Getting to the Point Strategies to Prevent Zoster



















- Slides: 29

Getting to the Point: Strategies to Prevent Zoster Kenneth Mc. Call, Pharm. D, BCGP, RPh, FAPh. A Associate Professor

Zoster Prevention - Objectives • Discuss the presentation and complications of herpes zoster • Recognize the ACIP recommendations for zoster vaccines • Describe the differences between the GSK zoster vaccine and the Merck zoster vaccine in terms of FDA indication, storage, preparation, administration, dosing, and contraindications

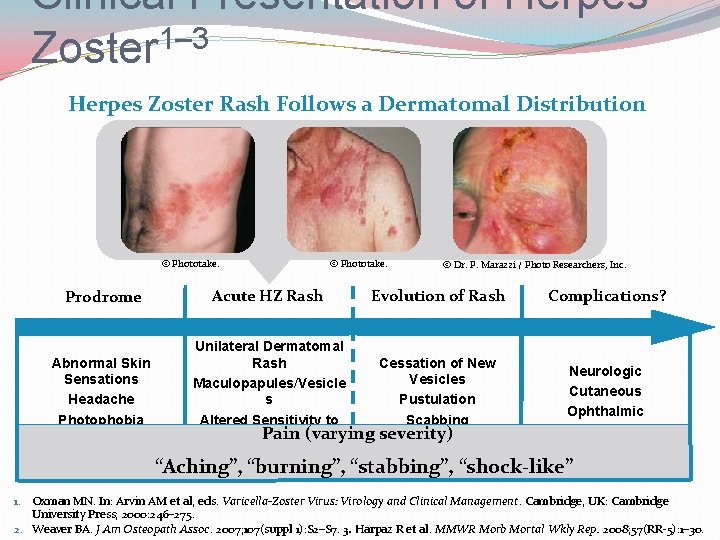
Clinical Presentation of Herpes Zoster 1– 3 Herpes Zoster Rash Follows a Dermatomal Distribution © Phototake. Prodrome Abnormal Skin Sensations Headache Photophobia Malaise Acute HZ Rash © Phototake. © Dr. P. Marazzi / Photo Researchers, Inc. Evolution of Rash Unilateral Dermatomal Rash Cessation of New Vesicles Maculopapules/Vesicle s Pustulation Altered Sensitivity to Scabbing Pain (varying severity) Touch Cutaneous Healing Unbearable Itching Complications? Neurologic Cutaneous Ophthalmic Visceral (rare) “Aching”, “burning”, “stabbing”, “shock-like” 1. Oxman MN. In: Arvin AM et al, eds. Varicella-Zoster Virus: Virology and Clinical Management. Cambridge, UK: Cambridge University Press; 2000: 246– 275. 2. Weaver BA. J Am Osteopath Assoc. 2007; 107(suppl 1): S 2–S 7. 3. Harpaz R et al. MMWR Morb Mortal Wkly Rep. 2008; 57(RR-5): 1– 30.


Herpes Zoster is the reactivation of which virus? 1. 2. 3. 4. Measles Mumps Rubella Varicella

What is the most common long-term complication of herpes zoster? 1. 2. 3. 4. Bell’s palsy Postherpetic neuralgia Vision loss Myocarditis

Prevention of Zoster and Postherpetic Neuralgia ZOSTAVAX® (Zoster Vaccine Live) 7

ZEST & Shingles Prevention Study (SPS) Results Vaccine Efficacy (%) 95% CI >80 yrs (n=1, 263) 1 (-29 to 48) 70 -79 yrs (n=7, 621) 1 60 -69 yrs (n=10, 370) 1 2 50 -59 yrs (n=11, 211) 0 18% 41% (28 to 52) (56 to 71) 64% 70% (54 to 81) 10 20 30 40 50 60 70 1. Oxman et al. New England Journal of Medicine. 2005. 352 (22): 2271 2. Zostavax® [package insert]. Whitehouse Station, NJ: Merck; April 2011. 80

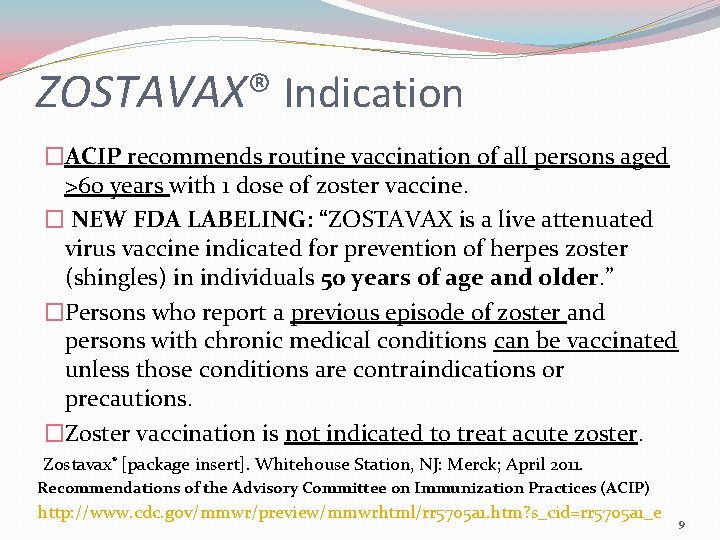
ZOSTAVAX® Indication �ACIP recommends routine vaccination of all persons aged >60 years with 1 dose of zoster vaccine. � NEW FDA LABELING: “ZOSTAVAX is a live attenuated virus vaccine indicated for prevention of herpes zoster (shingles) in individuals 50 years of age and older. ” �Persons who report a previous episode of zoster and persons with chronic medical conditions can be vaccinated unless those conditions are contraindications or precautions. �Zoster vaccination is not indicated to treat acute zoster. Zostavax® [package insert]. Whitehouse Station, NJ: Merck; April 2011. Recommendations of the Advisory Committee on Immunization Practices (ACIP) http: //www. cdc. gov/mmwr/preview/mmwrhtml/rr 5705 a 1. htm? s_cid=rr 5705 a 1_e 9

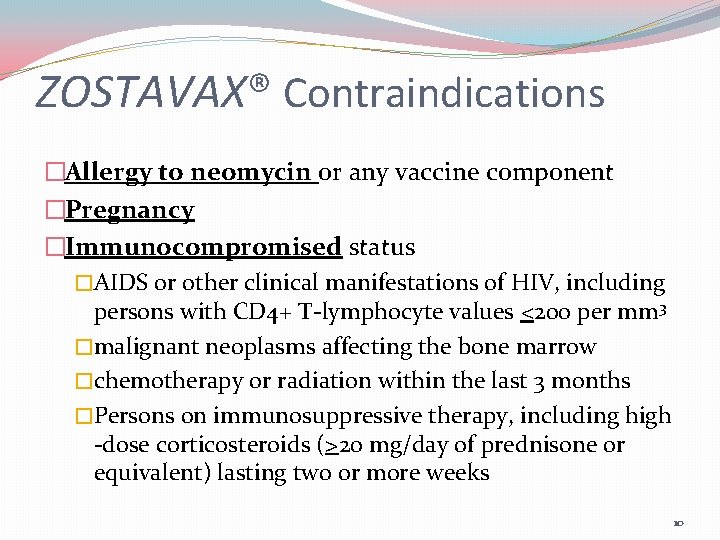
ZOSTAVAX® Contraindications �Allergy to neomycin or any vaccine component �Pregnancy �Immunocompromised status �AIDS or other clinical manifestations of HIV, including persons with CD 4+ T-lymphocyte values <200 per mm 3 �malignant neoplasms affecting the bone marrow �chemotherapy or radiation within the last 3 months �Persons on immunosuppressive therapy, including high -dose corticosteroids (>20 mg/day of prednisone or equivalent) lasting two or more weeks 10

ZOSTAVAX® Storage and Handling �Zoster vaccine must be stored frozen �The vaccine must be discarded if not used within 30 minutes after reconstitution. �New labeling: Zostavax may be stored and/or transported at fridge temp for up to 72 hours prior to reconstitution. Any unused vaccine at fridge temp should be discarded. Zostavax® [package insert]. Whitehouse Station, NJ: Merck; April 2011. 11

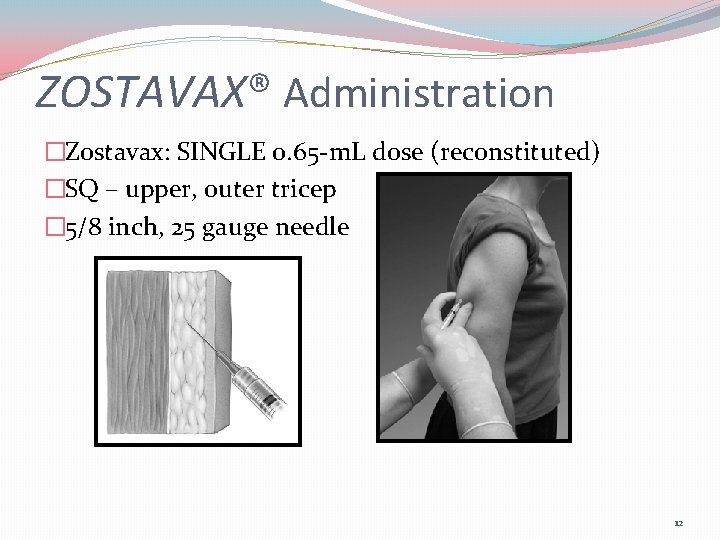
ZOSTAVAX® Administration �Zostavax: SINGLE 0. 65 -m. L dose (reconstituted) �SQ – upper, outer tricep � 5/8 inch, 25 gauge needle 12

What is the maximum length of time between reconstitution and administration of Zostavax? 1. 2. 3. 4. 10 minutes 30 minutes 1 hour 1 day

Prevention of Zoster and Postherpetic Neuralgia Shingrix® (Herpes Zoster Adjuvanted Subunit Vaccine) 14

Now Approved: Shingrix® �Inactivated, recombinant zoster subunit vaccine �Manufactured by Glaxo. Smith. Kline


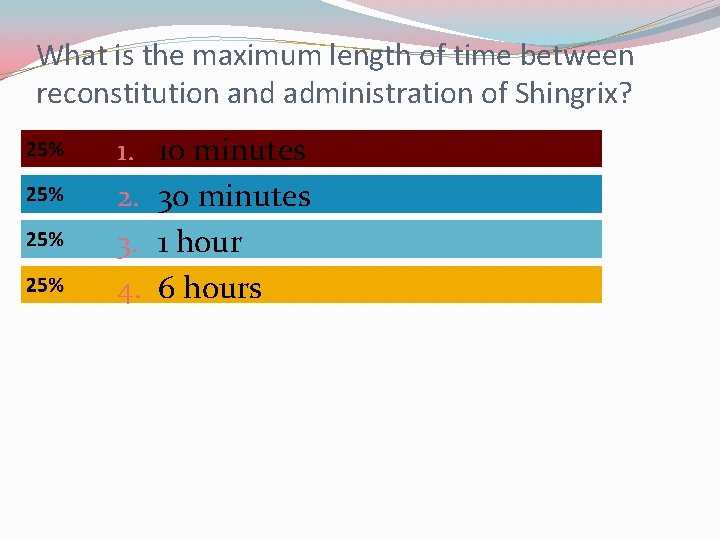
Shingrix® Storage and Preparation �Stored in the refrigerator at 4 o C �Suspension that must be reconstituted (o. 5 ml)* *For use within 6 hours

Shingrix® (Herpes Zoster Adjuvanted Subunit Vaccine)

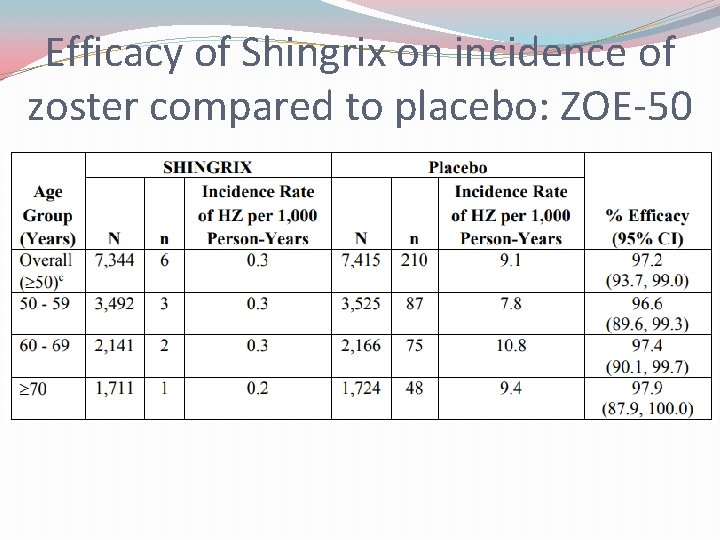
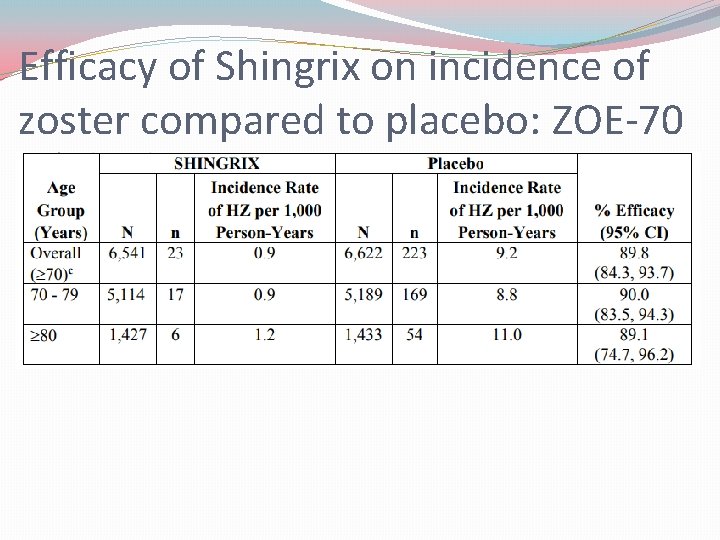
Shingrix® Trials �Trials: �ZOE-50: Shingrix reduced risk of herpes zoster by 97. 2% in patients 50 -59 year olds � (Zostavax had only 70% efficacy for the same population group) �ZOE-70: Shingrix reduced risk of herpes zoster by 90% in patients ≥ 70 years old � (Zostavax had only 38% efficacy for the same population group) �Zoster-048: Shingrix demonstrates appropriate immunogeneicity and safety data in all approved populations regardless of if the patient has received a previous Zostavax vaccine greater than 5 years earlier

Efficacy of Shingrix on incidence of zoster compared to placebo: ZOE-50

Efficacy of Shingrix on incidence of zoster compared to placebo: ZOE-70

Recommendations of the ACIP for Use of Herpes Zoster Vaccines �Recombinant zoster vaccine (RZV) is recommended for the prevention of herpes zoster and related complications for immunocompetent adults aged ≥ 50 years. �Whereas RZV is licensed for all persons aged ≥ 50 years, immunocompromised persons and those on moderate to high doses of immunosuppressive therapy were excluded from the efficacy studies (ZOE 50 and ZOE-70), and thus, ACIP has not made recommendations regarding the use of RZV in these patients. https: //www. cdc. gov/mmwr/volumes/67/wr/mm 6703 a 5. htm? s_cid=mm 6703 a 5_w

Recommendations of the ACIP for Use of Herpes Zoster Vaccines �RZV is recommended for the prevention of herpes zoster and related complications for immunocompetent adults who previously received zoster vaccine live (ZVL). �Based on expert opinion, RZV should not be given <2 months after receipt of ZVL. MMWR / January 26, 2018 / 67(3); 103– 108 https: //www. cdc. gov/mmwr/volumes/67/wr/mm 6703 a 5. htm? s_cid=mm 6703 a 5_w

Recommendations of the ACIP for Use of Herpes Zoster Vaccines �RZV may be used in adults aged ≥ 50 years, irrespective of prior receipt of varicella vaccine or ZVL. �RZV is preferred over ZVL for the prevention of herpes zoster and related complications. MMWR / January 26, 2018 / 67(3); 103– 108 https: //www. cdc. gov/mmwr/volumes/67/wr/mm 6703 a 5. htm? s_cid=mm 6703 a 5_w

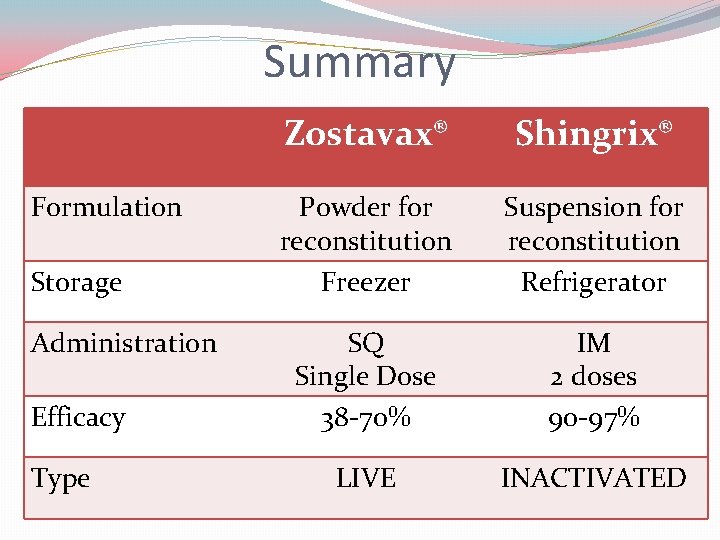
Summary Formulation Storage Administration Efficacy Type Zostavax® Shingrix® Powder for reconstitution Freezer Suspension for reconstitution Refrigerator SQ Single Dose 38 -70% IM 2 doses 90 -97% LIVE INACTIVATED

What is the maximum length of time between reconstitution and administration of Shingrix? 1. 2. 3. 4. 10 minutes 30 minutes 1 hour 6 hours

A 55 yo woman requests a zoster vaccine. Select the best option to prevent shingles as recommended by ACIP. A. B. C. D. Shingrix SQ x 1 dose Shingrix IM x 2 doses Zostavax SQ x 1 dose Zostavax IM x 2 doses

A 72 yo man previously received Zostavax 7 years ago. Despite being vaccinated, he experienced a shingles attack in January 2018. Select the best option to prevent shingles. A. B. C. D. Shingrix SQ x 1 dose Shingrix IM x 2 doses Zostavax booster SQ x 1 dose Both Shingrix and Zostavax are contraindicated

Questions and Discussion kmccall@une. edu
 The secret of getting ahead is getting started
The secret of getting ahead is getting started Getting from point a to point b
Getting from point a to point b Herpes zoster clasificacion
Herpes zoster clasificacion Varicella zoster complications
Varicella zoster complications Herpes zoster cicatriz
Herpes zoster cicatriz Varicela zoster
Varicela zoster Dendritic keratitis
Dendritic keratitis Valaciclovir dosis
Valaciclovir dosis Zoster eye disease study
Zoster eye disease study Zoster eye disease study
Zoster eye disease study Arjan hogewoning
Arjan hogewoning Practical strategies to prevent bullying
Practical strategies to prevent bullying Hát kết hợp bộ gõ cơ thể
Hát kết hợp bộ gõ cơ thể Frameset trong html5
Frameset trong html5 Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Gấu đi như thế nào
Gấu đi như thế nào Chụp phim tư thế worms-breton
Chụp phim tư thế worms-breton Chúa yêu trần thế alleluia
Chúa yêu trần thế alleluia Môn thể thao bắt đầu bằng chữ đua
Môn thể thao bắt đầu bằng chữ đua Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công thức tiính động năng
Công thức tiính động năng Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Mật thư tọa độ 5x5
Mật thư tọa độ 5x5 101012 bằng
101012 bằng độ dài liên kết
độ dài liên kết Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra