingles (Herpes Zoster)

42 The national shingles immunisation programme From 1 September 2016 shingles vaccine should be offered to: • patients aged 70 years on 1 September 2016 • patients aged 78 years on 1 September 2016 In addition, patients who were eligible for immunisation in the first three years of the programme but have not been vaccinated against shingles remain eligible until their 80 th birthday. These cohorts are: Withdrawn April 2018 • patients aged 71 to 73 on 1 September 2016 • patients aged 79 on 1 September 2016 Until 31 August 2016, GPs should continue to offer shingles vaccine to all those who were aged 70 or 78 on 1 September 2015 Vaccination against shingles (Herpes Zoster)

43 The national shingles vaccine programme - how do you know who is eligible? Withdrawn April 2018 Look at PHE Immunisation website Shingles: guidance and vaccination programme page for PHE/NHS England letters and eligibility chart www. gov. uk/government/collections/ shingles-vaccination-programme Vaccination against shingles (Herpes Zoster)

44 Maximising uptake • every effort should be made by healthcare professionals to maximise the uptake of the shingles vaccine • be able to provide clear, concise and accurate information to individuals eligible to receive shingles vaccine Withdrawn April 2018 • as Zostavax® can be administered concomitantly with flu vaccine, the appointment for administration of flu vaccine is an appropriate opportunity to also provide Zostavax® • however, any opportunity to offer the vaccine should be used Think of checking shingles vaccine status when an eligible patient presents for another reason Vaccination against shingles (Herpes Zoster)

45 Maximising uptake (cont’d) • for the 2015/16 programme, by the end of March 2016 just under half of eligible 70 and 78 -year-olds had been vaccinated against shingles • this represents a significant achievement but one that can be improved on Withdrawn April 2018 • ongoing efforts to identify and vaccinate all eligible individuals are needed to achieve a continuing reduction in the number of cases of this debilitating and painful condition • take every opportunity to offer shingles vaccination to eligible patients to help to protect as many elderly people as possible from this serious illness Vaccination against shingles (Herpes Zoster)

46 Resources • PHE and NHS England Letter (2016) Shingles immunisation programme from 1 September 2016 https: //www. gov. uk/government/collections/shingles-vaccinationprogramme Withdrawn April 2018 • PHE Immunisation against infectious disease (the Green Book) Shingles (herpes zoster) chapter 28 a https: //www. gov. uk/government/collections/immunisation-againstinfectious-disease-the-green-book • PHE Shingles: questions and answers for healthcare professionals, patient information leaflets, eligibility chart and other resources https: //www. gov. uk/government/collections/shingles-vaccinationprogramme Vaccination against shingles (Herpes Zoster)

47 Key messages • shingles can lead to a severe painful illness in older people that can persist for several months or even years • the severity of the illness increases with age and older people aged 70 years and over are at an increased risk • over 50, 000 cases of shingles occur in people aged 70 years and over each year in England Wales with approximately 50 cases resulting in death Withdrawn April 2018 • shingles vaccine is now offered routinely to individuals aged 70 years to reduce the incidence and severity of shingles and shingles related complications in older people It is important that healthcare professionals encourage and offer vaccination to all eligible patients Vaccination against shingles (Herpes Zoster)

48 About Public Health England exists to protect and improve the nation's health and wellbeing, and reduce health inequalities. It does this through world-class science, knowledge and intelligence, advocacy, partnerships and the delivery of specialist public health services. PHE is an operationally autonomous executive agency of the Department of Health. Public Health England Wellington House 133 -155 Waterloo Road London SE 1 8 UG Tel: 020 7654 8000 www. gov. uk/phe Twitter: @PHE_uk Facebook: www. facebook. com/Public. Health. England Withdrawn April 2018 © Crown copyright 2016 You may re-use this information (excluding logos) free of charge in any format or medium, under the terms of the Open Government Licence v 3. 0. To view this licence, visit OGL or email psi@nationalarchives. gsi. gov. uk. Where we have identified any third party copyright information you will need to obtain permission from the copyright holders concerned. Published July 2016 PHE publications gateway number: 2016154 Vaccination against shingles (Herpes Zoster)
 How to mix shingrix vaccine
How to mix shingrix vaccine Herpes zoster
Herpes zoster Herpes zoster clasificacion
Herpes zoster clasificacion Valaciclovir dosis
Valaciclovir dosis Herpes zoster cicatriz
Herpes zoster cicatriz Herpes zoster infection
Herpes zoster infection Varicela zoster
Varicela zoster Zoster eye disease study
Zoster eye disease study Zoster eye disease study
Zoster eye disease study Kinematic equations examples
Kinematic equations examples Shingles medscape
Shingles medscape Shingles older adults
Shingles older adults Shingles and pomalidimide
Shingles and pomalidimide Shingling of documents
Shingling of documents Luke autbeloe drops a pile of roof shingles
Luke autbeloe drops a pile of roof shingles Vagus nerve shingles
Vagus nerve shingles Luke drops a pile of roof shingles
Luke drops a pile of roof shingles Shingles vaccine side effects
Shingles vaccine side effects Sonya shingles
Sonya shingles Routine practices example
Routine practices example Diseases with vaccines
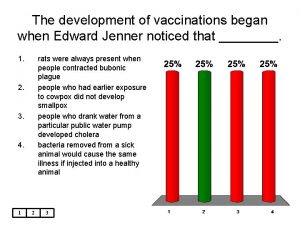
Diseases with vaccines A vaccination for smallpox was developed in 1796 by ____
A vaccination for smallpox was developed in 1796 by ____ Vaccination schedule in palestine
Vaccination schedule in palestine Mandatory vaccination
Mandatory vaccination Pcv vaccine route
Pcv vaccine route Laura henderson
Laura henderson Spay perry county
Spay perry county Intervax vaccine
Intervax vaccine Niccolo paganini fingers
Niccolo paganini fingers 10 rights of medication administration
10 rights of medication administration Vaccination a la defense
Vaccination a la defense Epi schedule in pakistan pdf
Epi schedule in pakistan pdf Poultry vaccination schedule
Poultry vaccination schedule Bankeryds vårdcentral influensavaccin
Bankeryds vårdcentral influensavaccin Vaccination bruxelles
Vaccination bruxelles Dada la siguiente secuencia rusia 2018 rusia 2018
Dada la siguiente secuencia rusia 2018 rusia 2018 Plexo pampiniforme ovario
Plexo pampiniforme ovario Herpes gladiatorum nhs
Herpes gladiatorum nhs Herpes simpleksserotipe 2
Herpes simpleksserotipe 2 Herpes genital glande
Herpes genital glande Bryonia 5ch posologia
Bryonia 5ch posologia Herpes simplex virus
Herpes simplex virus Site:slidetodoc.com
Site:slidetodoc.com Herpes simplex virus
Herpes simplex virus Simplex
Simplex Herpes genital tratamento definitivo
Herpes genital tratamento definitivo Herpes simpleksserotipe 2
Herpes simpleksserotipe 2 Cervicitis uteri
Cervicitis uteri Hsv encephalitis
Hsv encephalitis