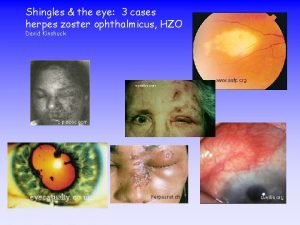
Herpes Zoster and the Zoster Eye Disease Study





















- Slides: 39

Herpes Zoster and the Zoster Eye Disease Study (ZEDS) Elisabeth J Cohen, MD Professor of Ophthalmology New York University So. M NYU Langone Health

Financial Disclosures I have no financial disclosures or conflicts of interest.

Why Should We Care About Shingles? • Herpes Zoster (HZ)/shingles is a common, serious, and preventable disease caused by reactivation of varicella zoster virus (VZV) in persons with a history of chicken pox • Shingles causes • Severe acute pain for average 30 days • Severe, debilitating chronic pain unresponsive to treatment • Chronic and/or recurrent unilateral eye disease and vision loss • Unilateral hearing loss • Fatal strokes and other serious neurological diseases • Risk of stroke 2 x after HZO than HZ elsewhere • Probably temporal/giant cell arteritis and heart attacks • It can ruin your life or kill you

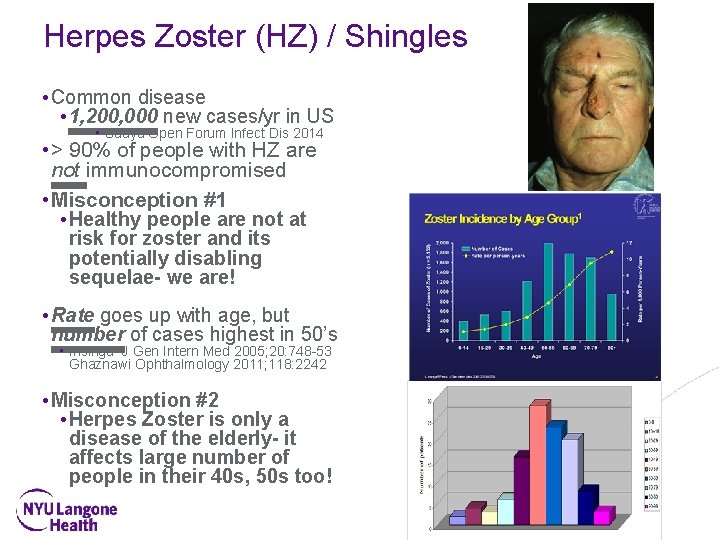
Herpes Zoster (HZ) / Shingles • Common disease • 1, 200, 000 new cases/yr in US • Suaya Open Forum Infect Dis 2014 • > 90% of people with HZ are not immunocompromised • Misconception #1 • Healthy people are not at risk for zoster and its potentially disabling sequelae- we are! • Rate goes up with age, but number of cases highest in 50’s • Insinga J Gen Intern Med 2005; 20: 748 -53 Ghaznawi Ophthalmology 2011; 118: 2242 • Misconception #2 • Herpes Zoster is only a disease of the elderly- it affects large number of people in their 40 s, 50 s too!

Role of Varicella Vaccination in Zoster Increase • Many think varicella/chicken pox vaccination cause of increase in zoster But rise in zoster began before, and rate of increase has not increased • Kawai Clin Infect Dis 2016; 63: 221 -6; Harpaz JID 2018: 218 (Suppl 2) S 57 -61. Wolfson Clin Infect Dis 2019 Apr 24; Harpaz Expert Rev Vaccines 2019 Jul 18 epub • Routine varicella vaccination not widespread in Europe (ex France, UK!) • Rates of HZ similar in these countries without varicella vaccination • Cause of increasing incidence of zoster is unknown!

Two Vaccines against Herpes Zoster • First: Zostavax contains live attenuated virus • Recommended by CDC since 2008 for immunocompetent age 60+ • In Ontario, publicly funded since 2016 or age 65 -70 only • New and better: Shingrix contains viral protein in novel adjuvant • The bottom line: Adults age 50 years and older should get Shingrix ASAP!

New Shingrix Vaccine Recombinant Zoster Vaccine (RZV) • Contains a viral protein in a new immunogenic solution • 2 injections 2 months apart in immunocompetent adults age 50+ • 90 -97% efficacy for all age groups • Acute local and systemic symptoms in ~15% • Efficacy persists: 85% at year 4 • FDA approved Shingrix 2017 age 50+ • CDC recommended 2018 for immunocompetent 50+ Presentation Title Goes Here 7

FDA Label for Shingrix • Indicated for prevention HZ in adults age 50+ • Not just immunocompetent adults! • Immunosuppressive treatment may reduce effectiveness • 2 shots given 2 to 6 months apart • Can give at same time as flu vaccine • Only contraindication: severe allergic reaction to vaccine • Acute adverse reactions in ~ 10% • Less common age 70+ than age 50 -69 years

CDC Recommendations • Shingrix recommended for immunocompetent adults age 50 years+ • Recommended if previously received Zostavax • Shingrix should be given to people with a past history of zoster • Shingrix is preferred over Zostavax • Dooling, Guo, Pater, Lee, Moore, Belongia, Harpaz. MMWR Jan 26, 2018; 67: 103 -108

Canada’s National Advisory Co on Immunization (NACI) Statement (Can Commun Dis Rep. 2018; 44: 220 -5) • RZV should be offered to populations/individuals 50+ yrs without contraindications (strong recommendation) • Including previously vaccinated ZVL (strong) • One year after ZVL (discretionary recommendation) • Including previous episode of HZ (strong) • One year after HZ (discretionary) • RZV may be considered for immunocompromised age 50+ case by case (discretionary) • ZVL may be considered for immunocompetent 50+ yrs when RZV contraindicated, unavailable, or inaccessible (discretionary) • Both cost-effective age 50+ vs no vaccine, RZV more cost effective

Evectiveness and Cost-effectiveness of Vaccination against Herpes Zoster in Canada (Drolet M CMAJ 2019 Aug 26 epub) • Purpose to compare ZVL and RZV. • Outcome Incremental Cost per Quality-Adjusted Life-Year (QALY) • Results • Number needed to vaccinate to prevent 1 case HZ or PHN: • ZVL > RZV at all ages, difference increases with age • Cost-effectivenesss for RZV: cost saving ~$26, 000 • Cut-off used of $45, 000/QALY in Canada • Interpretation: RZV cost-effective age 60 years and older • And likely more cost-effective than ZVL, even though RZV cost higher • Age 50 s: cost/QALY< $45, 000 if cost RZV $100, <$100, 000 cost 180 • Age 60: cost/QALY~$25, 000 Age 65 -70: most cost-effective • Current status vaccination against HZ in Canada • Quebec: RZV age 65+ recommended • Ontario: ZVL covered age 65 -70 • British Columbia: Vaccination recommended age 50+, no public funds • EJC OPINION: NEED TO DO BETTER!

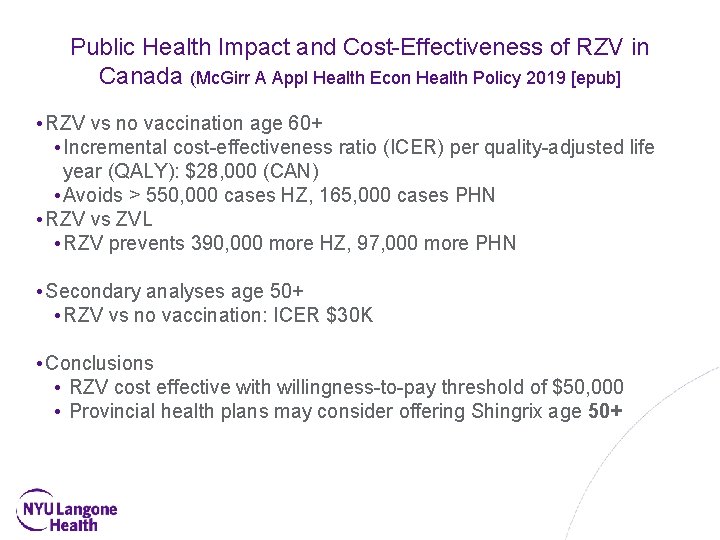
Public Health Impact and Cost-Effectiveness of RZV in Canada (Mc. Girr A Appl Health Econ Health Policy 2019 [epub] • RZV vs no vaccination age 60+ • Incremental cost-effectiveness ratio (ICER) per quality-adjusted life year (QALY): $28, 000 (CAN) • Avoids > 550, 000 cases HZ, 165, 000 cases PHN • RZV vs ZVL • RZV prevents 390, 000 more HZ, 97, 000 more PHN • Secondary analyses age 50+ • RZV vs no vaccination: ICER $30 K • Conclusions • RZV cost effective with willingness-to-pay threshold of $50, 000 • Provincial health plans may consider offering Shingrix age 50+

When Zoster Hits Close to Home • Anecdote 2017: 58 year old friend Last year I was told by a doctor friend to get a shingles shot. My thought was that’s for old people- boy was I wrong!! Six months later I had a pain in my neck that kept me up at night and 2 days later I had a rash from the top of my back around my neck to my chest. I knew it was SHINGLES. I was so mad at myself for not getting the vaccine. It’s been 4 months and it is still painful and it feels like it is going to stay with me forever. I tell everyone I meet, please go to your doctor and get the vaccine as soon as you can. I have reached out to friends around the world, and will keep telling everyone this is nothing to fool with! • Evidence • Impact of personal zoster awareness on zoster vaccine uptake in the US • Harpaz R Vaccine 2017; 35: 3457 -60 • Vaccine uptake marked increased immediately after zoster in a spouse • Relative incidence vaccination 7 x during month post vs 6 months pre • Relative incidence 14 x when spouse severe zoster requiring opiates • No increase in pneumonia vaccination during month post HZ

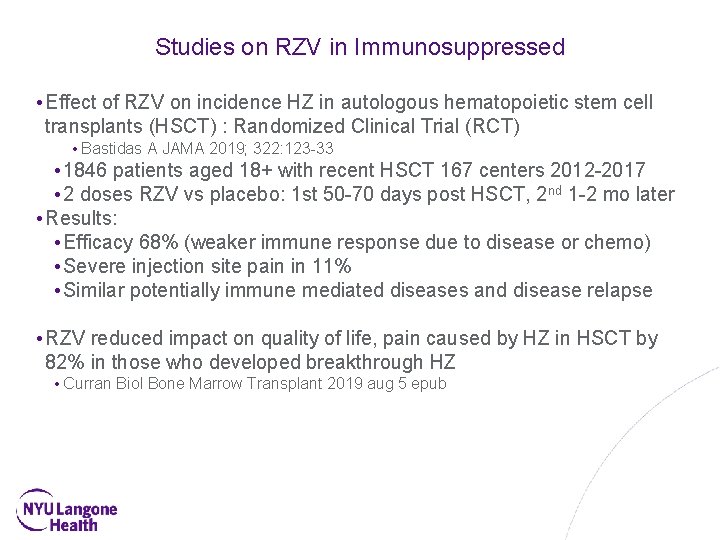
Studies on RZV in Immunosuppressed • Effect of RZV on incidence HZ in autologous hematopoietic stem cell transplants (HSCT) : Randomized Clinical Trial (RCT) • Bastidas A JAMA 2019; 322: 123 -33 • 1846 patients aged 18+ with recent HSCT 167 centers 2012 -2017 • 2 doses RZV vs placebo: 1 st 50 -70 days post HSCT, 2 nd 1 -2 mo later • Results: • Efficacy 68% (weaker immune response due to disease or chemo) • Severe injection site pain in 11% • Similar potentially immune mediated diseases and disease relapse • RZV reduced impact on quality of life, pain caused by HZ in HSCT by 82% in those who developed breakthrough HZ • Curran Biol Bone Marrow Transplant 2019 aug 5 epub

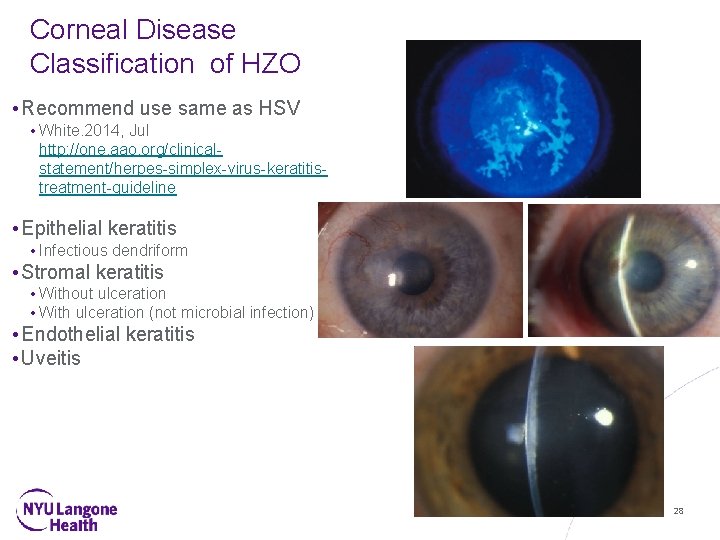
Herpes Zoster Ophthalmicus (HZO) • 10 -20% of HZ cases involve Vth trigeminal cranial nerve • HZO refers to zoster affecting trigeminal nerve and the eye • Can damage all parts of the eye and surrounding tissues • Anterior segment disease • Dendriform epithelial keratitis (pseudodendrites) • Stromal keratitis • Endothelial keratitis (disciform) • Uveitis • Neurotrophic keratopathy Presentation Title Goes Here 15

Development of American Academy of Ophthalmology (AAO) Preferred Practice Patterns • Quality of studies • I: Meta-analyses, Randomized Controlled Trials (RCTs) • II: Case-control or cohort studies • III: Case reports, case series • Quality of recommendations • Good: further research unlikely to change estimate of effect • Moderate: further research likely to impact estimate of effect • Insufficient: estimate of effect is very uncertain • Key recommendations • Strong: benefits clearly outweigh risks or do not • Discretionary: when trade-offs less certain • Based on evidence without mention of expert opinion!

Current Role of Antiviral Treatment for HZO • Acute antiviral treatment of herpes zoster is FDA approved and CDC recommended based on RCTs for 7 -10 day course within 72 hours of onset of rash with • Acyclovir 800 mg 5 x a day • Valacyclovir 1000 mg 3 x a day • Famciclovir 500 mg 3 x a day

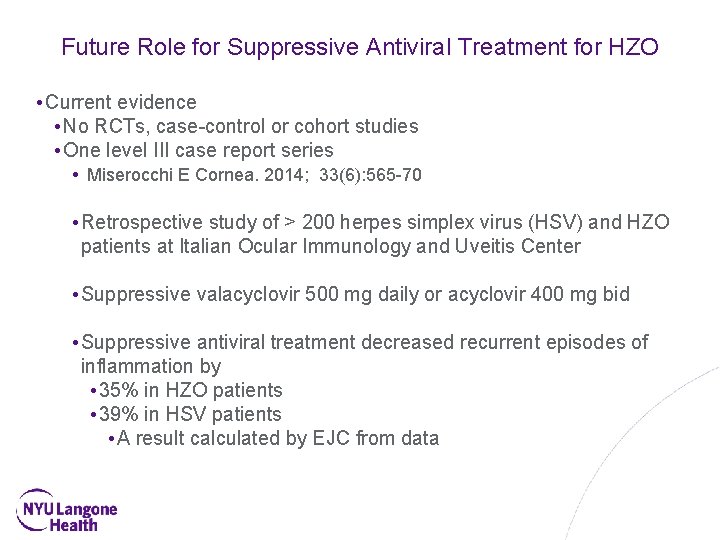
Future Role for Suppressive Antiviral Treatment for HZO • Current evidence • No RCTs, case-control or cohort studies • One level III case report series • Miserocchi E Cornea. 2014; 33(6): 565 -70 • Retrospective study of > 200 herpes simplex virus (HSV) and HZO patients at Italian Ocular Immunology and Uveitis Center • Suppressive valacyclovir 500 mg daily or acyclovir 400 mg bid • Suppressive antiviral treatment decreased recurrent episodes of inflammation by • 35% in HZO patients • 39% in HSV patients • A result calculated by EJC from data

Possible New Treatment for HZO • The Zoster Eye Disease Study (ZEDS) funded by NEI in 2016 to conduct a multicenter, Randomized, placebo controlled Clinical Trial (RCT) to determine whether prolonged, suppressive valacyclovir treatment reduces complications of Herpes Zoster Ophthalmicus (HZO), including eye disease and/or postherpetic neuralgia Presentation Title Goes Here 19

ZEDS Study Structure • > 60 Participating Clinical Centers (PCCs) in USA • 2019 adding centers in USA, Canada, Brazil and UK and satellites • Coordinating Center (CC) at NYU Langone Health • CC project manager: Mee. Lee Tom • Zeds. cta@nyulangone. org • Study Chair: Elisabeth Cohen, Co-Chair Bennie Jeng, U MD • Multiple PIs: Judith Hochman, Andrea Troxel CC NYUSo. M, NYULH • Executive Co: Stephen Mc. Loed, Todd Margolis, James Chodosh, Chris Rapuano, Alice Matoba, Anat Galor, Shahzad Mian • Chair Clinical Event Review Co: Kathryn Colby Presentation Title Goes Here 20

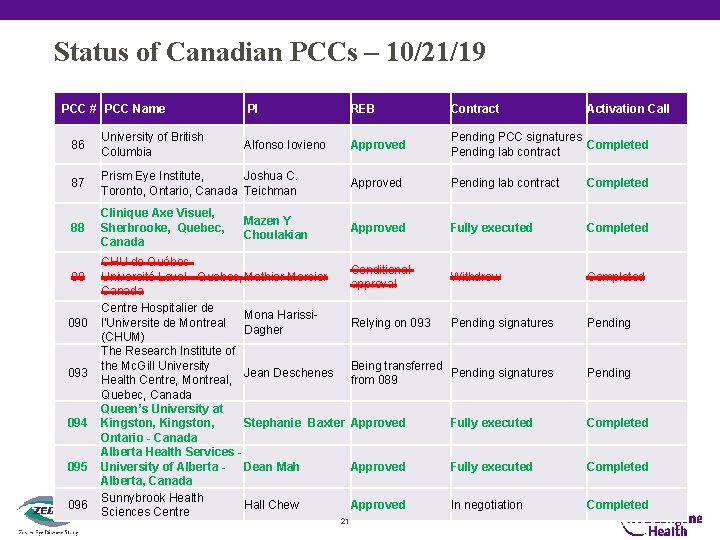
Status of Canadian PCCs – 10/21/19 PCC # PCC Name PI REB Contract Activation Call Alfonso Iovieno Approved Pending PCC signatures Completed Pending lab contract 86 University of British Columbia 87 Prism Eye Institute, Joshua C. Toronto, Ontario, Canada Teichman Approved Pending lab contract Completed 88 Clinique Axe Visuel, Sherbrooke, Quebec, Canada Approved Fully executed Completed Conditional approval Withdrew Completed Relying on 093 Pending signatures Pending Being transferred Pending signatures from 089 Pending Approved Fully executed Completed Approved In negotiation Completed 89 090 093 094 095 096 Mazen Y Choulakian CHU de Québec. Université Laval - Quebec, Mathier Mercier Canada Centre Hospitalier de Mona Harissil'Universite de Montreal Dagher (CHUM) The Research Institute of the Mc. Gill University Jean Deschenes Health Centre, Montreal, Quebec, Canada Queen’s University at Kingston, Stephanie Baxter Ontario - Canada Alberta Health Services University of Alberta - Dean Mah Alberta, Canada Sunnybrook Health Hall Chew Sciences Centre 21

Background and Rationale of ZEDS • Acute high dose oral antiviral treatment is recommended for Herpes Zoster Ophthalmicus (HZO), but there is no standard approach to antiviral treatment for ocular complications of HZO. • Rationale of the Zoster Eye Disease Study (ZEDS) • First: • Relatively recent knowledge of infectious pathogenesis of complications of Herpes Zoster and HZO • Second: • Significant benefit of suppressive antiviral treatment in reducing recurrent Herpes Simplex Virus (HSV) eye disease • HZO and HSV keratitis, caused by different herpes viruses, are analogous in many ways 22

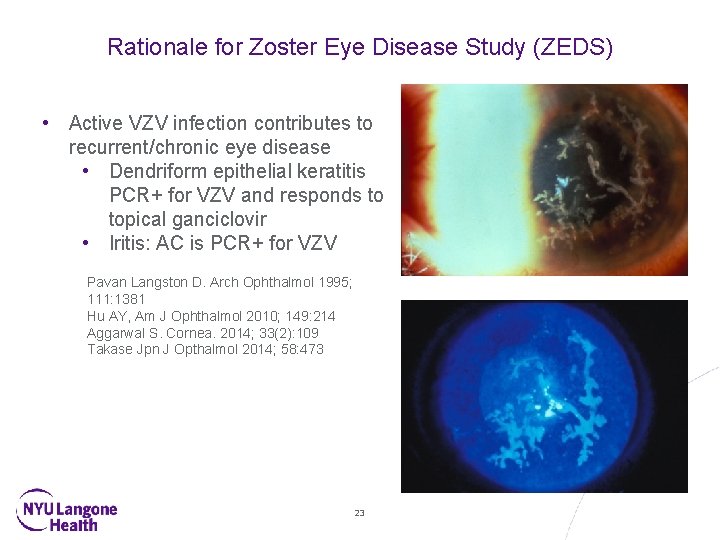
Rationale for Zoster Eye Disease Study (ZEDS) • Active VZV infection contributes to recurrent/chronic eye disease • Dendriform epithelial keratitis PCR+ for VZV and responds to topical ganciclovir • Iritis: AC is PCR+ for VZV Pavan Langston D. Arch Ophthalmol 1995; 111: 1381 Hu AY, Am J Ophthalmol 2010; 149: 214 Aggarwal S. Cornea. 2014; 33(2): 109 Takase Jpn J Opthalmol 2014; 58: 473 23

Rationale of Zoster Eye Disease Study • Herpetic Eye Disease Study (HEDS) Acyclovir Prevention Trial (APT) • • HEDS Study Group. N Eng J Med 1998; 339: 300 -306 HEDS Study Group. Arch Ophthalmol 2000; 118: 1030 -36. • Long-term suppressive treatment with oral acyclovir resulted in 45% reduction in recurrent Herpes Simplex Virus disease at 1 yr • Suppressive antiviral treatment is now standard of care for HSV keratitis, and has improved outcomes • ZEDS trial analogous to the HEDS APT study for ocular disease caused by varicella zoster virus (VZV) • Valacyclovir, prodrug of acyclovir, has higher plasma concentration than acyclovir • Similar trial design: RCT of 1 yr of suppressive valacyclovir vs. placebo with follow-up every 3 months for 18 months 24

Overview of Study Design • Immunocompetent HZO patients, 18 years and older • History of typical unilateral vesicular rash in V 1 distribution • Episode in medical record within the year prior to enrollment of • Dendriform epithelial keratitis • Stromal keratitis • Endothelial keratitis • Iritis • Keratitis and iritis can be called by wide variety of names: pseudodendrites, keratouveitis, disciform keratitis, uveitis… • Randomized 1: 1 ratio to double masked valacyclovir 1000 mg or placebo daily for 1 yr • Randomized in 4 strata • Age of onset HZO: < 60 years vs 60 years or more • Time since onset HZO < 6 months vs 6 months or more • Enroll 1050 study participants over 3 years 25

Exclusion Criteria • Immunocompromise by CDC criteria for impaired cellular immunity due to disease or treatment • Refractive surgery, other than at time of cataract surgery, within 5 years, corneal transplant in eye with HZO • Renal insufficiency with e. GFR) < 45 • Pregnant, nursing, or do not agree to use contraception for one year • Requires valacyclovir, or famciclovir, for genital/eye HSV • Vaccination against zoster within 1 month of enrollment (just delay) • Incarceration, unable to give informed consent, comply with protocol, or complete 18 months of follow-up 26

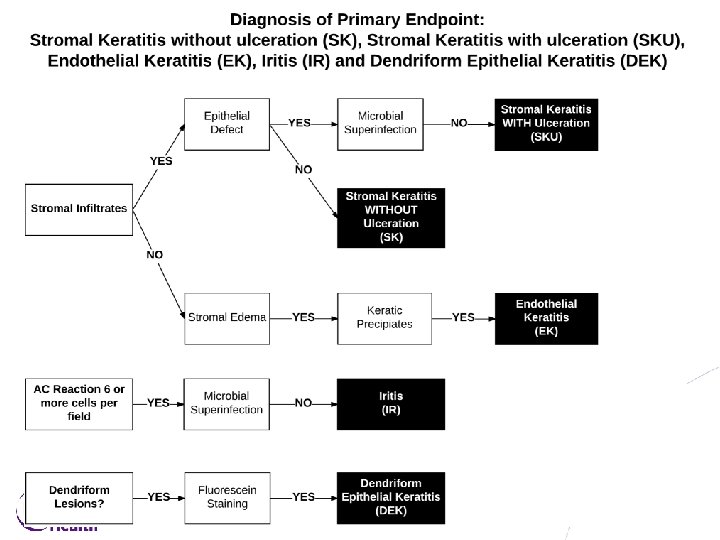
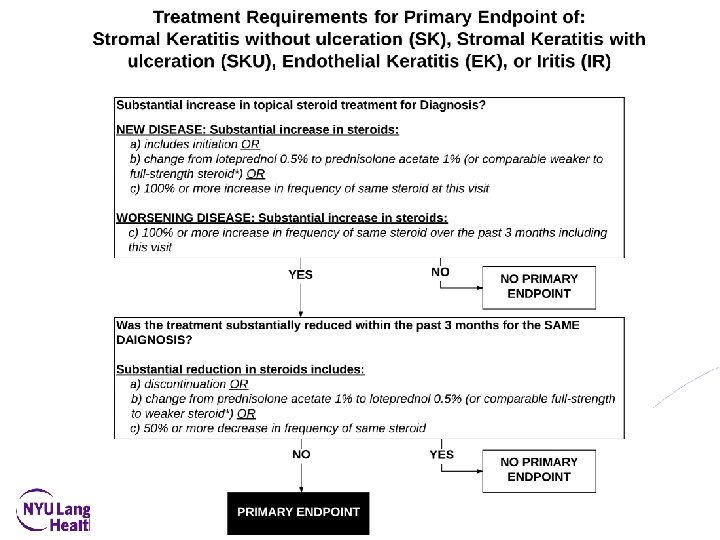
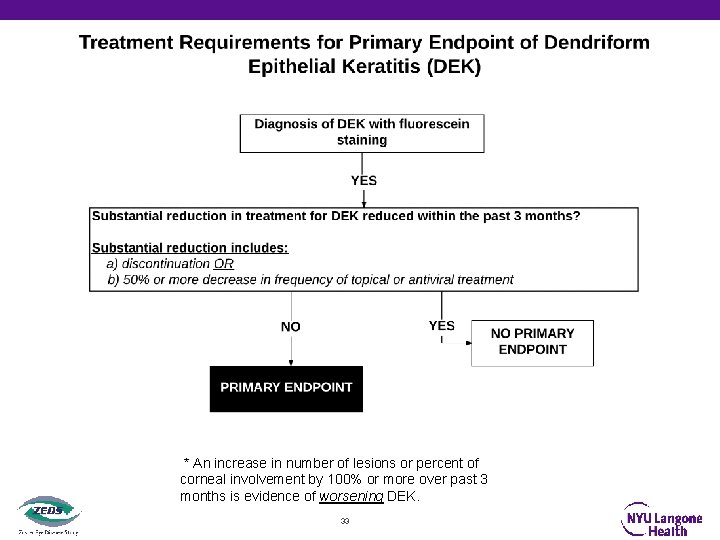
Primary Objective and Endpoint • To evaluate whether or not suppressive valacyclovir treatment compared with placebo will delay time to first occurrence by 12 months of new or worsening • • • Dendriform epithelial keratitis (DEK) Stromal keratitis without ulceration(SK) Endothelial keratitis (EK) Iritis (IR) OR Stromal keratitis with ulceration (SKU) • With pre-specified treatment requirements to be a primary endpoint 27

Corneal Disease Classification of HZO • Recommend use same as HSV • White. 2014, Jul http: //one. aao. org/clinicalstatement/herpes-simplex-virus-keratitistreatment-guideline • Epithelial keratitis • Infectious dendriform • Stromal keratitis • Without ulceration • With ulceration (not microbial infection) • Endothelial keratitis • Uveitis Presentation Title Goes Here 28

Definitions Endpoints of Stromal Keratitis With or Without Ulceration, Endothelial Keratitis, Iritis and Dendriform Epithelial Keratitis • Stromal Keratitis without ulceration: Localized or diffuse stromal infiltrates with or without keratic precipitates, corneal edema, or corneal neovascularization, and is without an epithelial defect by fluorescein staining. • Endothelial Keratitis: Disciform (localized) or diffuse corneal stromal, and usually epithelial edema, with keratic precipitates out of proportion to anterior chamber reaction (none or trace: 5 cells or less per field). • Iritis: Anterior chamber reaction graded ≥ 1+ (6 or more cells/field using 1 x 1 mm slit beam) in the absence of suspected or proven microbial keratitis. • Stromal Keratitis with ulceration: Stromal infiltrates with an overlying epithelial defect by fluorescein staining due to active stromal keratitis and not neurotrophic keratopathy or suspected or proven microbial superinfection. Microbial superinfection should be ruled out by negative corneal smears and cultures and/or lack of response to intensive antibiotic treatment every hour. • Dendriform Epithelial Keratitis: Linear or elongated, often branching lesion(s) on the corneal surface that stain at least minimally with fluorescein. They are usually elevated, non-ulcerated, and without terminal bulbs. 29

30

31

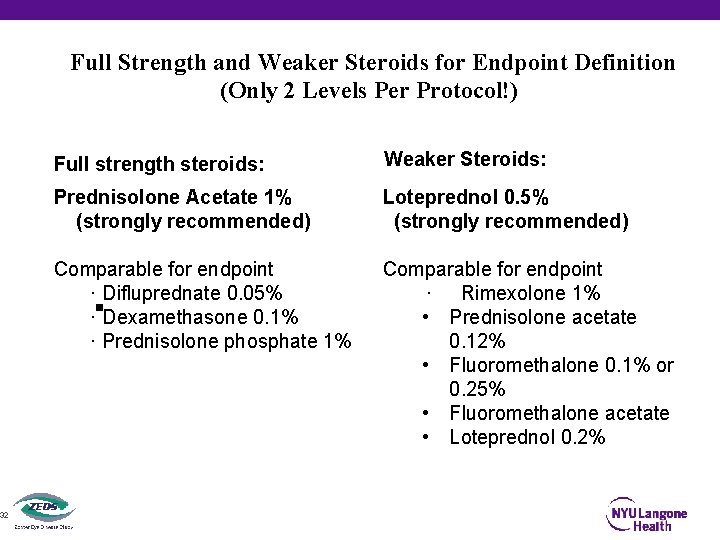
32 Full Strength and Weaker Steroids for Endpoint Definition (Only 2 Levels Per Protocol!) Full strength steroids: Weaker Steroids: Prednisolone Acetate 1% (strongly recommended) Loteprednol 0. 5% (strongly recommended) Comparable for endpoint · Difluprednate 0. 05% · Dexamethasone 0. 1% · Prednisolone phosphate 1% Comparable for endpoint · Rimexolone 1% • Prednisolone acetate 0. 12% • Fluoromethalone 0. 1% or 0. 25% • Fluoromethalone acetate • Loteprednol 0. 2%

* An increase in number of lesions or percent of corneal involvement by 100% or more over past 3 months is evidence of worsening DEK. 33

Secondary Study Objectives and Endpoints • To evaluate whether or not the effect of treatment on primary endpoint persists for 6 months after treatment by comparing rates of new or worsening DEK, SK, EK, IR or SKU by 18 months follow-up in participants randomized to valacyclovir or placebo • To test hypothesis that suppressive valacyclovir treatment reduces the incidence, severity, duration of postherpetic neuralgia (PHN) compared to placebo at 12 and 18 months 34

Other Secondary Endpoints • Development of specific disease manifestations of HZO • Primary endpoints: DEK, SK, EK, IR and SKU • Neurotrophic keratopathy with and without melting, perforation, presumed or proven microbial superinfection • Episcleritis, Scleritis • Effect of past history of specific manifestations on their recurrence • Glaucoma: by IOP (22 mm Hg+ and 6 mm. Hg+ rise) and medical/surgical treatment required • Impact of vaccination against zoster on HZO endpoints by treatment group (valacyclovir vs. placebo) 35

Study Visits and Treatment • Prescreening review of medical records including ED and discussion with potential study participant by investigator, coordinator • Screening Study Visit 1 • Consent obtained, then e. GFR and pregnancy tests ordered • Enrollment/randomization Study Visit 2 (within 30 days of tests) • Study medication consisting of 3 mos supply of masked valacyclovir and placebo pills dispensed, to be taken 2 pills once daily • Valacyclovir 500 mg used because 1000 mg too big for encapsulation (and option for bid and daily dosing if side effects) • Follow up visits every 3 months for 18 months • 12 month study visit: study medication discontinued • Open label antiviral treatment is allowed after new or worsening DEK, SK, EK, IR, or SKU is diagnosed Presentation Title Goes Here 36

Case Report Forms for Electronic Data Capture 37

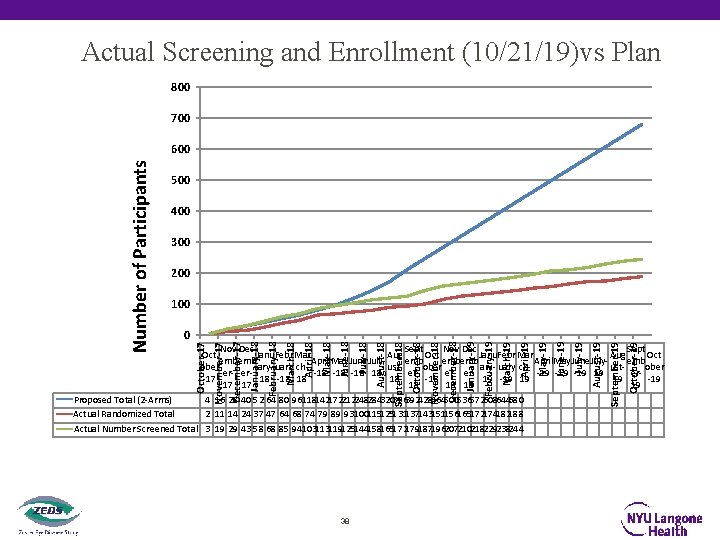
Actual Screening and Enrollment (10/21/19)vs Plan 800 700 500 400 300 200 October-19 September-19 August-19 July-19 June-19 May-19 April-19 March-19 February-19 January-19 December-18 November-18 October-18 September-18 August-18 July-18 June-18 May-18 April-18 March-18 February-18 January-18 December-17 0 November-17 100 October-17 Number of Participants 600 Nov Dec Sept Oct Janu. Febr Mar Aug Oct April. May. June Julyemb emb emb ober ary- uary chustober er-18 -18 18 erer- er-19 -19 19 er-17 18 -18 18 18 -18 19 -19 19 19 -19 17 17 18 18 18 19 Proposed Total (2 -Arms) 4 16 28 40 52 64 80 96118142172212248284320356392428464500536572608644680 Actual Randomized Total 2 11 14 24 37 47 64 68 74 79 89 93100115125131137143151156165172174182188 Actual Number Screened Total 3 19 29 43 58 68 85 94103113119125144158165172179187196207210218229238244 38

Thank You for Supporting the Zoster Eye Disease Study! • Please refer all your HZO patients to the ZEDS Participating Clinical Center at University of British Columbia for evaluation for possible current or future eligibility for enrollment so we can determine new evidence based standard of care to reduce complications of HZO! • PI: Alfonso Iovieno • Co-I: Sonia Yeung • Coordinator: Aleksandra Kuzmanovic • Please contact us if you have any questions • Elisabeth. cohen@nyulangone. org • ZEDS. cta@nyulangone. org • http: //med. nyu. edu/research/zoster-eye-disease-study/
 Zoster eye disease study
Zoster eye disease study Zoster eye disease study
Zoster eye disease study Herpes zoster clasificacion
Herpes zoster clasificacion Que es diagnostico diferencial
Que es diagnostico diferencial How to mix shingrix vaccine
How to mix shingrix vaccine Dendritic keratitis
Dendritic keratitis Valaciclovir dosis
Valaciclovir dosis Herpes zoster infection
Herpes zoster infection Staphylococcus
Staphylococcus Communicable disease and non communicable disease
Communicable disease and non communicable disease Examples of bird's eye view
Examples of bird's eye view Thyroid eye disease
Thyroid eye disease Thyroid eye disease
Thyroid eye disease Polyarteritis nodosa symptoms
Polyarteritis nodosa symptoms Disease
Disease Mikrobial
Mikrobial Enroth sign
Enroth sign Hammurabi mesopotamia
Hammurabi mesopotamia Hammurabi code eye for an eye
Hammurabi code eye for an eye Dr anees nephrologist
Dr anees nephrologist Code of hammurabi activity
Code of hammurabi activity An eye for an eye a tooth for a tooth sister act
An eye for an eye a tooth for a tooth sister act Explain
Explain An eye for an eye meaning
An eye for an eye meaning Behold he is coming
Behold he is coming Herpes og graviditet
Herpes og graviditet Historia natural de la enfermedad del herpes genital
Historia natural de la enfermedad del herpes genital Herpes simpleksserotipe 2
Herpes simpleksserotipe 2 Herpes simpleksserotipe 2
Herpes simpleksserotipe 2 Herpes simpleksserotipe 2
Herpes simpleksserotipe 2 Corrimento amarelo na gravidez
Corrimento amarelo na gravidez Site:slidetodoc.com
Site:slidetodoc.com Genial herpes
Genial herpes Herpes rugbiorum
Herpes rugbiorum Herpes simplex virus
Herpes simplex virus Glositis intersticial
Glositis intersticial Varicela incubatie
Varicela incubatie Mulluscum contagiosum
Mulluscum contagiosum Papilomavírus humano
Papilomavírus humano Herpes simplex virus 2
Herpes simplex virus 2