PHCC VACCINATION SCHEDULE AND DEFAULTER SCHEDULE ALI IS





















































- Slides: 67

PHCC VACCINATION SCHEDULE AND DEFAULTER SCHEDULE


ALI IS A 6 -MONTH OLD BABY VISITING THE WELL-BABY CLINIC TODAY. HE RECEIVED HIS VACCINES AT BIRTH, 2 -MONTHS AND 4 -MONTHS VISIT. - WHAT VACCINES DO YOU WANT TO ADMINISTER TODAY? - ARE THERE ANY CONTRAINDICATIONS? - HOW ARE YOU GOING TO ADMINISTER THEM?

10 -Rights of Medication Administration 1)Right Patient 2)Right Medication 3)Right Dosage 4)Right Route 5)Right Time 6) Right Documentation 7) Right Education 8) Right to Refuse 9) Right Assessment 10) Right Evaluation

General Principles Visibly dirty skin need only be washed with soap + water. If alcohol and other disinfecting agents are used, skin must be allowed to dry as these could inactivate live vaccines.

General Principles There is no upper limit to the number of vaccines that may be administered at a single visit. The vaccines should not be mixed in the same syringe unless the combination has been specifically approved by the USFDA.

General Principles Increasing the interval between doses of a multidose vaccine does not diminish the effectiveness of the vaccine. Decreasing the interval between doses of a multidose vaccine may interfere with antibody response and protection.

General Principles • Two or more inactivated vaccines may be administered at the same visit or at any interval between doses. • In persons who require both PCV 13 and PPSV 23, PCV 13 should be administered at least eight weeks before PPSV 23.

General Principles Inactivated and live vaccines may be administered at the same visit or at any interval between doses.

General Principles Two or more live injectable vaccines (eg, MMR vaccine, varicella vaccine) or live intranasal vaccines (eg, live attenuated influenza vaccine) may be administered on the same day or ≥ 28 days apart. Live oral rotavirus vaccine and live oral typhoid vaccine may be administered on the same day as or at any interval before inactivated or live injectable vaccines.

General Principles When multiple injectable vaccines are required at a single visit, separate limbs should be used if possible, particularly for injections more likely to cause a local reaction (eg, DTa. P and PCV 13). If necessary, two or more vaccines can be given in the same limb (usually the anterolateral thigh). If possible, the injections should be separated by at least 2. 5 cm to avoid overlap of local reactions.

General Principles When multiple vaccines are required at one visit, administering the least painful vaccines first may lessen immunization-related pain and distress. For infants who require rotavirus vaccine, we suggest administering rotavirus vaccine before injectable vaccines. The oral rotavirus vaccine is flavored with sucrose, which may reduce the pain associated with subsequent injections


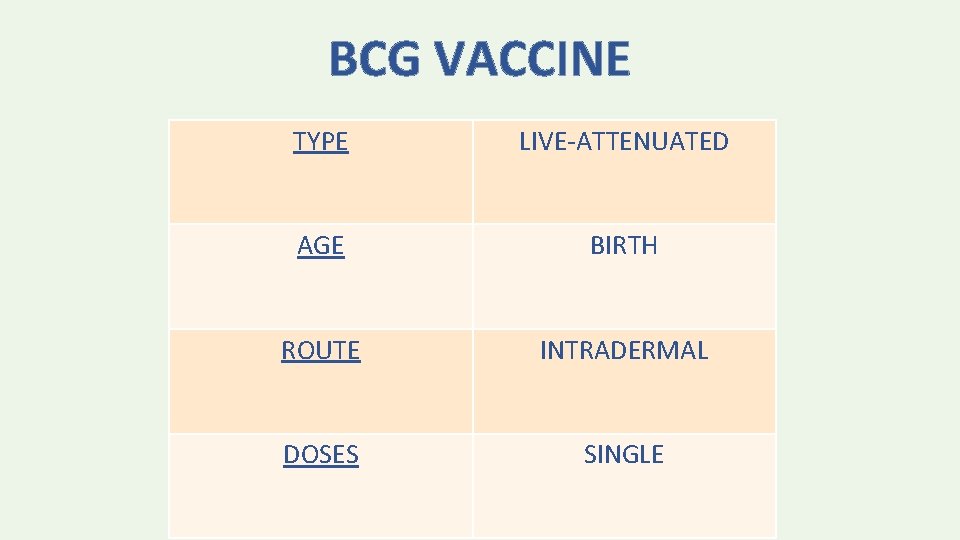
Injection Site INTRAMUSCULAR: < 3 years: Anterolateral thigh “Vastus lateralis” > 3 years: Deltoid SUBCUTANEOUS: <12 months: Fatty tissue over anterolateral thigh ≥ 12 months: Fatty tissue over upper-outer triceps BCG VACCINE: Intradermal “deltoid region”


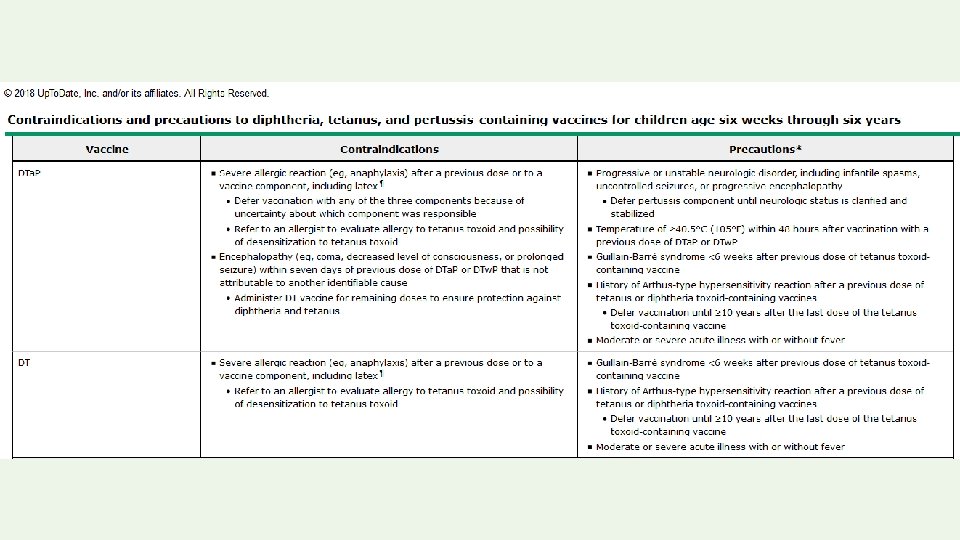
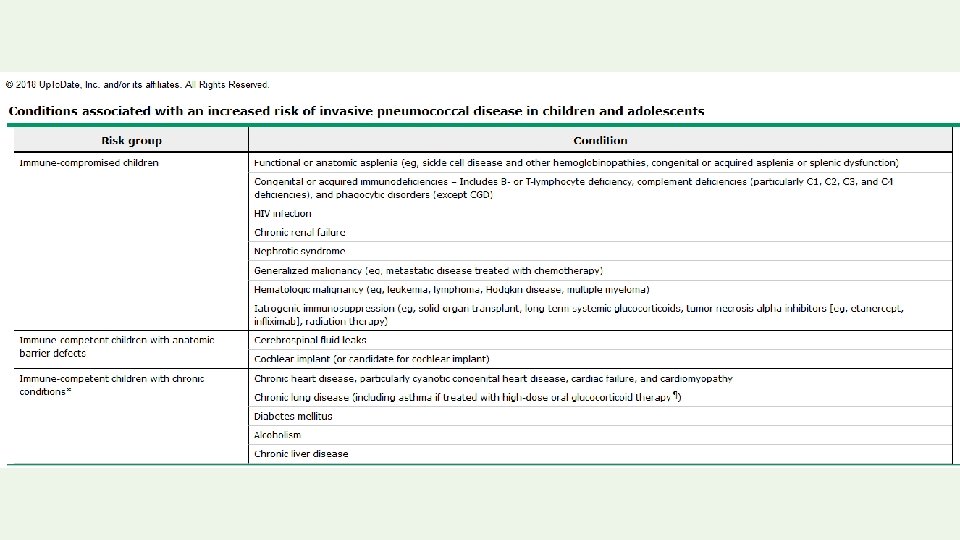
Permanent contraindications to vaccination: • Severe allergic reaction to a vaccine component or following a prior dose • Encephalopathy not due to another identifiable cause occurring within 7 days of pertussis vaccination • Severe combined immunodeficiency (rotavirus vaccine) • History of intussusception (rotavirus vaccine)

Temporary precautions to vaccination: • Moderate or severe acute illness (all vaccines) • Recent receipt of an antibody-containing blood product. • The latter precaution applies only to MMR and varicellacontaining (except zoster) vaccines. Temporary contraindications to vaccination with live vaccines: Pregnancy and Immunosuppression.

Screening Questions • Is the child sick today? • Does the child have allergies to medications, food, or any vaccine? • Has the child had a serious reaction to a vaccine in the past? • Has the child had a seizure, brain or nerve problem? • Has the child had a health problem with asthma, lung disease, heart disease, kidney disease, metabolic disease such as diabetes, or a blood disorder? • Does the child have cancer, leukemia, AIDS, or any other immune system problem? • Has the child taken cortisone, prednisone, other steroids, or anticancer drugs, or had xray treatments in the past 3 months? • Has the child received a transfusion of blood or blood products, or been given a medicine called immune (gamma) globulin in the past year? • Is the person pregnant or is there a chance she could become pregnant during the next month? • Has the child received vaccinations in the past 4 weeks?

BCG VACCINE TYPE LIVE-ATTENUATED AGE BIRTH ROUTE INTRADERMAL DOSES SINGLE

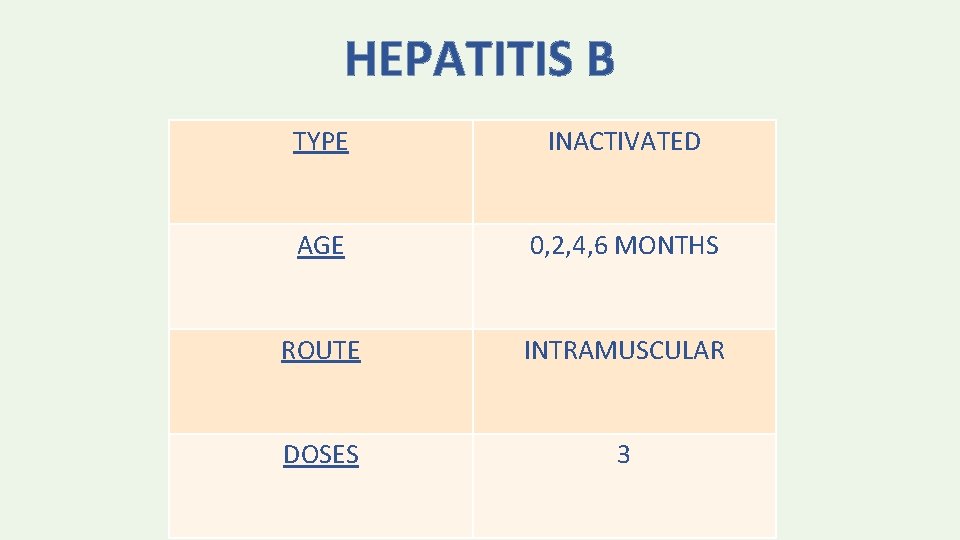
HEPATITIS B TYPE INACTIVATED AGE 0, 2, 4, 6 MONTHS ROUTE INTRAMUSCULAR DOSES 3

• The minimum age for the final (3 rd or 4 th) dose of Hep. B vaccine is 24 weeks. • The minimum interval is 4 weeks between the 1 st and 2 nd dose; 8 weeks between the 2 nd and 3 rd dose; and 16 weeks between the 1 st and 3 rd dose.

• If the mother is HBs. Ag-negative, the birth weight is <2 kg and the infant is medically stable, the infant is immunized at one month of age. • HBs. Ag-positive mother or mother with other evidence of HBV infection — As soon as possible and within 12 hours after birth, infants should receive: - The first dose of single-antigen Hep. B vaccine (IM) - HBIG 0. 5 m. L IM (in different limbs)

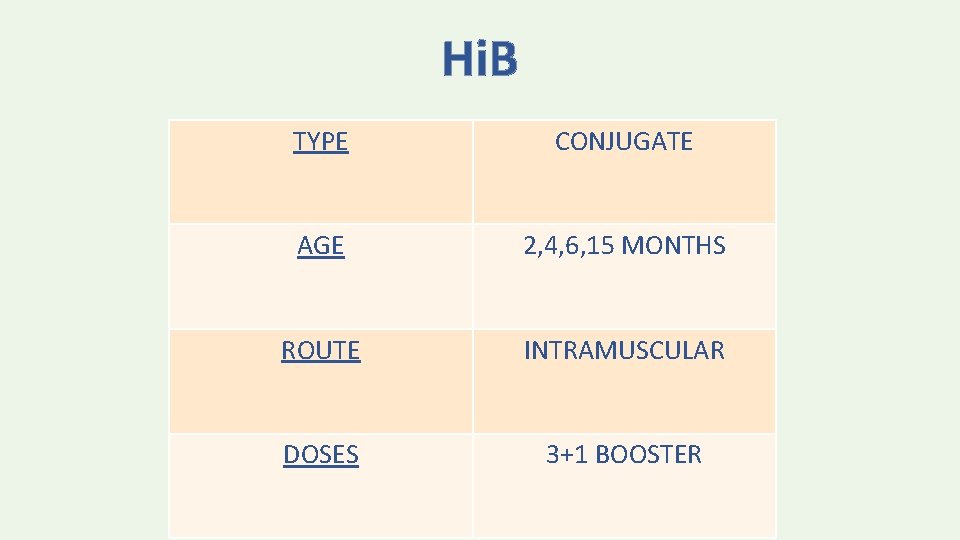
Hi. B TYPE CONJUGATE AGE 2, 4, 6, 15 MONTHS ROUTE INTRAMUSCULAR DOSES 3+1 BOOSTER

• The minimum age for the first dose is 6 weeks. • Doses administered before 6 weeks should not be counted as valid. • The recommended interval between doses in the primary series is 8 weeks; however, doses separated by at least 4 weeks may be counted as valid. • The minimum age for the final dose is 12 months

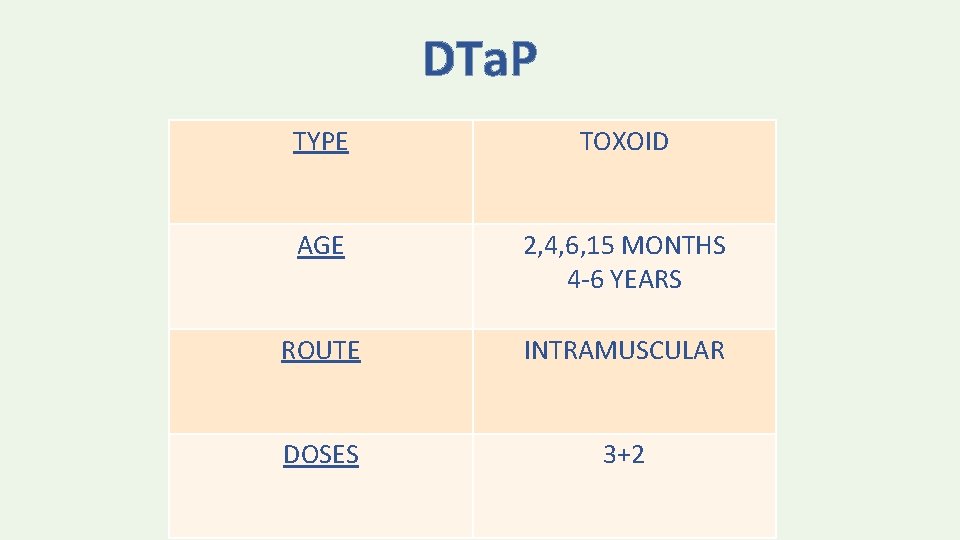
DTa. P TYPE TOXOID AGE 2, 4, 6, 15 MONTHS 4 -6 YEARS ROUTE INTRAMUSCULAR DOSES 3+2

DTa. P – Diphtheria and tetanus toxoids, and acellular pertussis DTw. P – Diphtheria and tetanus toxoids, and whole-cell pertussis DT – Diphtheria and tetanus toxoids; also called pediatric DT Tdap – Tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis Td – Tetanus toxoid, reduced diphtheria toxoid; also called adult Td, d. T, and adult d. T

• DTa. P vaccines must be distinguished from Tdap vaccines, which generally are used for a single booster dose during adolescence or adulthood. • DTa. P vaccines typically contain more diphtheria toxoid and pertussis antigens, and may contain more tetanus toxoid than Tdap vaccines

• DT vaccine is available for children <7 years who have contraindications or precautions to pertussis-containing vaccines. • DT vaccine must be distinguished from Td vaccine, which is typically used for booster doses in adolescents and adults. DT contains higher doses of diphtheria toxoid and may contain higher doses of tetanus toxoid than Td



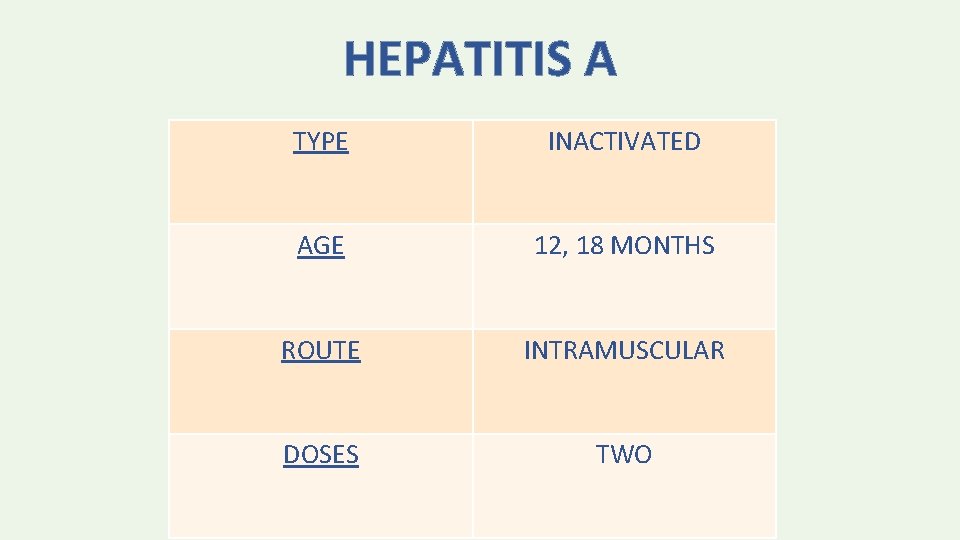
HEPATITIS A TYPE INACTIVATED AGE 12, 18 MONTHS ROUTE INTRAMUSCULAR DOSES TWO

• The minimum interval between the doses is 6 months.

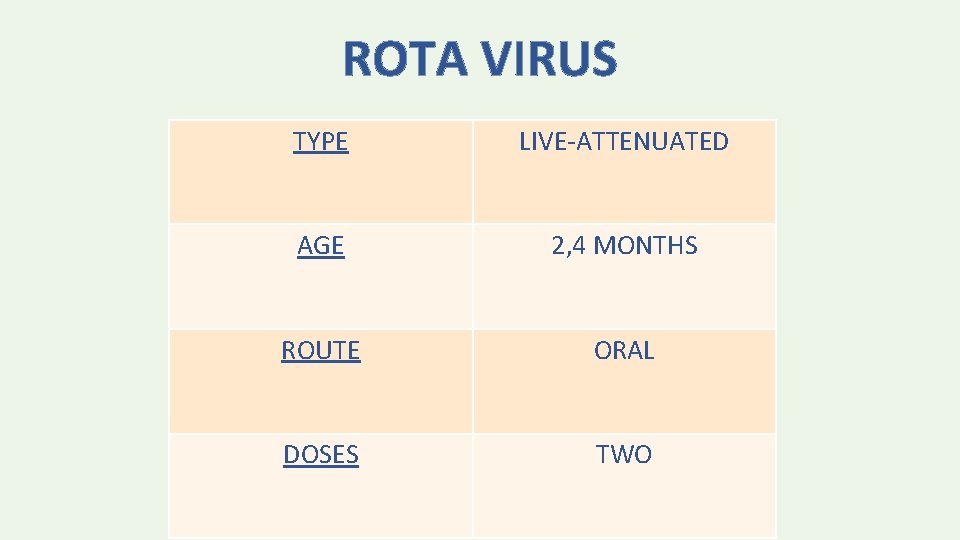
ROTA VIRUS TYPE LIVE-ATTENUATED AGE 2, 4 MONTHS ROUTE ORAL DOSES TWO

• The vaccine series should not be initiated in infants who are older than 14 weeks, 6 days of age • Minimum interval is 4 weeks • Series should be completed by 24 weeks

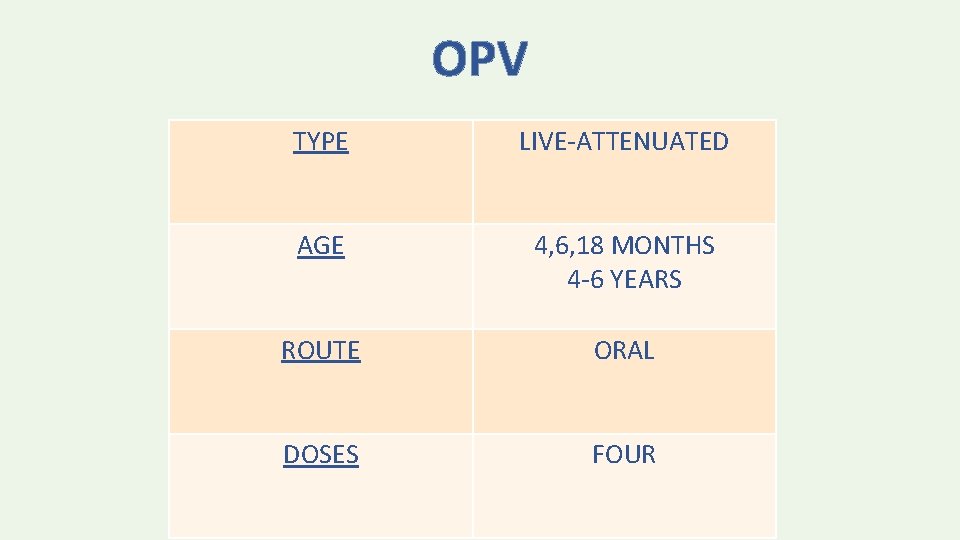
OPV TYPE LIVE-ATTENUATED AGE 4, 6, 18 MONTHS 4 -6 YEARS ROUTE ORAL DOSES FOUR

IPV TYPE INACTIVATED AGE 2, 4 MONTHS ROUTE INTRAMUSCULAR DOSES TWO

The Global Polio Eradication Initiative The GPEI "endgame" strategy calls for cessation of circulation of wild-type poliovirus and type 2 circulating vaccine-derived poliovirus, replacement of trivalent oral polio vaccine with bivalent oral polio vaccine for an interim period after April 2016, introduction of inactivated polio vaccine for routine childhood immunization, and eventual discontinuation of all oral poliovirus vaccine use once complete eradication of all types is certified by the World Health Organization.

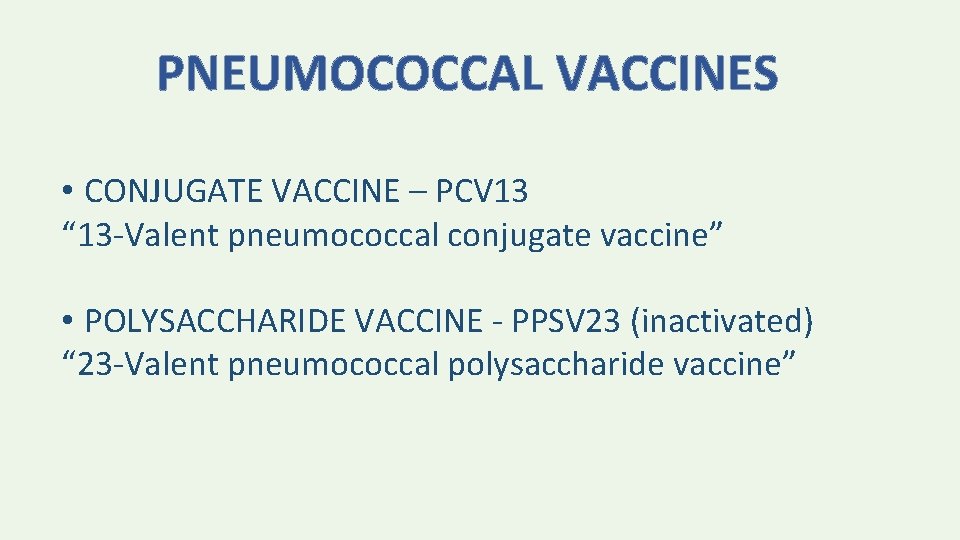
PNEUMOCOCCAL VACCINES • CONJUGATE VACCINE – PCV 13 “ 13 -Valent pneumococcal conjugate vaccine” • POLYSACCHARIDE VACCINE - PPSV 23 (inactivated) “ 23 -Valent pneumococcal polysaccharide vaccine”

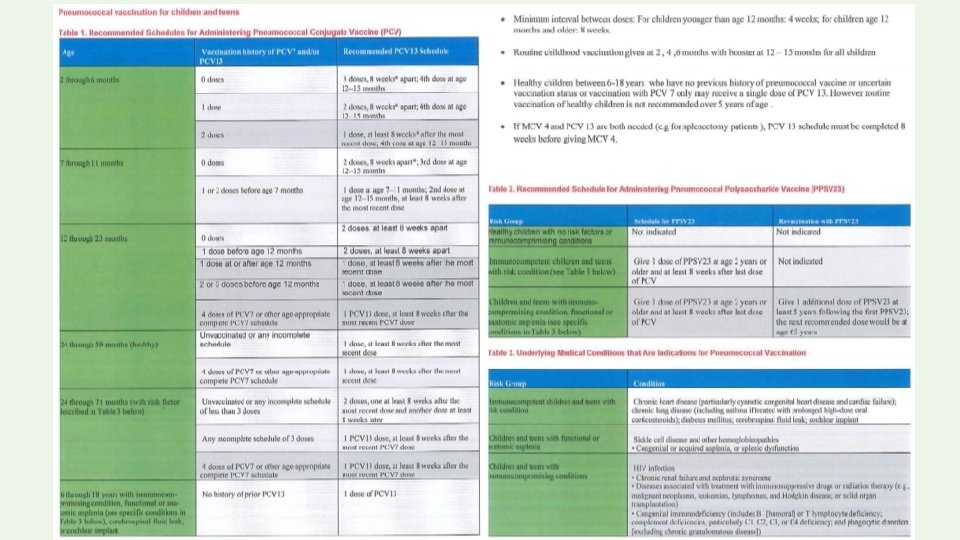
• PPSV 23 is licensed for use in people ≥ 2 years of age. Polysaccharide vaccines are poorly immunogenic in children younger than two years • The first dose of PCV 13 must be administered at the age of 2 months and should not be given for children more than 59 months (5 years) of age for healthy children • High risk children may still receive PCV 13 over 5 years age

PCV 13 TYPE SUBUNIT (CONJUGATE SEROTYPE VACCINE) AGE 2, 4, 6 MONTHS 15 MONTHS BOOSTER ROUTE INTRAMUSCULAR DOSES 3 + 1 booster



MMR TYPE LIVE-ATTENUATED AGE 12 , 18 MONTHS ROUTE SUBCUTANEOUS DOSES TWO

• Tuberculosis testing — Tuberculin testing should be performed before, at the same visit as, or four to six weeks after MMR vaccination. MMR may temporarily reduce tuberculin skin test sensitivity • Severe allergic reaction to neomycin or gelatin is a contraindication. • Minimum Interval is 4 weeks.

The receipt of parenterally administered immune globulin or antibody-containing blood products can blunt or block the host response to certain live-virus vaccines. For most children and adolescents, the suggested interval between receipt of immune globulin or antibody containing blood products and administration of measles, mumps, and rubella combination vaccine (MMR) varies between 3 and 11 months depending upon the product

LACK OF ASSOCIATION WITH AUTISM — Concern has been raised about a possible link between measles, mumps, and rubella vaccination and autism and other chronic diseases. Multiple studies have failed to demonstrate any such association. However, there is an association between congenital rubella syndrome and autism, highlighting a potential role for rubella immunization in the prevention of autism spectrum disorders

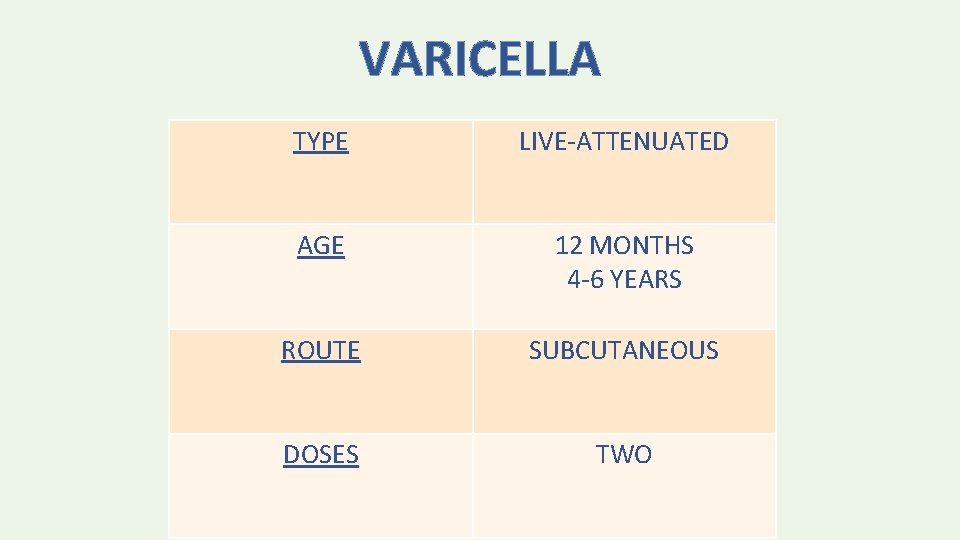
VARICELLA TYPE LIVE-ATTENUATED AGE 12 MONTHS 4 -6 YEARS ROUTE SUBCUTANEOUS DOSES TWO

• Antiviral medications — Antiviral drugs active against herpesviruses (eg, acyclovir, valacyclovir) may interfere with response to varicella-containing vaccines and should be discontinued ≥ 24 hours before administration of varicella-containing vaccines. • They also should be avoided for 14 days after vaccination.

• Children <13 years — The minimum age for 1 st dose is 12 months. Doses administered before age 12 months should not be counted and the child should be revaccinated at 1215 months of age (and ≥ 3 months after the initial dose). • The second dose may be administered earlier than age 4 years (eg, during a varicella outbreak). • For children <13 years, the recommended minimum interval between doses is 3 months.

• A second dose of varicella vaccine is recommended for adolescents and adults who previously received only one dose. • The minimum interval between doses for people ≥ 13 years is 28 days

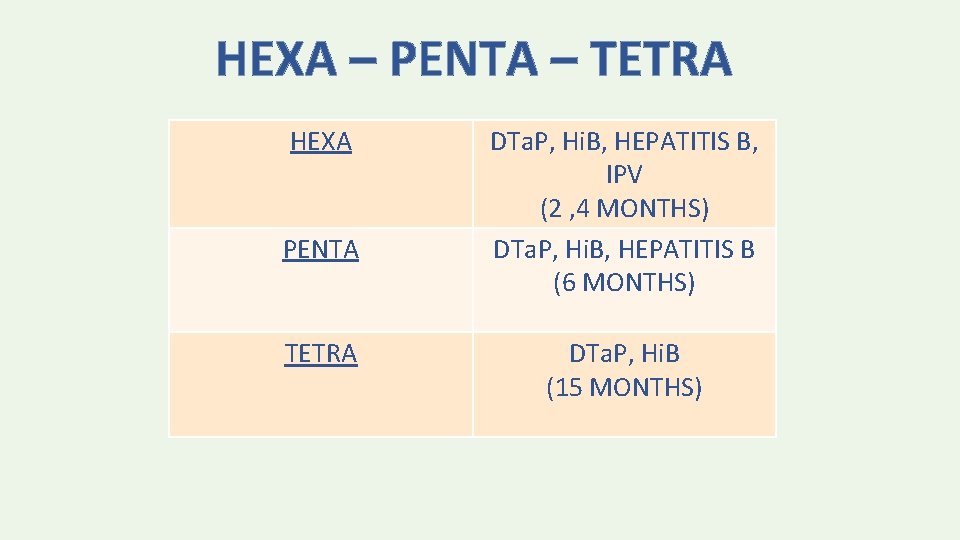
HEXA – PENTA – TETRA HEXA PENTA TETRA DTa. P, Hi. B, HEPATITIS B, IPV (2 , 4 MONTHS) DTa. P, Hi. B, HEPATITIS B (6 MONTHS) DTa. P, Hi. B (15 MONTHS)

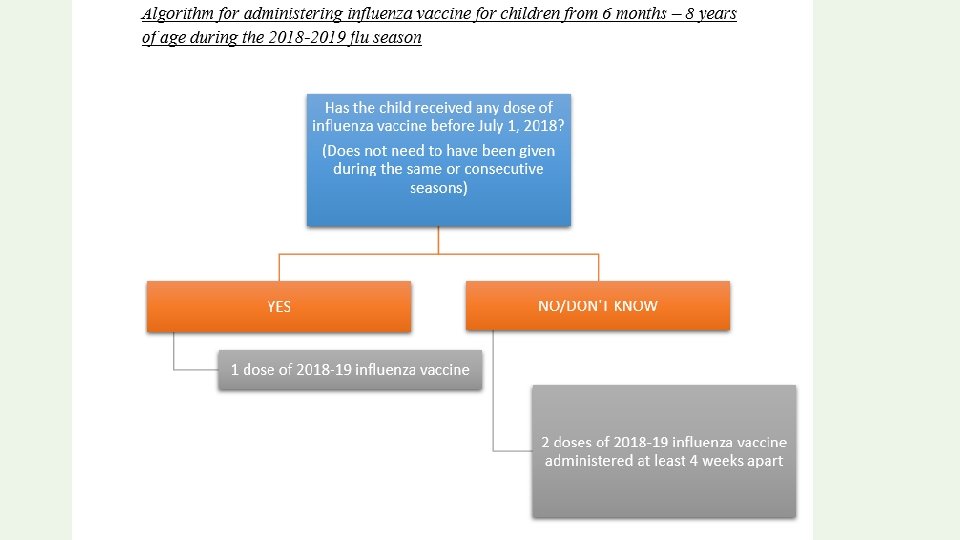
INFLUENZA VACCINE • The ACIP and the AAP recommend universal annual influenza vaccination for persons ≥ 6 months. • Inactivated influenza vaccine (IIV) can be administered to all persons 6 months of age and older, including healthy and persons with underlying medical conditions. • Live attenuated influenza vaccine (LAIV 4) is licensed only for healthy, nonpregnant persons age 2 through 49 years.

There are two types of IIV 1. QUADRIVALENT INFLUENZA VACCINE recommended for children 3 years and older 2. TRIVALENT INFLUENZA VACCINE Recommended for children 6 months of age and older IIV dose: 0. 25 ml 6 -35 months and 0. 5 ml age 3 years and older.

Children ≥ 9 years: require only a single dose of influenza vaccine. Children 6 months-8 years: require two doses of influenza vaccine during the first season in which they are vaccinated to optimize immune response


A 6 year old boy with a history of bronchial asthma is coming to receive annual influenza vaccine. He has a history of allergy to eggs ‘”Hives”. How would you approach? What if he had history of anaphylaxis to eggs?



VACCINATION DEFAULTERS SCHEDULE

Infants or children from 3 months-18 years of age who : 1. Start vaccination late or have delayed vaccination for more than 1 month according to National Immunization Schedule 2. Have not completed vaccination series of recommended vaccines in National Immunization Schedule 3. Lack reliable evidence of immunity or immunizations

MMR 2 doses VARICELLA 2 doses HEPATITIS B 3 doses HEPATITIS A 2 doses between 12 -24 months and above 24 months for travelers to endemic areas ROTAVIRUS 2 doses before 6 months DTa. P 3 months-4 years: 5 doses 4 years-5 years : 4 doses After 5 th birthday-7 years : 3 doses Tda. P 1 dose above 7 years followed by 2 doses of Td if no doses or incomplete doses of DTa. P were given before 7 years Booster dose at 13 -16 years IPV/OPV 1 -2 doses of IPV, 3 -4 doses of OPV






CDC PINK BOOK https: //www. cdc. gov/vaccines/pubs/pinkbook/index. html UPTODATE QATAR NATIONAL IMMUNIZATION PROGRAM
 Defaulter vaccination schedule
Defaulter vaccination schedule Poultry vaccination schedule
Poultry vaccination schedule Vaccination schedule in palestine
Vaccination schedule in palestine Epi schedule pakistan 2021
Epi schedule pakistan 2021 Meaning of variolation
Meaning of variolation Spay perry county
Spay perry county Vaccination bruxelles
Vaccination bruxelles Mandatory vaccination
Mandatory vaccination Vaccination xard
Vaccination xard Vaccination a la defense
Vaccination a la defense Vaccination dose
Vaccination dose A vaccination for smallpox was developed in 1796 by ____
A vaccination for smallpox was developed in 1796 by ____ Sleeping princesses
Sleeping princesses Mark anthony fernandez vaccination
Mark anthony fernandez vaccination Lokstallarna jönköping vaccination
Lokstallarna jönköping vaccination Format kertas kerja pemeriksaan
Format kertas kerja pemeriksaan Embedded systems
Embedded systems What is this in english
What is this in english Aruza veda kimin eseri
Aruza veda kimin eseri Rafayet ali
Rafayet ali Ali afzal malik
Ali afzal malik Nevjera ali umire
Nevjera ali umire Ali sekmen
Ali sekmen Ali
Ali Mohamed father in law
Mohamed father in law Abu rayhon beruniyning pedagogik qarashlari ppt
Abu rayhon beruniyning pedagogik qarashlari ppt Ya aliyu ya azim
Ya aliyu ya azim Ali darejeh
Ali darejeh Ali kemal göğüş
Ali kemal göğüş Kıssaların en güzeli
Kıssaların en güzeli On three days ali throws darts at a target
On three days ali throws darts at a target Spajanje ali sinteza
Spajanje ali sinteza Riwayat hidup ali bin abu talib
Riwayat hidup ali bin abu talib Dengesel olmayan koordinasyon testleri
Dengesel olmayan koordinasyon testleri Hz ali kadir gecesi
Hz ali kadir gecesi Mohammad ali javidian
Mohammad ali javidian Matematii
Matematii Ali osman alaca
Ali osman alaca Ali veríeis galantes pintados de preto e vermelho
Ali veríeis galantes pintados de preto e vermelho Mehmet ali uysal
Mehmet ali uysal Corrado govoni autoritratto
Corrado govoni autoritratto Ali imran 26 27
Ali imran 26 27 Enflemasyon
Enflemasyon Alexandre mercier-dalphond
Alexandre mercier-dalphond Mehmet ali lahur ticaret meslek lisesi
Mehmet ali lahur ticaret meslek lisesi Kandid ali optimizem obnova
Kandid ali optimizem obnova Complete the tag question your name is ali khalid
Complete the tag question your name is ali khalid Interview questions for kitchenhand
Interview questions for kitchenhand 21 ramadan hazrat ali
21 ramadan hazrat ali Ali sayir afosr
Ali sayir afosr Sobia ali
Sobia ali Paano nakamit ng imperyong mali
Paano nakamit ng imperyong mali Valj oglišča
Valj oglišča Ali madani md
Ali madani md Ali sekmen
Ali sekmen Ziarat imam ali in english
Ziarat imam ali in english Kısa dalga telsizler uzay teknolojisi
Kısa dalga telsizler uzay teknolojisi Dr ali saleh
Dr ali saleh Ali rezaei csulb
Ali rezaei csulb Tundra iklimi özellikleri
Tundra iklimi özellikleri Samir ould ali inserm
Samir ould ali inserm Ali obaidi
Ali obaidi Abrar ali saiyed
Abrar ali saiyed Walled world
Walled world Ali galip bayrak
Ali galip bayrak Sinir sisteminin tipləri
Sinir sisteminin tipləri Ali shahidi
Ali shahidi Ali hearn restorative practices
Ali hearn restorative practices