IMMUNIZATION UPDATE Amelia Arnold Pharm D Retail Pharmacy







![Comparison of HD vs. Traditional Fluzone HD [Prescribing Information]. Sanofi Pasteur, Swiftwater, Pennsylvania. July Comparison of HD vs. Traditional Fluzone HD [Prescribing Information]. Sanofi Pasteur, Swiftwater, Pennsylvania. July](https://slidetodoc.com/presentation_image_h/ba521655a3e3751ce1e6c521b20df9bb/image-10.jpg)















![Zanamivir (Relenza®) Relenza [Prescribing Information]. Glaxo. Smith. Kline, Research Triangle Park, North Carolina. August Zanamivir (Relenza®) Relenza [Prescribing Information]. Glaxo. Smith. Kline, Research Triangle Park, North Carolina. August](https://slidetodoc.com/presentation_image_h/ba521655a3e3751ce1e6c521b20df9bb/image-37.jpg)



















- Slides: 73

IMMUNIZATION UPDATE Amelia Arnold, Pharm. D Retail Pharmacy Operations Manager, Community Pharmacies, LP President-Elect, Maine Pharmacy Association March 2018

Disclosures ■ I have nothing to disclose concerning possible financial or personal relationships with commercial entities that may have a direct or indirect interest in the subject matter of this presentation.

Objectives ■ Recognize the changes made by the Advisory Committee on Immunization Practices (ACIP) to the 2018 immunization schedules ■ Discuss ACIP recommendations for use of the new herpes zoster vaccine Shringrix ■ Describe the impact of the 2017 -2018 influenza season in terms of vaccine efficacy and flu intensity ■ List the 2018 influenza antiviral recommendations

Flu Shot Options ■ Trivalent ■ Quadrivalent ■ Cell-based technology quadrivalent ■ Recombinant egg-free trivalent ■ Recombinant egg-free quadrivalent ■ High-dose trivalent ■ Trivalent with adjuvant ■ Live-attenuated, inhaled quadrivalent

Quad Flu Shot- Examples ■ Afluria ■ Fluarix ■ Flulaval ■ Fluzone ■ Agriflu ■ Flucelvax

Flucelvax® ■ Approved August 2016 ■ 0. 5 m. L given IM in deltoid ■ Approved for ages 4 years and older ■ ‘Cell-based’ flu vaccine ■ Original vaccine virus is still grown in eggs, but mass production is performed by growing viruses in animal cells Flucelvax [Prescribing Information]. Seqirus Inc. , Holly Springs, North Carolina. April 2017.

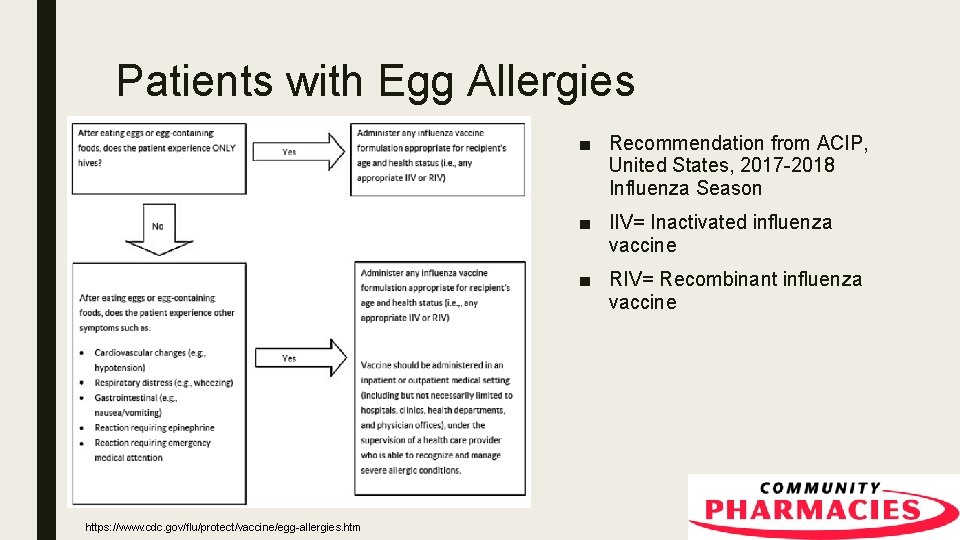
Patients with Egg Allergies ■ Recommendation from ACIP, United States, 2017 -2018 Influenza Season ■ IIV= Inactivated influenza vaccine ■ RIV= Recombinant influenza vaccine https: //www. cdc. gov/flu/protect/vaccine/egg-allergies. htm

Flu. Blok ® ■ FDA approved 2013 ■ 0. 5 m. L given IM in deltoid ■ Protein Sciences Corp- Available as trivalent and quadrivalent ■ Approved for 18 years and older ■ Only vaccine that is completely egg free Flu. Blok [Prescribing Information]. Protein Sciences Corporation, Meriden, Connecticut. March 2017.

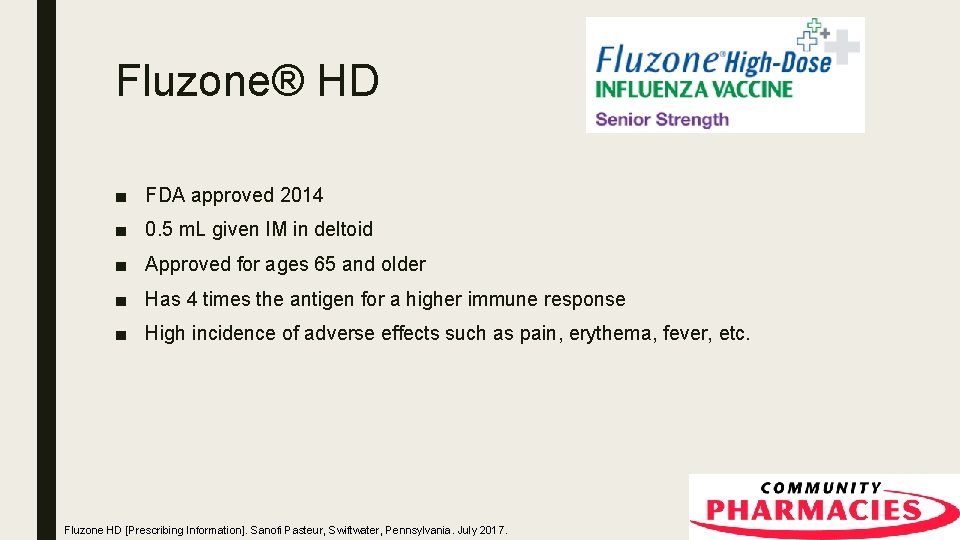
Fluzone® HD ■ FDA approved 2014 ■ 0. 5 m. L given IM in deltoid ■ Approved for ages 65 and older ■ Has 4 times the antigen for a higher immune response ■ High incidence of adverse effects such as pain, erythema, fever, etc. Fluzone HD [Prescribing Information]. Sanofi Pasteur, Swiftwater, Pennsylvania. July 2017.
![Comparison of HD vs Traditional Fluzone HD Prescribing Information Sanofi Pasteur Swiftwater Pennsylvania July Comparison of HD vs. Traditional Fluzone HD [Prescribing Information]. Sanofi Pasteur, Swiftwater, Pennsylvania. July](https://slidetodoc.com/presentation_image_h/ba521655a3e3751ce1e6c521b20df9bb/image-10.jpg)
Comparison of HD vs. Traditional Fluzone HD [Prescribing Information]. Sanofi Pasteur, Swiftwater, Pennsylvania. July 2017.

Fluad ™ ■ FDA approved in 2015 ■ Adjuvanted, inactivated, trivalent vaccine ■ 0. 5 m. L given IM in deltoid ■ Approved for 65 years and older Fluad [Prescribing Information]. Seqirus Inc. , Liverpool, United Kingdom. March 2017.

Immunology Review: Adjuvant ■ An adjuvant is an ingredient of a vaccine that helps create a stronger immune response in the patient’s body. ■ A vaccine made with adjuvants ensures the body produces an immune response strong enough to protect the patient from the germ he or she is being vaccinated against. https: //www. cdc. gov/vaccinesafety/concerns/adjuvants. html

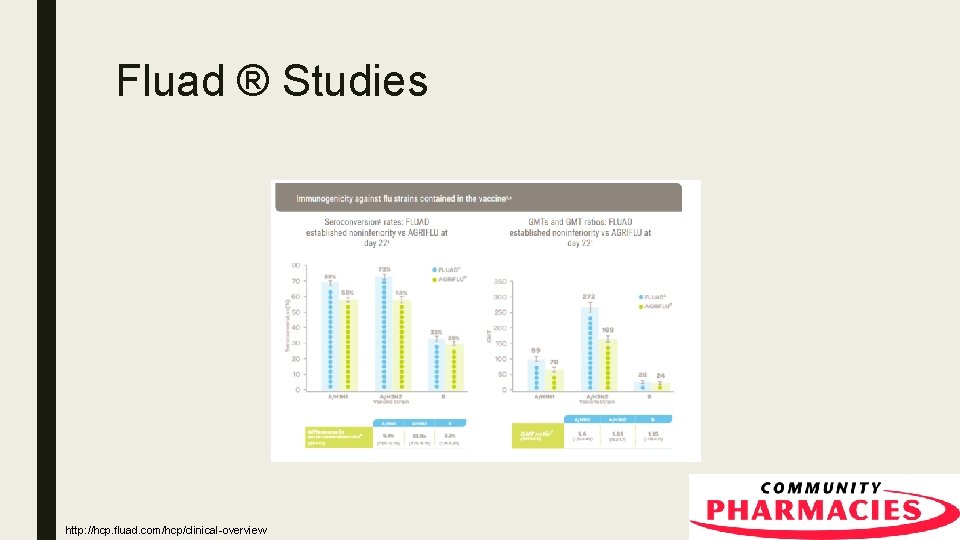
Fluad ™ ■ A proprietary adjuvant called MF 59® – MF 59 is an emulsion of the oil squalene, which is a naturally occurring substance in the body ■ Demonstrated noninferiority compared to standard trivalent flu shots in a study Fluad [Prescribing Information]. Seqirus Inc. , Liverpool, United Kingdom. March 2017.

Fluad ® Studies http: //hcp. fluad. com/hcp/clinical-overview

Flu. Mist ® ■ Intranasal, quadrivalent, live flu vaccine ■ 0. 2 m. L given in each nostril, 0. 4 m. L total ■ Approved in healthy, non-pregnant individuals from 2 years to 49 years ■ Depress plunger in nostril until the dose divider clips stop it, then remove and repeat in second nostril Flu. Mist [Prescribing Information]. Med. Immune, Gaithersburg, MD 20878. August 2017.

Flu. Mist® ■ For 17 -18 flu season, CDC voted to NOT recommend the Flu. Mist vaccine ■ In February 2018, CDC ACIP voted to recommend Flu. Mist for the 2018 -2019 flu season https: //www. cdc. gov/flu/about/season/flu-season-2017 -2018. htm https: //www. flumistquadrivalent. com/

Flu. Mist® https: //www. flumistquadrivalent. com/

Strains in 2017 -2018 ■ Trivalent formulation: – A/Michigan/45/2015 (H 1 N 1)pdm 09 -like virus – A/Hong Kong/4801/2014 (H 3 N 2)-like virus – B/Brisbane/60/2008 -like (B/Victoria lineage) virus ■ Additional strain in Quad: – B/Phuket/3073/2013 -like (B/Yamagata lineage) virus https: //www. cdc. gov/flu/about/season/flu-season-2017 -2018. htm

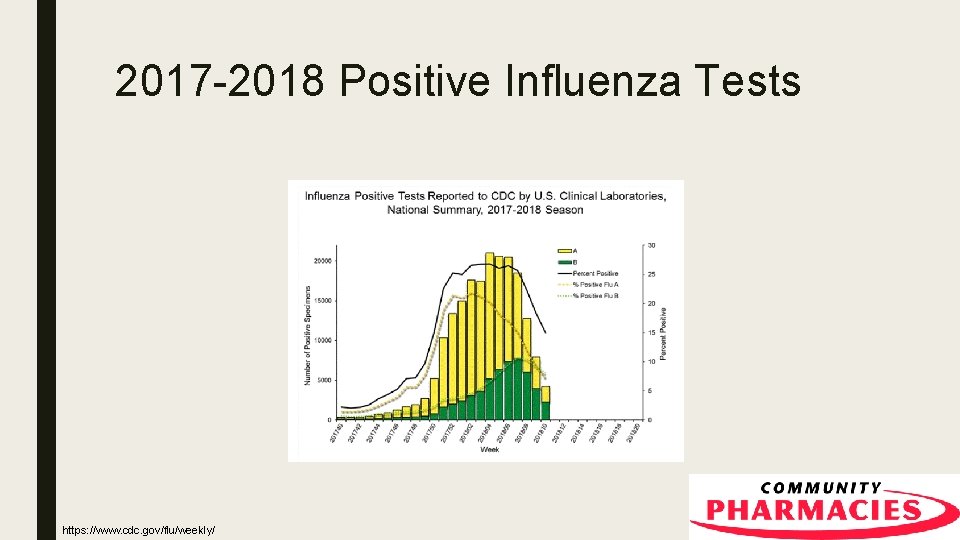
2017 -2018 Positive Influenza Tests https: //www. cdc. gov/flu/weekly/

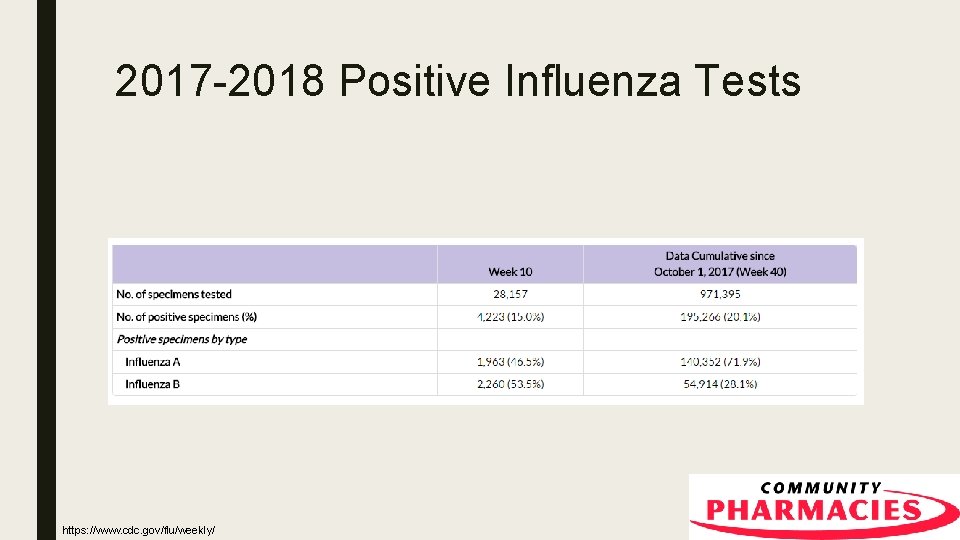
2017 -2018 Positive Influenza Tests https: //www. cdc. gov/flu/weekly/

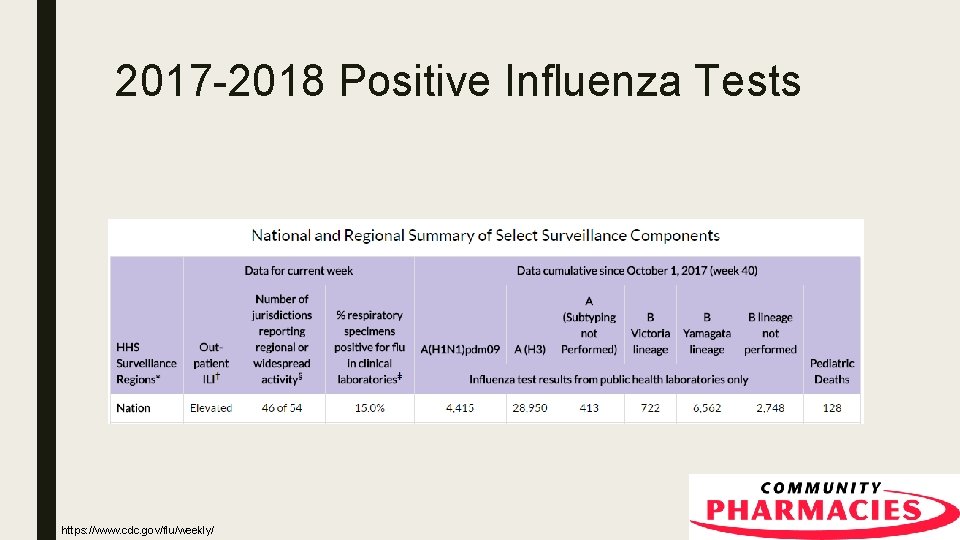
2017 -2018 Positive Influenza Tests https: //www. cdc. gov/flu/weekly/index. htm

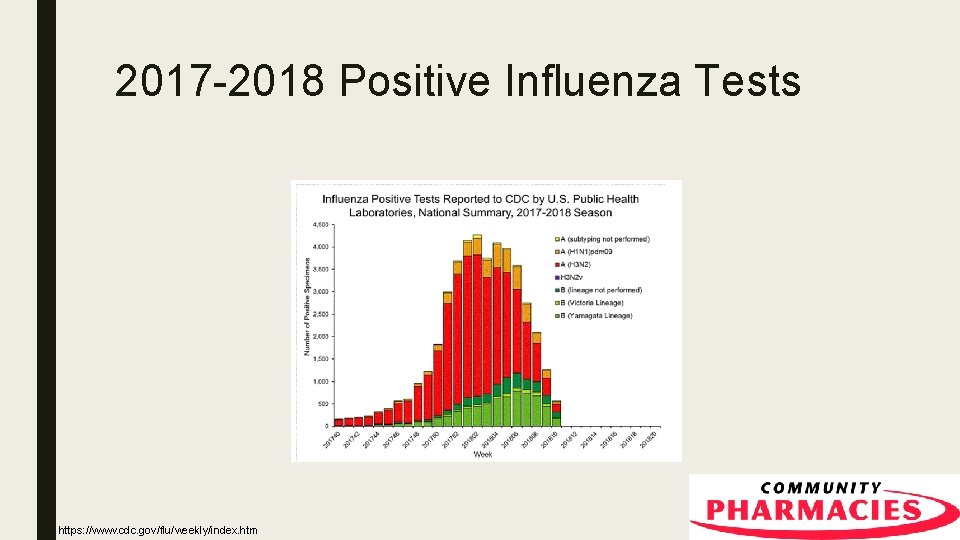
2017 -2018 Positive Influenza Tests https: //www. cdc. gov/flu/weekly/

SO HOW DID THE VACCINE DO?

Strains in 2017 -2018 ■ Trivalent formulation: – A/Michigan/45/2015 (H 1 N 1)pdm 09 -like virus – A/Hong Kong/4801/2014 (H 3 N 2)-like virus – B/Brisbane/60/2008 -like (B/Victoria lineage) virus ■ Additional strain in Quad: – B/Phuket/3073/2013 -like (B/Yamagata lineage) virus https: //www. cdc. gov/flu/about/season/flu-season-2017 -2018. htm

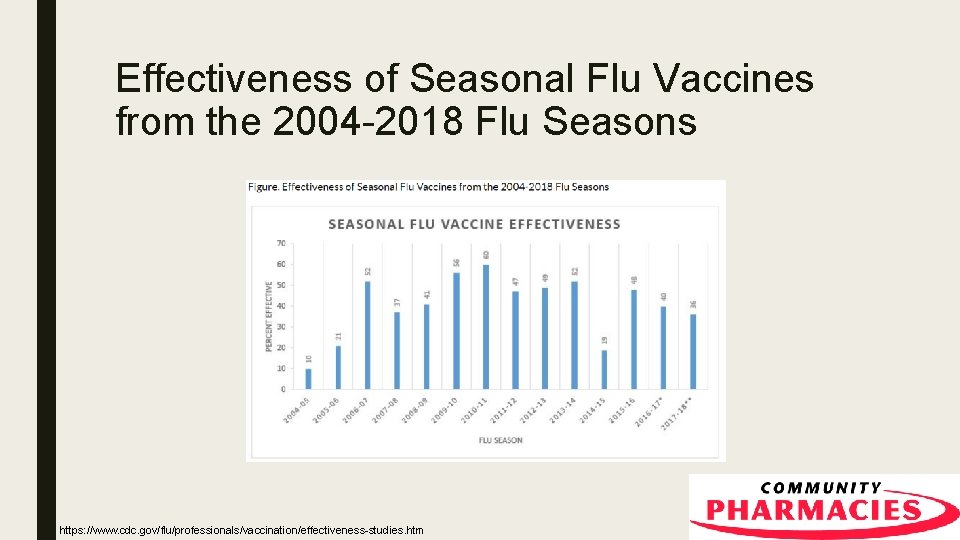
Vaccine Efficacy ■ According to the CDC interim estimates: – B Strains: 42% (CI = 25% to 56%) – H 1 N 1 Efficacy: 67% (CI = 54% to 76%) – H 3 N 2 Efficacy: 25% (CI = 13% to 36%)

2017 -2018 Positive Influenza Tests https: //www. cdc. gov/flu/weekly/index. htm

Effectiveness of Seasonal Flu Vaccines from the 2004 -2018 Flu Seasons https: //www. cdc. gov/flu/professionals/vaccination/effectiveness-studies. htm

Strains in 2018 -2019 ■ World Health Organization voted on March 1 for the vaccine composition for next year – Trivalent: ■ ■ ■ A/Michigan/45/2015 (H 1 N 1)pdm 09 -like virus A/Singapore/INFIMH-16 -0019/2016 (H 3 N 2)-like virus B/Colorado/06/2017 -like virus (B/Victoria/2/87 lineage) – In Quad: ■ B/Phuket/3073/2013 -like virus (B/Yamagata/16/88 lineage) http: //www. who. int/influenza/vaccines/virus/recommendations/2018_19_north/en/

WHAT’S THE BEST FLU SHOT? The one that is available!

WHAT HAPPENS AFTER YOU GET THE FLU?

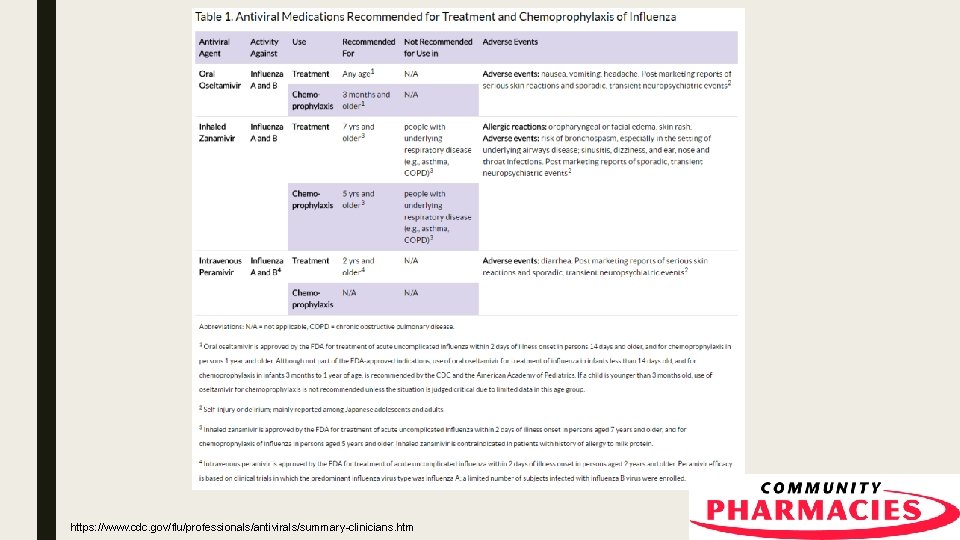
Antivirals ■ Can shorten illness by 1 or 2 days ■ CDC urges consideration of antiviral use for high risk populations ■ Prevent serious flu complications such as ear infections in children, pneumonia, hospitalization and death in adults https: //www. cdc. gov/flu/professionals/antivirals/summary-clinicians. htm

“High Risk Populations” ■ Asthma ■ Adults 65 and older ■ Neurological and neurodevelopment conditions ■ Children younger than 5 and especially younger than 2 ■ Blood disorders ■ Pregnant women ■ Chronic lung disease ■ Women 2 weeks after the end of pregnancy ■ Endocrine disorder (ex. Diabetes) ■ ■ Heart disease People with weakened immune system due to disease or medications ■ Kidney disorders ■ American Indians and Alaska Natives ■ Liver disorders ■ People younger than 19 on long term aspirin therapy ■ Metabolic disorders ■ ■ BMI >= 40 People in nursing facilities and long term care homes https: //www. cdc. gov/flu/professionals/antivirals/summary-clinicians. htm

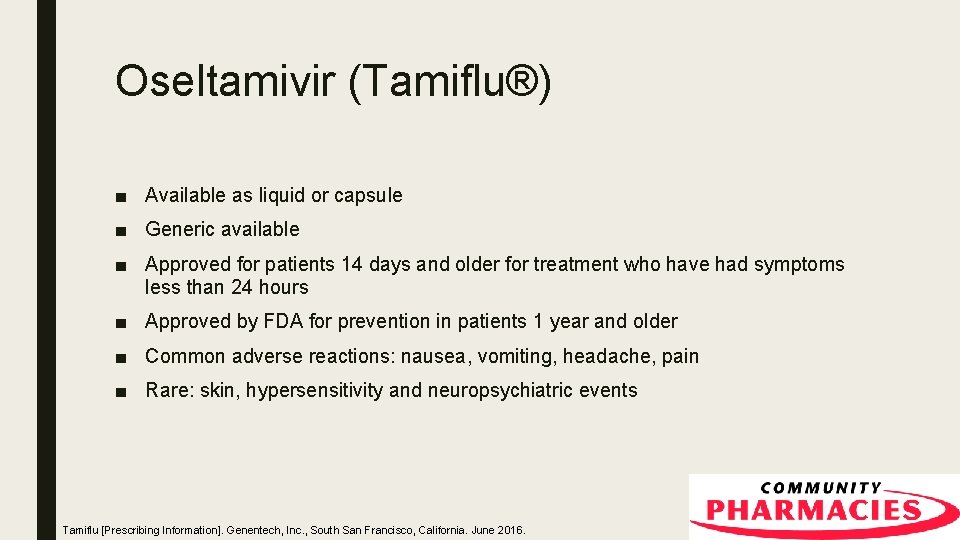
Oseltamivir (Tamiflu®) ■ Available as liquid or capsule ■ Generic available ■ Approved for patients 14 days and older for treatment who have had symptoms less than 24 hours ■ Approved by FDA for prevention in patients 1 year and older ■ Common adverse reactions: nausea, vomiting, headache, pain ■ Rare: skin, hypersensitivity and neuropsychiatric events Tamiflu [Prescribing Information]. Genentech, Inc. , South San Francisco, California. June 2016.

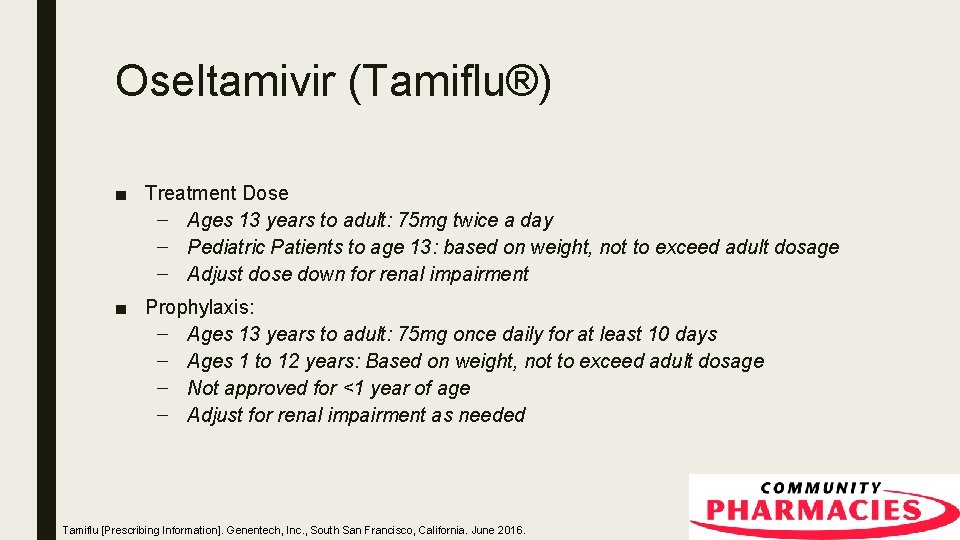
Oseltamivir (Tamiflu®) ■ Treatment Dose – Ages 13 years to adult: 75 mg twice a day – Pediatric Patients to age 13: based on weight, not to exceed adult dosage – Adjust dose down for renal impairment ■ Prophylaxis: – Ages 13 years to adult: 75 mg once daily for at least 10 days – Ages 1 to 12 years: Based on weight, not to exceed adult dosage – Not approved for <1 year of age – Adjust for renal impairment as needed Tamiflu [Prescribing Information]. Genentech, Inc. , South San Francisco, California. June 2016.

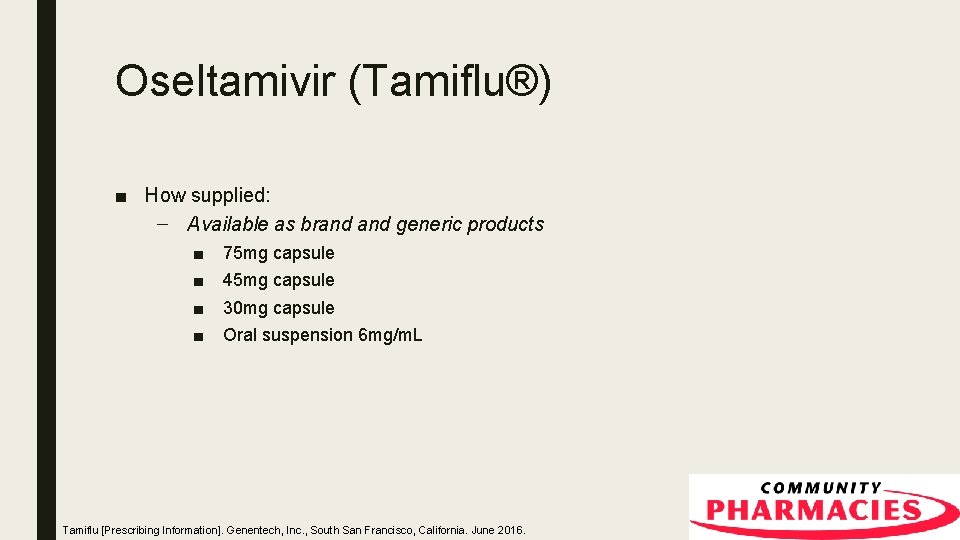
Oseltamivir (Tamiflu®) ■ How supplied: – Available as brand generic products ■ ■ 75 mg capsule 45 mg capsule 30 mg capsule Oral suspension 6 mg/m. L Tamiflu [Prescribing Information]. Genentech, Inc. , South San Francisco, California. June 2016.

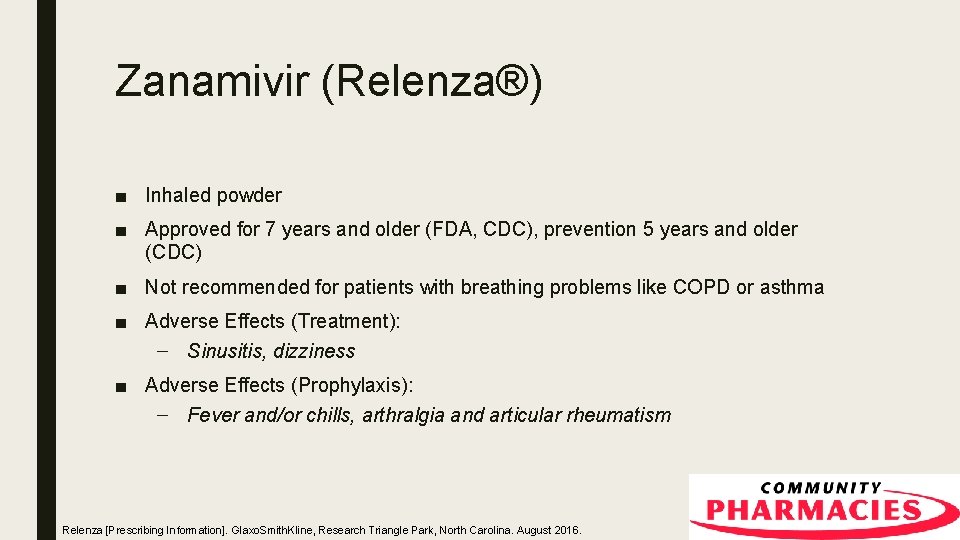
Zanamivir (Relenza®) ■ Inhaled powder ■ Approved for 7 years and older (FDA, CDC), prevention 5 years and older (CDC) ■ Not recommended for patients with breathing problems like COPD or asthma ■ Adverse Effects (Treatment): – Sinusitis, dizziness ■ Adverse Effects (Prophylaxis): – Fever and/or chills, arthralgia and articular rheumatism Relenza [Prescribing Information]. Glaxo. Smith. Kline, Research Triangle Park, North Carolina. August 2016.
![Zanamivir Relenza Relenza Prescribing Information Glaxo Smith Kline Research Triangle Park North Carolina August Zanamivir (Relenza®) Relenza [Prescribing Information]. Glaxo. Smith. Kline, Research Triangle Park, North Carolina. August](https://slidetodoc.com/presentation_image_h/ba521655a3e3751ce1e6c521b20df9bb/image-37.jpg)
Zanamivir (Relenza®) Relenza [Prescribing Information]. Glaxo. Smith. Kline, Research Triangle Park, North Carolina. August 2016.

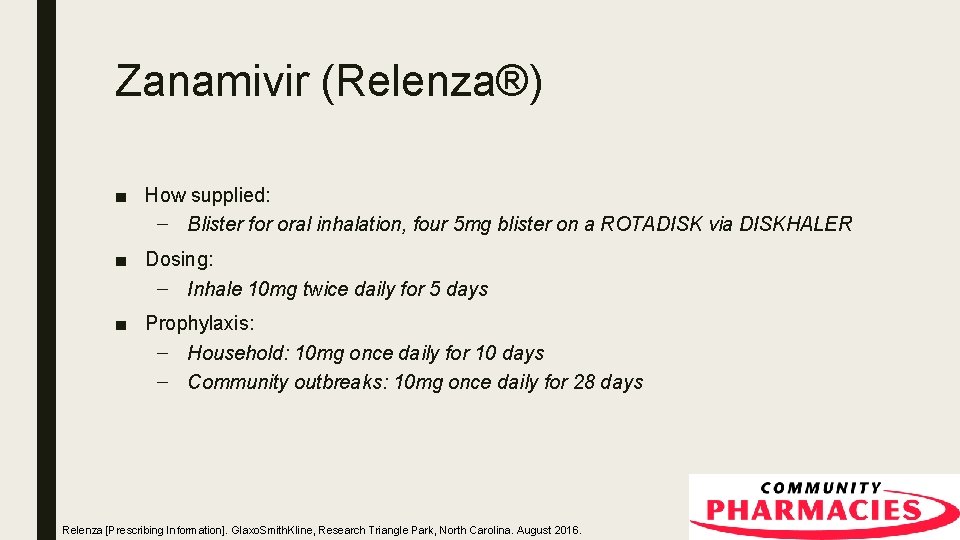
Zanamivir (Relenza®) ■ How supplied: – Blister for oral inhalation, four 5 mg blister on a ROTADISK via DISKHALER ■ Dosing: – Inhale 10 mg twice daily for 5 days ■ Prophylaxis: – Household: 10 mg once daily for 10 days – Community outbreaks: 10 mg once daily for 28 days Relenza [Prescribing Information]. Glaxo. Smith. Kline, Research Triangle Park, North Carolina. August 2016.

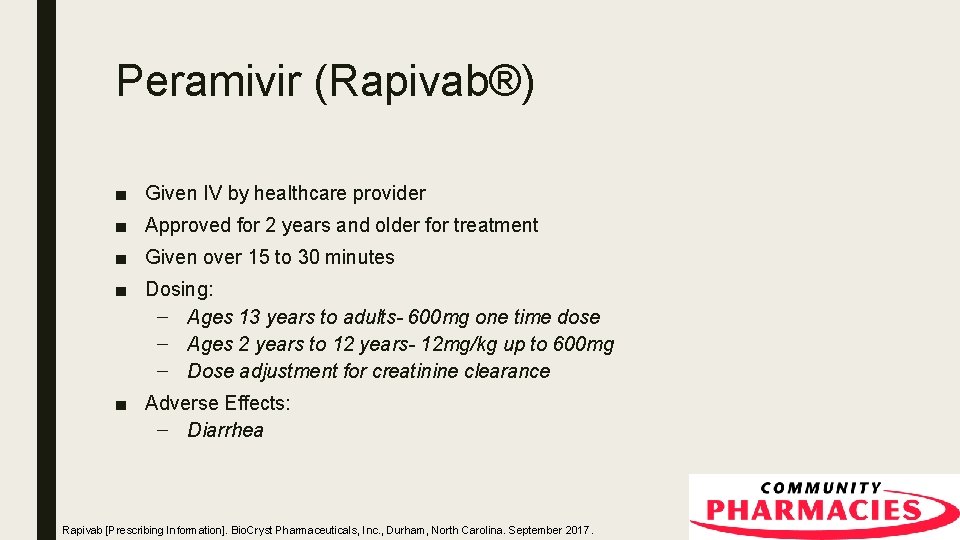
Peramivir (Rapivab®) ■ Given IV by healthcare provider ■ Approved for 2 years and older for treatment ■ Given over 15 to 30 minutes ■ Dosing: – Ages 13 years to adults- 600 mg one time dose – Ages 2 years to 12 years- 12 mg/kg up to 600 mg – Dose adjustment for creatinine clearance ■ Adverse Effects: – Diarrhea Rapivab [Prescribing Information]. Bio. Cryst Pharmaceuticals, Inc. , Durham, North Carolina. September 2017.

https: //www. cdc. gov/flu/professionals/antivirals/summary-clinicians. htm

Patient Case ■ 62 year old woman presents to pharmacy to pick up a prescription for oseltamivir 75 mg once daily. During counseling the patient discloses that she will be traveling out of state to visit her daughter and grandchildren, who have tested positive today for influenza. What would you tell the patient? ■ Patient currently taking 25 mg HCTZ daily, atorvastatin 40 mg daily, Ventolin HFA 2 puffs PRN, and metformin 500 mg BID.

QUESTIONS?

HERPES ZOSTER AND SHINGRIX™ Written and Developed by: Steven Garhartt Pharm. D/MBA Candidate 2018


Disclaimer ■ All recommendations and data in this slide were developed prior to March 21 st, 2018 and revolve around CDC and ACIP guidelines ■ All information in this presentation is subject to change based on new guideline recommendations by the aforementioned governing bodies

Learning Objectives ■ Discuss ACIP recommendations for use of the new herpes zoster vaccine Shringrix ■ Recognize the changes made by the Advisory Committee on Immunization Practices (ACIP) to the 2018 immunization schedules

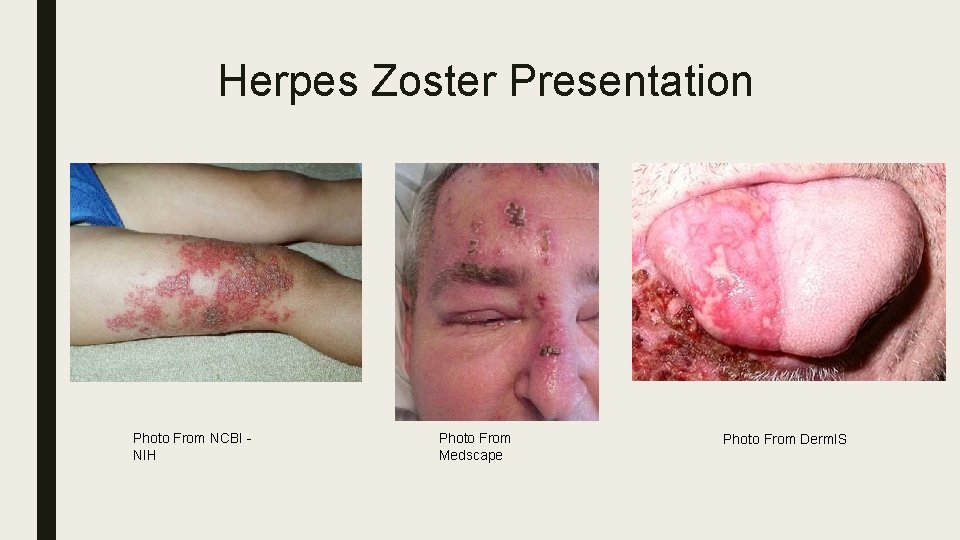
Epidemiology and Postherpetic Neuralgia 1, 2 ■ 1 in every 3 persons in the United States will develop shingles – Estimated 1, 000 cases annually ■ Equating to 4 per 1000 ■ Incidence of Herpes Zoster (HZ) increases with age – Less than 1 per 1000 in children – Greater than 15 per 1000 in adults over 80 ■ Following the clearing of the shingles rash, patients may experience PHN, a severe and debilitating pain in the same localized areas – 10 -18% of patients with shingles over 50 years old will have PHN and this number increases with age

Herpes Zoster Presentation Photo From NCBI - NIH Photo From Medscape Photo From Derm. IS

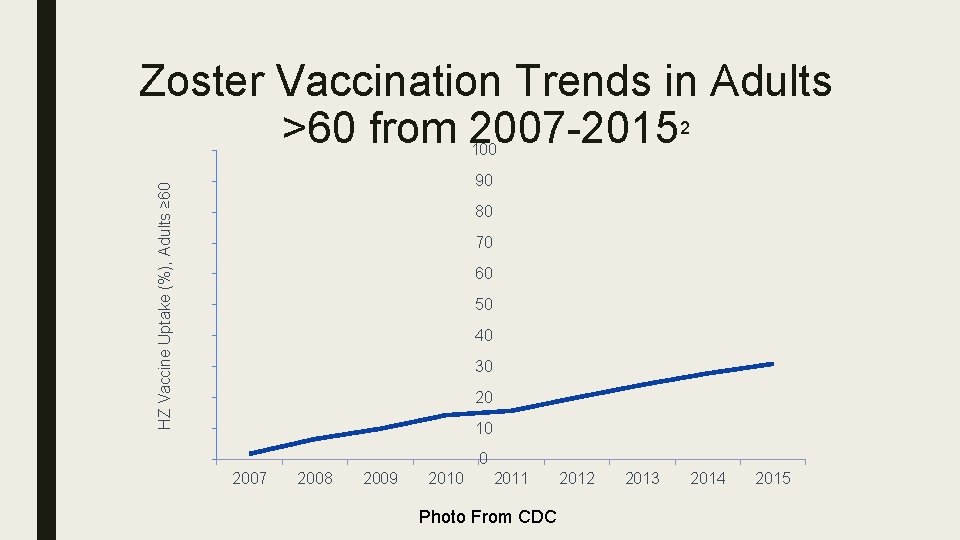
Zoster Vaccination Trends in Adults >60 from 2007 -20152 100 HZ Vaccine Uptake (%), Adults ≥ 60 90 80 70 60 50 40 30 20 10 0 2007 2008 2009 2010 2011 Photo From CDC 2012 2013 2014 2015

Shingrix™ (Highlights)1, 2, 3 ■ Licensed by the FDA on Oct 20, 2017 ■ An adjuvanted recombinant protein subunit vaccine – 2 components ■ ■ ■ Adjuvant ASO 1 B Glycoprotein E Phase III clinical trials contained more than 29, 000 people – >17, 000 people have received at least 1 dose of HZ/su in safety studies ■ Refrigerator stable, requires reconstitution prior to injection (Do Not Shake) – Administer within 6 hours of reconstitution ■ 2 doses, – ■ IM at 0 and 2 -6 months (DNE: 6 mo) Limitation: Not for the prevention of Chicken Pox

SAFETY FOR SHINGRIX™

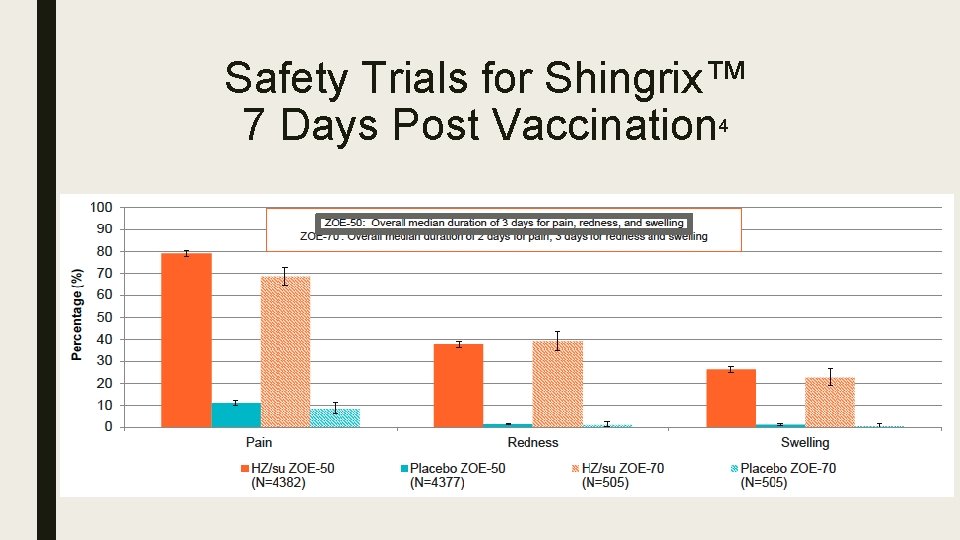
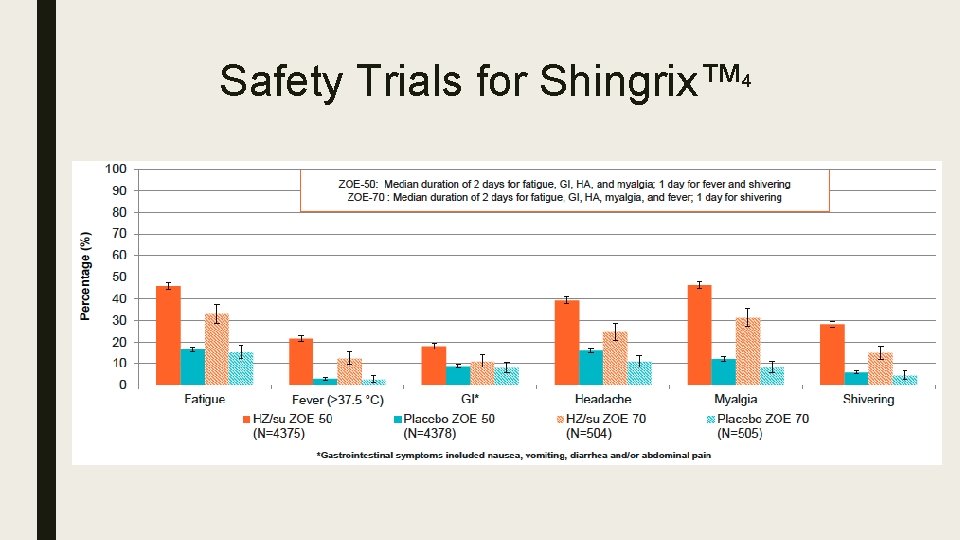
Safety Trials for Shingrix™ 4 ZOE-50 ZOE-70 ■ June 2015 ■ October 2016 ■ Participants – Active: 7, 695 – Placebo: 7, 710 ■ Participants – Active: 6, 950 – Placebo: 6, 950

Safety Trials for Shingrix™ 7 Days Post Vaccination 4

Safety Trials for Shingrix™ 4

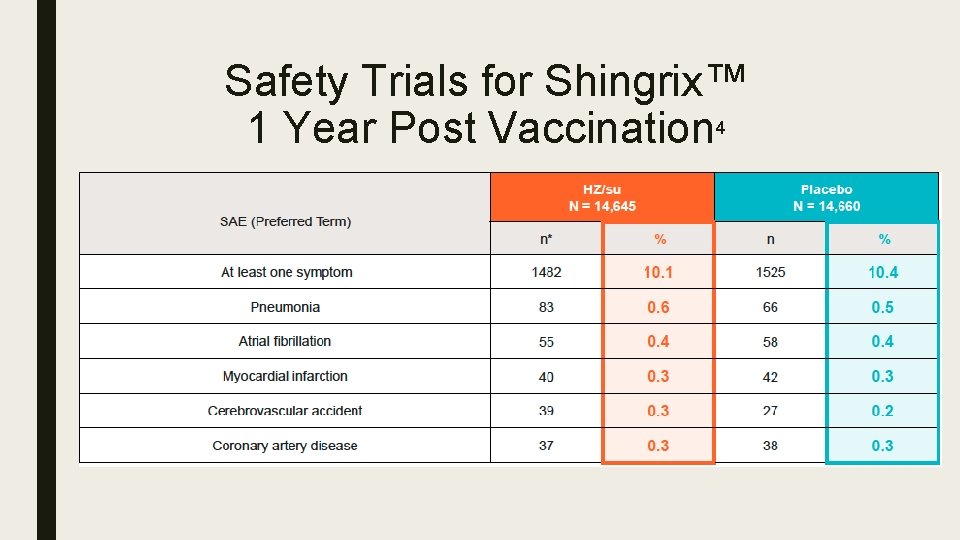
Safety Trials for Shingrix™ 1 Year Post Vaccination 4

EFFECTIVENESS OF SHINGRIX™

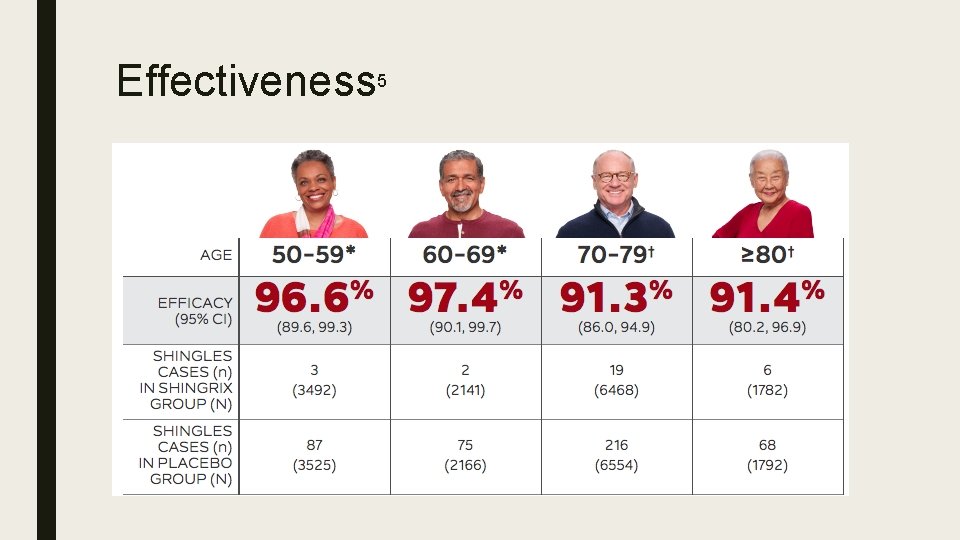
Effectiveness 5

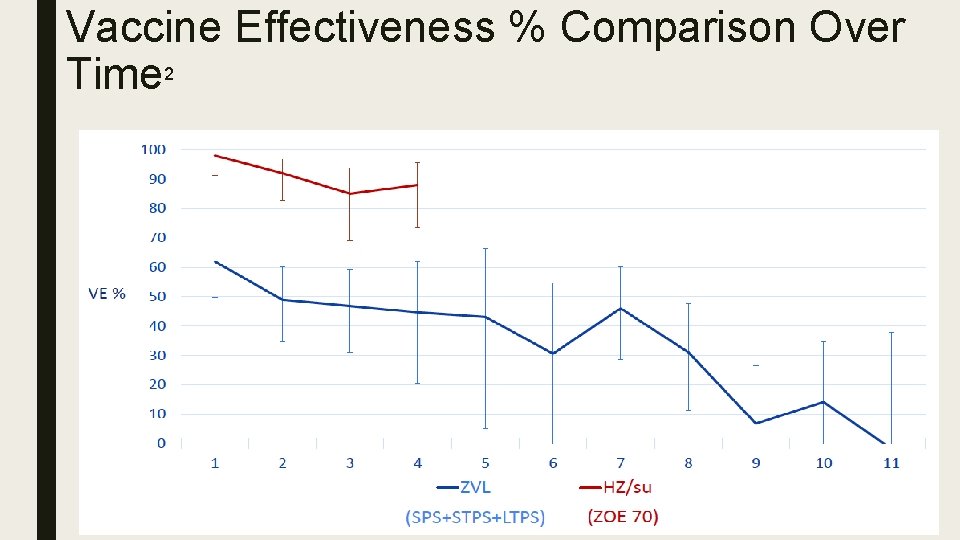
Vaccine Effectiveness % Comparison Over Time 2

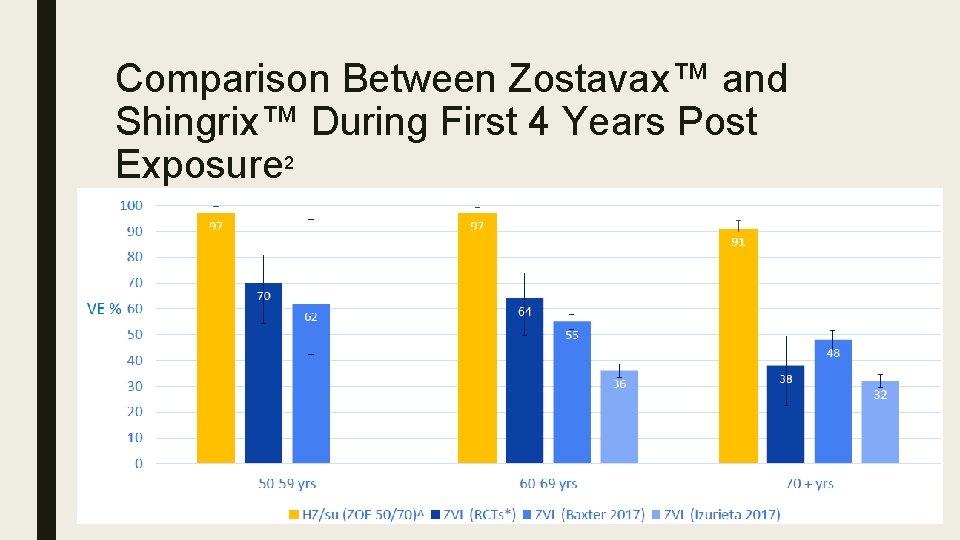
Comparison Between Zostavax™ and Shingrix™ During First 4 Years Post Exposure 2

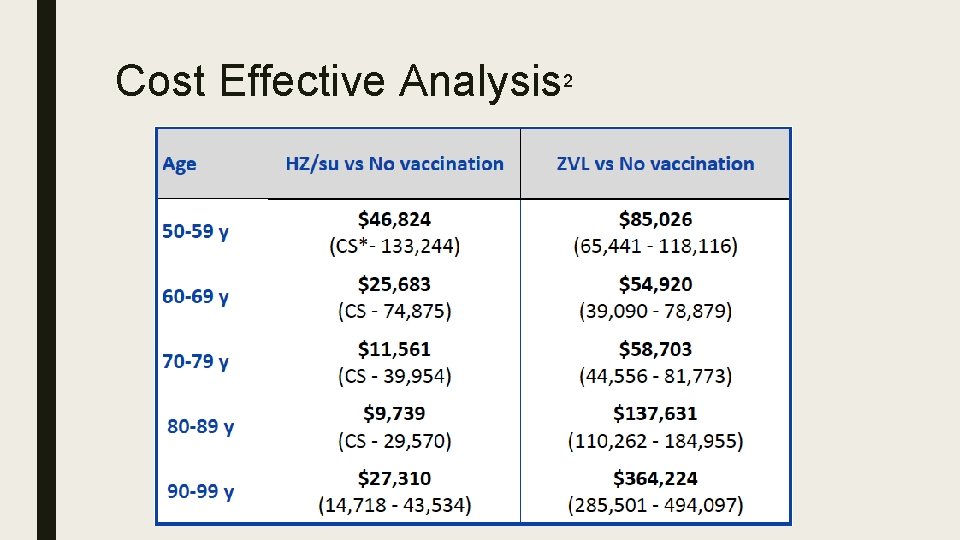
Cost Effective Analysis 2

UPDATES TO VACCINATION SCHEDULES Written and Developed by: Steven Garhartt Pharm. D/MBA Candidate 2018

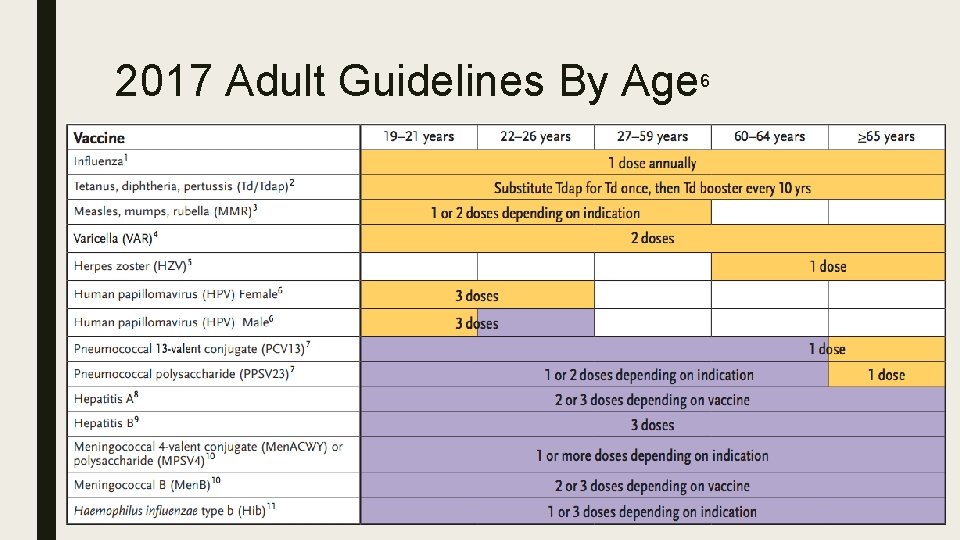
2017 Adult Guidelines By Age 6

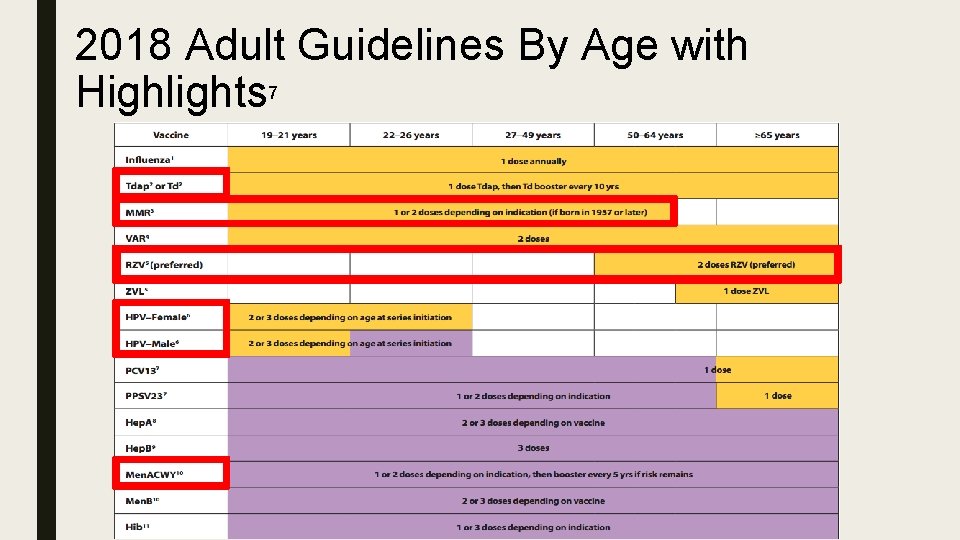
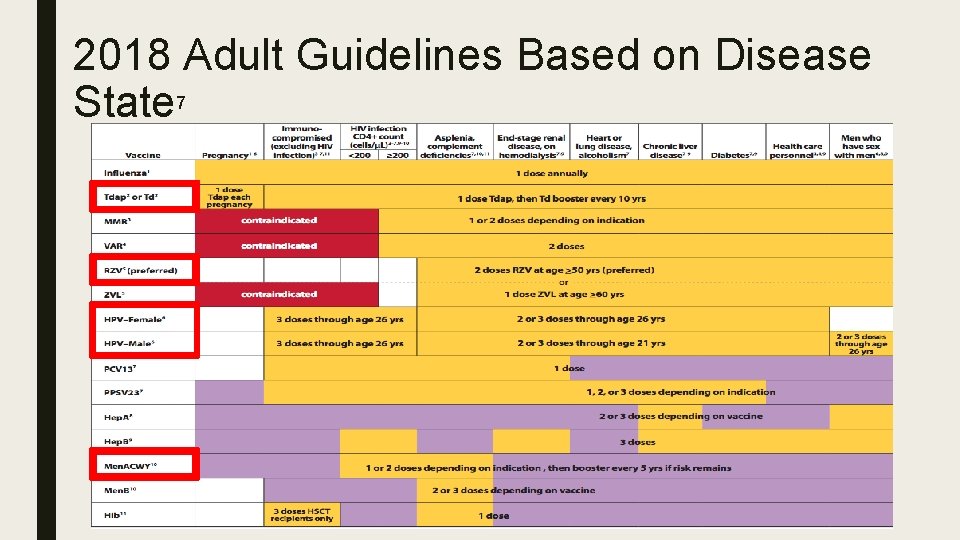
2018 Adult Guidelines By Age with Highlights 7

Summary of Changes 7 ■ Tdap or Td – Grammatical Change; Confusion on the administration of the correct vaccine ■ MMR – “Administer 1 dose of MMR to adults who previously received ≤ 2 doses of mumpscontaining vaccine and are identified by a public health authority to be at increased risk during a mumps outbreak. ” Added 1957 or later ■ Zoster – RZV Vs ZVL – New recommendation for RZV vaccine ■ HPV – Changed from 3 doses, to 2 -3 based on age ■ Men. ACWY – MPSV 4 is no longer available, therefore it was removed from the guideline

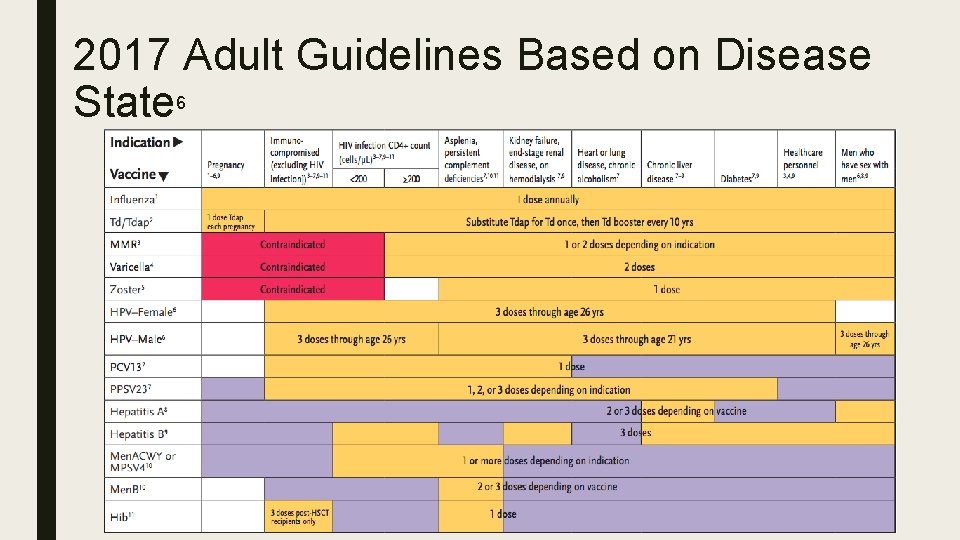
2017 Adult Guidelines Based on Disease State 6

2018 Adult Guidelines Based on Disease State 7

FURTHER DETAIL COURTESY OF THE CDC COMPARISON BETWEEN 2017 AND 20188





QUESTIONS? ? ? Steven Garhartt Pharm. D/MBA Candidate 2018

References 1. Cdc. gov. (2018). Shingles | Clinical Overview - Varicella Vaccine | Herpes Zoster | CDC. [online] Available at: https: //www. cdc. gov/shingles/hcp/clinical-overview. html [Accessed 21 Mar. 2018]. 2. Dooling, K. (2017). Considerations for the use of herpes zoster vaccines. [online] CDC. Available at: https: //www 2. cdc. gov/vaccines/ed/ciinc/archives/17/downloads/11_8_17/Zoster_CIINC_Dooling_20171106. pdf [Accessed 21 Mar. 2018]. 3. Fda. gov. (2017). Shingrix Package Insert. [online] Available at: https: //www. fda. gov/downloads/Biologics. Blood. Vaccines/Approved. Products/UCM 581605. pdf [Accessed 21 Mar. 2018]. 4. Cdc. gov. (2017). Safety Summary of Investigational Vaccine: SHINGRIX* (HZ/su). [online] Available at: https: //www. cdc. gov/vaccines/acip/meetings/downloads/slides-2017 -02/zoster-02 -gsk. pdf [Accessed 21 Mar. 2018]. 5. Gsksource. com. (2018). Efficacy | SHINGRIX | GSKSource. [online] Available at: https: //www. gsksource. com/pharma/content/gsk/source/us/en/brands/shingrix/pi/efficacy. html [Accessed 21 Mar. 2018]. 6. Immunize. org. (2017). Recommended Adult Immunization Schedule – United States, 2017. [online] Available at: http: //www. immunize. org/shop/views/adultsched_pg 2. pdf [Accessed 21 Mar. 2018]. 7. Cdc. gov. (2018). Recommended Immunization Schedule for Adults Aged 19 Years or Older, United States, 2018. [online] Available at: https: //www. cdc. gov/vaccines/schedules/downloads/adult-combined-schedule. pdf [Accessed 21 Mar. 2018]. 8. Cdc. gov. (2017). Updates – 2018 Adult Immunization Schedule. [online] Available at: https: //www. cdc. gov/vaccines/acip/meetings/downloads/slides-2017 -10/adult-immunization-02 -kim. pdf [Accessed 21 Mar. 2018].