Inactivated Polio Vaccine IPV Comprehensive technical module Rationale






- Slides: 44

Inactivated Polio Vaccine (IPV) Comprehensive technical module Rationale for IPV introduction & OPV withdrawal in relation to Objective 2 of The Polio Eradication & Endgame Strategic Plan Immunization Systems Management Group (IMG) Version date: February 10, 2014

Glossary of terms & abbreviations § § § c. VDPV Circulating Vaccine-Derived Poliovirus DTP 3 Diphtheria Tetanus Pertussis (third dose) GPEI Global Polio Eradication Initiative IMG Immunization Systems Management Group IPV Inactivated Polio Vaccine OPV Oral polio vaccine - b. OPV (bivalent, contains types 1 and 3) m. OPV (monovalent) OPV 1 (type 1 component of OPV) OPV 2 (type 2 component of OPV) OPV 3 (type 3 component of OPV) SAGE Strategic Advisory Group of Experts on Immunization VAPP Vaccine-associated paralytic poliomyelitis VDPV Vaccine-derived poliovirus WHA World Health Assembly WHO World Health Organization WPV Wild poliovirus 10/29/2020 IPV introduction 2

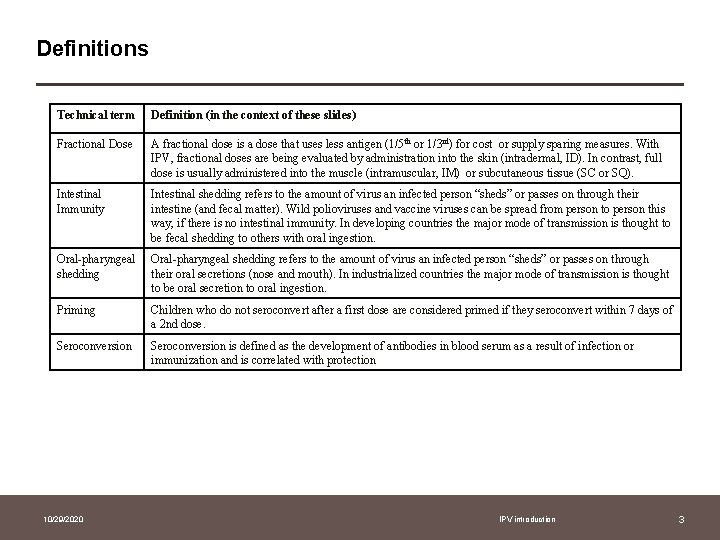
Definitions Technical term Definition (in the context of these slides) Fractional Dose A fractional dose is a dose that uses less antigen (1/5 th or 1/3 rd) for cost or supply sparing measures. With IPV, fractional doses are being evaluated by administration into the skin (intradermal, ID). In contrast, full dose is usually administered into the muscle (intramuscular, IM) or subcutaneous tissue (SC or SQ). Intestinal Immunity Intestinal shedding refers to the amount of virus an infected person “sheds” or passes on through their intestine (and fecal matter). Wild polioviruses and vaccine viruses can be spread from person to person this way, if there is no intestinal immunity. In developing countries the major mode of transmission is thought to be fecal shedding to others with oral ingestion. Oral-pharyngeal shedding refers to the amount of virus an infected person “sheds” or passes on through their oral secretions (nose and mouth). In industrialized countries the major mode of transmission is thought to be oral secretion to oral ingestion. Priming Children who do not seroconvert after a first dose are considered primed if they seroconvert within 7 days of a 2 nd dose. Seroconversion is defined as the development of antibodies in blood serum as a result of infection or immunization and is correlated with protection 10/29/2020 IPV introduction 3

Objectives of this module 1. Provide Technical background on Polio & Polio vaccines as it relates to Objective 2 of GPEI’s Polio Eradication & Endgame Strategic Plan - Review Rationale for OPV cessation Review Rationale for IPV introduction 2. Review of SAGE recommendations for IPV introduction Note: this is a comprehensive stand-alone deck of slides with an accompanying document 10/29/2020 IPV introduction 4

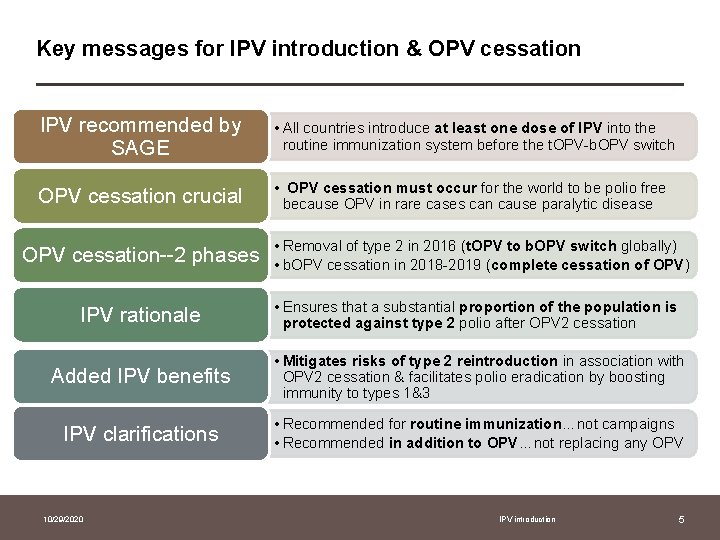
Key messages for IPV introduction & OPV cessation IPV recommended by SAGE • All countries introduce at least one dose of IPV into the routine immunization system before the t. OPV-b. OPV switch OPV cessation crucial • OPV cessation must occur for the world to be polio free because OPV in rare cases can cause paralytic disease OPV cessation--2 phases IPV rationale • Removal of type 2 in 2016 (t. OPV to b. OPV switch globally) • b. OPV cessation in 2018 -2019 (complete cessation of OPV) • Ensures that a substantial proportion of the population is protected against type 2 polio after OPV 2 cessation Added IPV benefits • Mitigates risks of type 2 reintroduction in association with OPV 2 cessation & facilitates polio eradication by boosting immunity to types 1&3 IPV clarifications • Recommended for routine immunization…not campaigns • Recommended in addition to OPV…not replacing any OPV 10/29/2020 IPV introduction 5

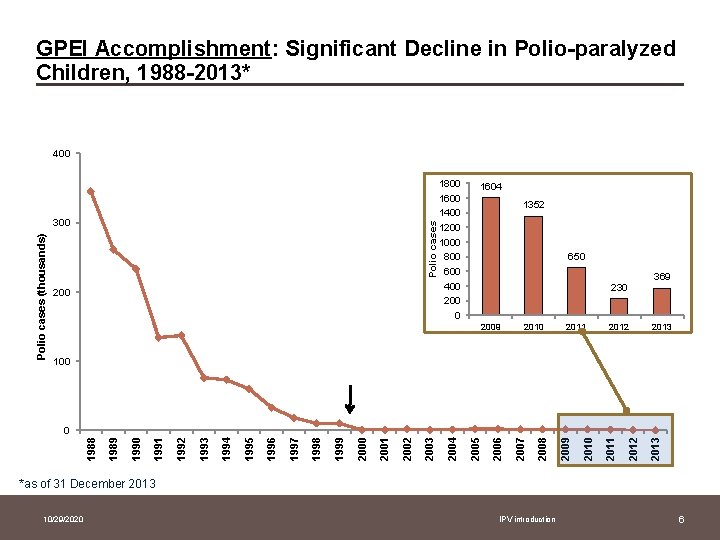
GPEI Accomplishment: Significant Decline in Polio-paralyzed Children, 1988 -2013* 400 Polio cases (thousands) 300 200 1800 1600 1400 1200 1000 800 600 400 200 0 1604 1352 650 230 2009 2010 2011 2012 369 2013 100 2013 2012 2011 2010 2009 2008 2007 2006 2005 2004 2003 2002 2001 2000 1999 1998 1997 1996 1995 1994 1993 1992 1991 1990 1989 1988 0 *as of 31 December 2013 10/29/2020 IPV introduction 6

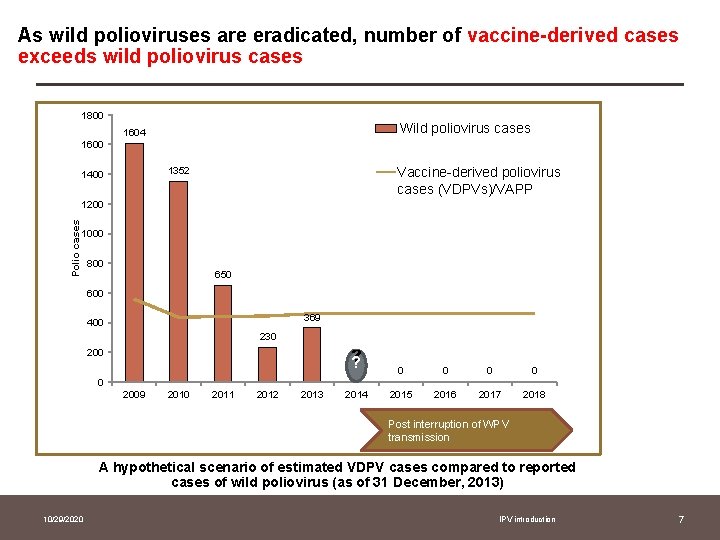
As wild polioviruses are eradicated, number of vaccine-derived cases exceeds wild poliovirus cases 1800 Wild poliovirus cases 1604 1600 Vaccine-derived poliovirus cases (VDPVs)/VAPP 1352 1400 Polio cases 1200 1000 800 650 600 369 400 230 200 ? ? 0 0 2014 2015 2016 2017 2018 0 2009 2010 2011 2012 2013 Post interruption of WPV transmission A hypothetical scenario of estimated VDPV cases compared to reported cases of wild poliovirus (as of 31 December, 2013) 10/29/2020 IPV introduction 7

The Polio Eradication & Endgame Strategic Plan 2013 -2018 The Plan differs from previous eradication plans “complete the eradication and containment of all wild, vaccine-related, and Sabin polioviruses such that no child ever again suffers paralytic poliomyelitis. ” 10/29/2020 IPV introduction 8

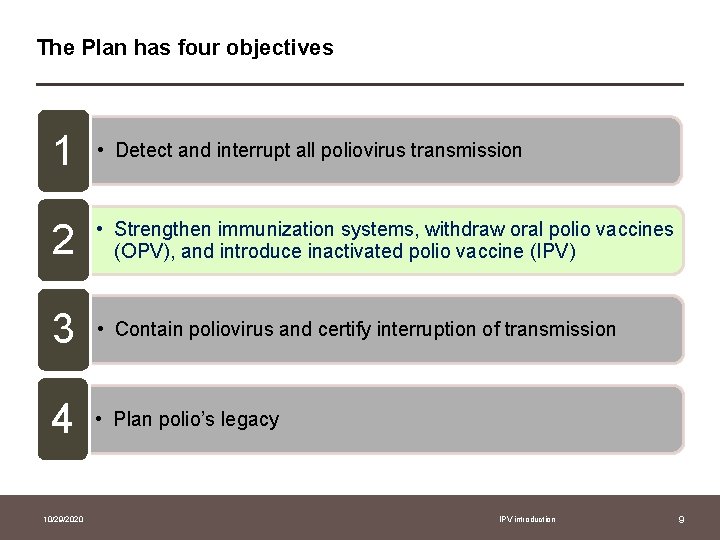
The Plan has four objectives 1 • Detect and interrupt all poliovirus transmission 2 • Strengthen immunization systems, withdraw oral polio vaccines (OPV), and introduce inactivated polio vaccine (IPV) 3 • Contain poliovirus and certify interruption of transmission 4 • Plan polio’s legacy 10/29/2020 IPV introduction 9

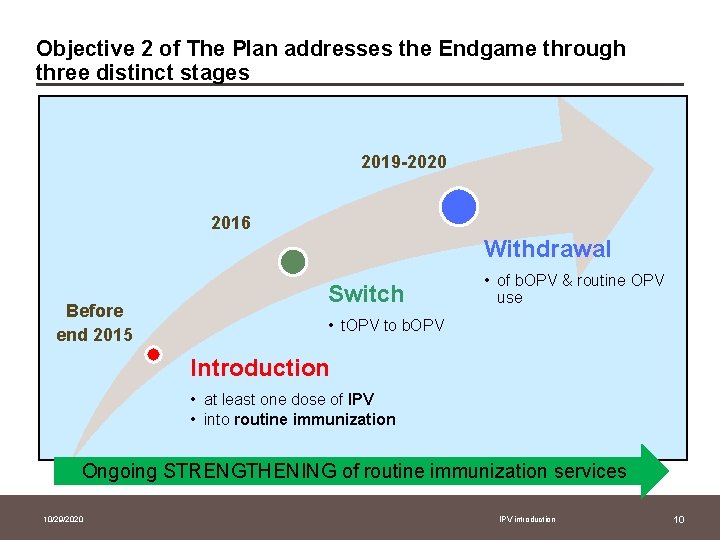
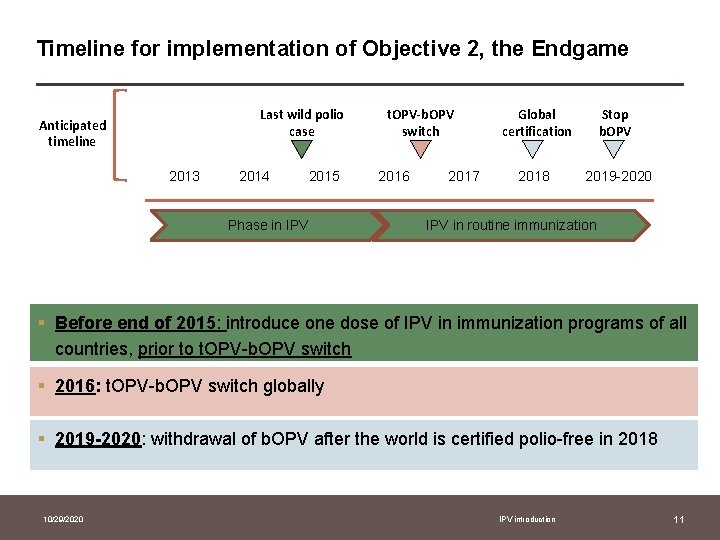
Objective 2 of The Plan addresses the Endgame through three distinct stages 2019 -2020 2016 Withdrawal Before end 2015 Switch • of b. OPV & routine OPV use • t. OPV to b. OPV Introduction • at least one dose of IPV • into routine immunization Ongoing STRENGTHENING of routine immunization services 10/29/2020 IPV introduction 10

Timeline for implementation of Objective 2, the Endgame Anticipated timeline Last wild polio case t. OPV-b. OPV switch Global certification Stop b. OPV 2013 2014 2015 2016 2017 2018 2019 -2020 Phase in IPV in routine immunization § Before end of 2015: introduce one dose of IPV in immunization programs of all countries, prior to t. OPV-b. OPV switch § 2016: t. OPV-b. OPV switch globally § 2019 -2020: withdrawal of b. OPV after the world is certified polio-free in 2018 10/29/2020 IPV introduction 11

Technical Rationale for Oral Polio Vaccine (OPV) Cessation Key Messages § Because OPV in rare cases can cause paralytic disease, OPV cessation must occur for the world to be polio free. § OPV cessation will occur globally in two phases: - removal of type 2 component (switch from t. OPV to b. OPV) in 2016 - followed by b. OPV withdrawal and cessation of OPV use in 2018 -2019. 10/29/2020 IPV introduction 12

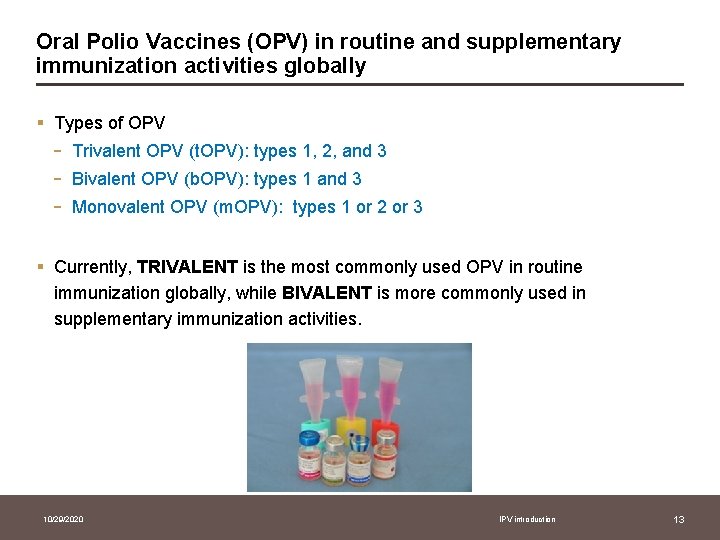
Oral Polio Vaccines (OPV) in routine and supplementary immunization activities globally § Types of OPV - Trivalent OPV (t. OPV): types 1, 2, and 3 Bivalent OPV (b. OPV): types 1 and 3 Monovalent OPV (m. OPV): types 1 or 2 or 3 § Currently, TRIVALENT is the most commonly used OPV in routine immunization globally, while BIVALENT is more commonly used in supplementary immunization activities. 10/29/2020 IPV introduction 13

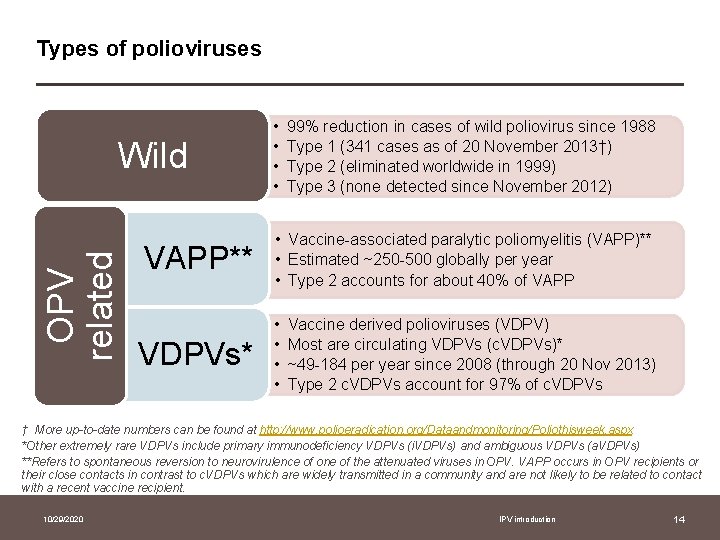
Types of polioviruses OPV related Wild VAPP** VDPVs* • • 99% reduction in cases of wild poliovirus since 1988 Type 1 (341 cases as of 20 November 2013†) Type 2 (eliminated worldwide in 1999) Type 3 (none detected since November 2012) • Vaccine-associated paralytic poliomyelitis (VAPP)** • Estimated ~250 -500 globally per year • Type 2 accounts for about 40% of VAPP • • Vaccine derived polioviruses (VDPV) Most are circulating VDPVs (c. VDPVs)* ~49 -184 per year since 2008 (through 20 Nov 2013) Type 2 c. VDPVs account for 97% of c. VDPVs † More up-to-date numbers can be found at http: //www. polioeradication. org/Dataandmonitoring/Poliothisweek. aspx *Other extremely rare VDPVs include primary immunodeficiency VDPVs (i. VDPVs) and ambiguous VDPVs (a. VDPVs) **Refers to spontaneous reversion to neurovirulence of one of the attenuated viruses in OPV. VAPP occurs in OPV recipients or their close contacts in contrast to c. VDPVs which are widely transmitted in a community and are not likely to be related to contact with a recent vaccine recipient. 10/29/2020 IPV introduction 14

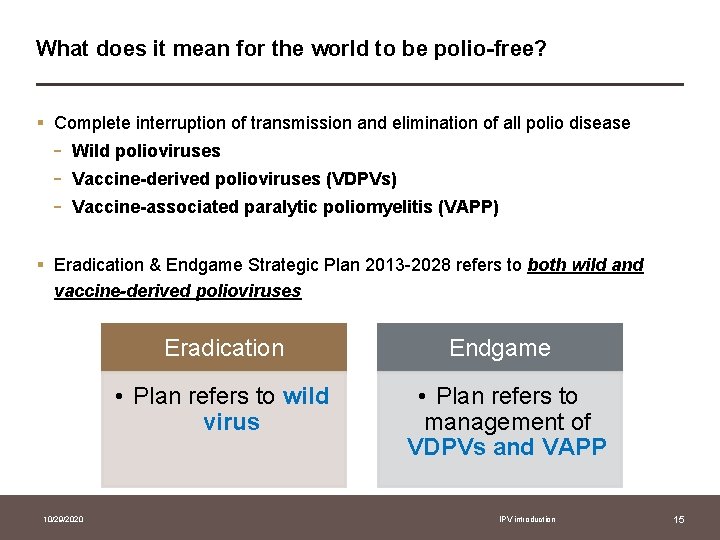
What does it mean for the world to be polio-free? § Complete interruption of transmission and elimination of all polio disease - Wild polioviruses Vaccine-derived polioviruses (VDPVs) Vaccine-associated paralytic poliomyelitis (VAPP) § Eradication & Endgame Strategic Plan 2013 -2028 refers to both wild and vaccine-derived polioviruses Eradication • Plan refers to wild virus 10/29/2020 Endgame • Plan refers to management of VDPVs and VAPP IPV introduction 15

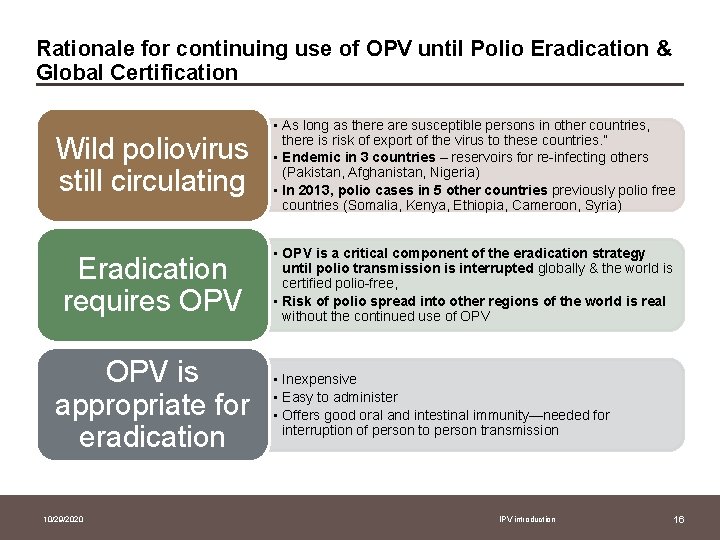
Rationale for continuing use of OPV until Polio Eradication & Global Certification Wild poliovirus still circulating • As long as there are susceptible persons in other countries, there is risk of export of the virus to these countries. ” • Endemic in 3 countries – reservoirs for re-infecting others (Pakistan, Afghanistan, Nigeria) • In 2013, polio cases in 5 other countries previously polio free countries (Somalia, Kenya, Ethiopia, Cameroon, Syria) Eradication requires OPV • OPV is a critical component of the eradication strategy until polio transmission is interrupted globally & the world is certified polio-free, • Risk of polio spread into other regions of the world is real without the continued use of OPV is appropriate for eradication 10/29/2020 • Inexpensive • Easy to administer • Offers good oral and intestinal immunity—needed for interruption of person to person transmission IPV introduction 16

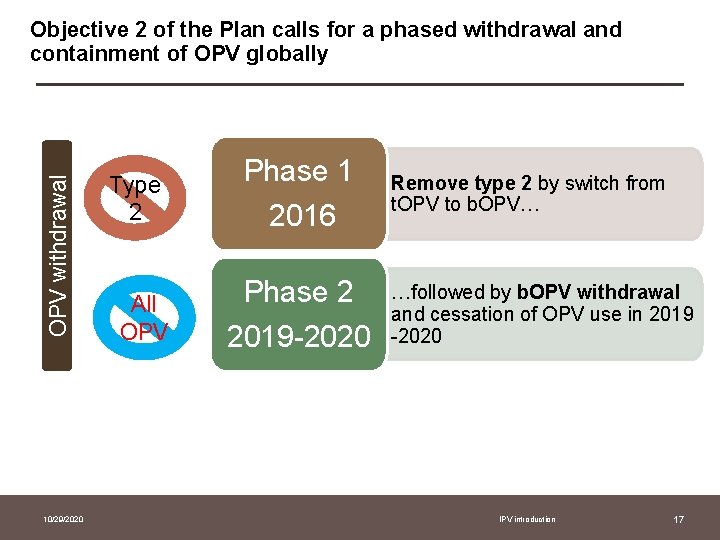
OPV withdrawal Objective 2 of the Plan calls for a phased withdrawal and containment of OPV globally 10/29/2020 Type 2 Phase 1 2016 All OPV Phase 2 2019 -2020 Remove type 2 by switch from t. OPV to b. OPV… …followed by b. OPV withdrawal and cessation of OPV use in 2019 -2020 IPV introduction 17

Rationale for removing type 2 component of OPV (OPV 2) § Risks of OPV 2 far outweigh the benefits - Thus, need to remove OPV 2, but need to maintain population immunity against type 2 with IPV prior to OPV 2 cessation § Type 2 poliovirus apparently eradicated since 1999 (last case detected in Aligarh, India) § New diagnostics and experience suggest that type 2 cause >95% of c. VDPVs § Type 2 causes 40% of VAPP today § Type 2 component of OPV interferes with immune response to types 1 and types 3 10/29/2020 IPV introduction 18

Rationale for retaining Types 1 & Types 3 components of OPV (bivalent OPV) until global certification of polio eradication § Type 1 causes all polio cases related to wild virus today - Few VDPV cases are type 1 Few VAPP cases in immunocompetent individuals § Type 3 last detected in November, 2012 (as of 20 November 2013) - Few VDPV cases are type 3 Most VAPP cases (60%) in immunocompetent persons are type 3 While lack of detection since November 2012 is promising, the period without detection to date is not long enough to assume eradication—due to potential silent transmission, certification of eradication requires at least 3 years without detection of virus. 10/29/2020 IPV introduction 19

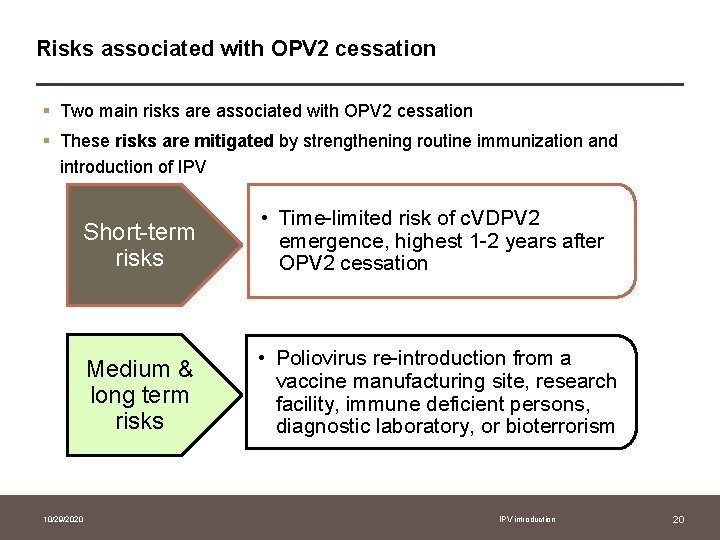
Risks associated with OPV 2 cessation § Two main risks are associated with OPV 2 cessation § These risks are mitigated by strengthening routine immunization and introduction of IPV Short-term risks • Time-limited risk of c. VDPV 2 emergence, highest 1 -2 years after OPV 2 cessation Medium & long term risks • Poliovirus re-introduction from a vaccine manufacturing site, research facility, immune deficient persons, diagnostic laboratory, or bioterrorism 10/29/2020 IPV introduction 20

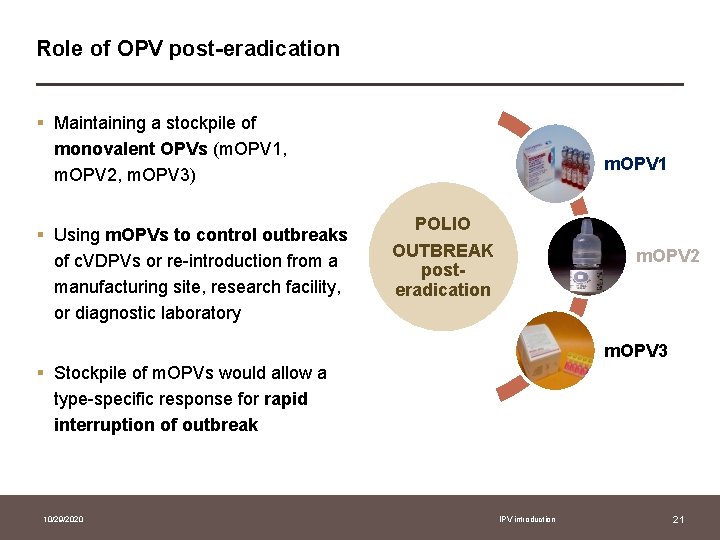
Role of OPV post-eradication § Maintaining a stockpile of monovalent OPVs (m. OPV 1, m. OPV 2, m. OPV 3) § Using m. OPVs to control outbreaks of c. VDPVs or re-introduction from a manufacturing site, research facility, or diagnostic laboratory m. OPV 1 POLIO OUTBREAK posteradication m. OPV 2 m. OPV 3 § Stockpile of m. OPVs would allow a type-specific response for rapid interruption of outbreak 10/29/2020 IPV introduction 21

Technical Rationale for Introduction for Inactivated Polio Vaccine (IPV) Key Messages § Introducing IPV before the t. OPV-b. OPV switch in 2016 will ensure that a substantial proportion of the population is protected against type 2 polio after OPV 2 cessation and mitigate risks associated with OPV 2 cessation § IPV is recommended for routine immunization programmes & not campaigns § IPV is given in addition to OPV doses and does not replace any OPV doses 10/29/2020 IPV introduction 22

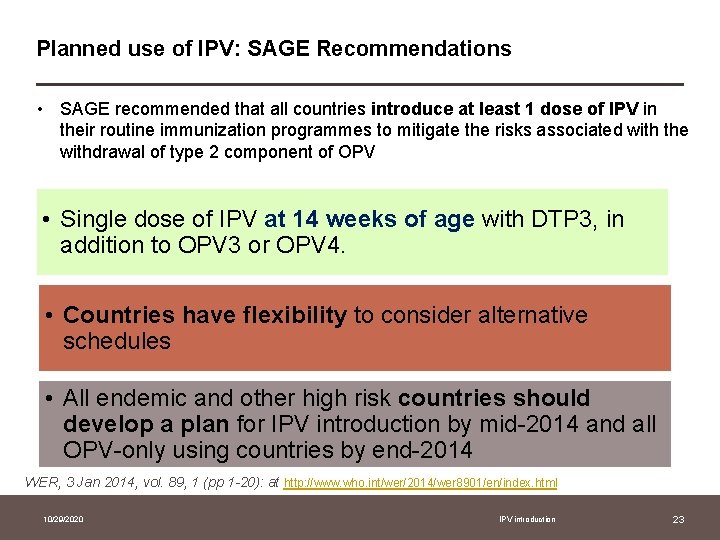
Planned use of IPV: SAGE Recommendations • SAGE recommended that all countries introduce at least 1 dose of IPV in their routine immunization programmes to mitigate the risks associated with the withdrawal of type 2 component of OPV • Single dose of IPV at 14 weeks of age with DTP 3, in addition to OPV 3 or OPV 4. • Countries have flexibility to consider alternative schedules • All endemic and other high risk countries should develop a plan for IPV introduction by mid-2014 and all OPV-only using countries by end-2014 WER, 3 Jan 2014, vol. 89, 1 (pp 1 -20): at http: //www. who. int/wer/2014/wer 8901/en/index. html 10/29/2020 IPV introduction 23

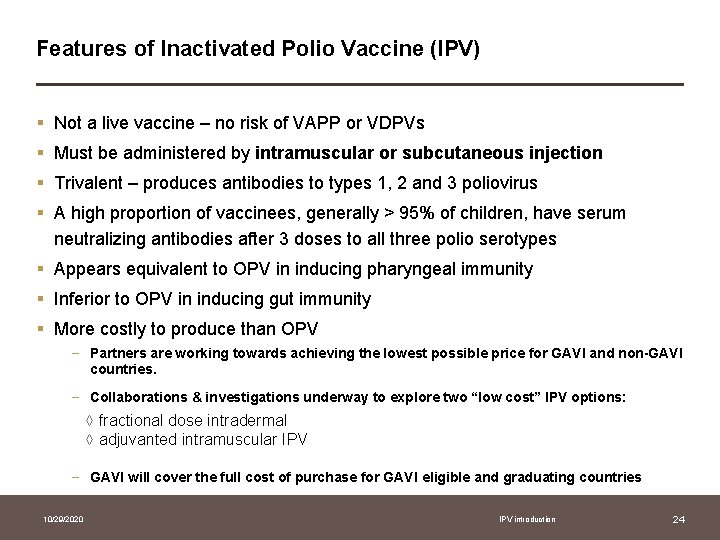
Features of Inactivated Polio Vaccine (IPV) § Not a live vaccine – no risk of VAPP or VDPVs § Must be administered by intramuscular or subcutaneous injection § Trivalent – produces antibodies to types 1, 2 and 3 poliovirus § A high proportion of vaccinees, generally > 95% of children, have serum neutralizing antibodies after 3 doses to all three polio serotypes § Appears equivalent to OPV in inducing pharyngeal immunity § Inferior to OPV in inducing gut immunity § More costly to produce than OPV − Partners are working towards achieving the lowest possible price for GAVI and non-GAVI countries. − Collaborations & investigations underway to explore two “low cost” IPV options: ◊ fractional dose intradermal ◊ adjuvanted intramuscular IPV − GAVI will cover the full cost of purchase for GAVI eligible and graduating countries 10/29/2020 IPV introduction 24

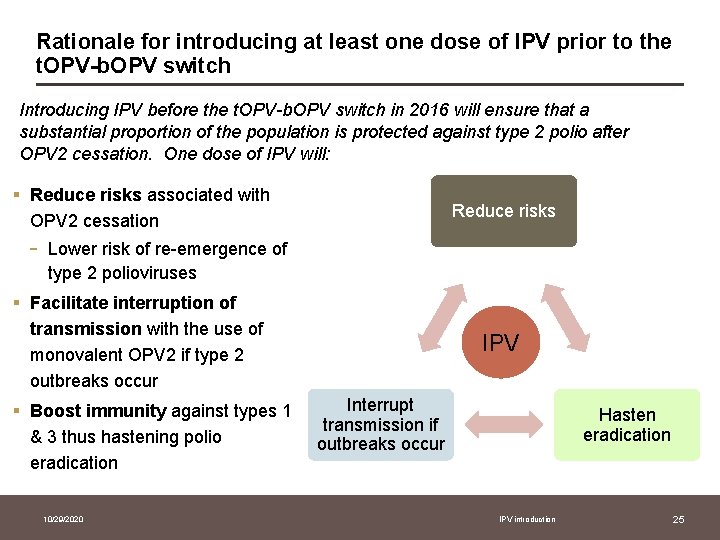
Rationale for introducing at least one dose of IPV prior to the t. OPV-b. OPV switch Introducing IPV before the t. OPV-b. OPV switch in 2016 will ensure that a substantial proportion of the population is protected against type 2 polio after OPV 2 cessation. One dose of IPV will: § Reduce risks associated with OPV 2 cessation - Reduce risks Lower risk of re-emergence of type 2 polioviruses § Facilitate interruption of transmission with the use of monovalent OPV 2 if type 2 outbreaks occur § Boost immunity against types 1 & 3 thus hastening polio eradication 10/29/2020 IPV Interrupt transmission if outbreaks occur Hasten eradication IPV introduction 25

Individual protection against paralytic disease induced by IPV – REDUCE RISKS Reduce risks IPV Interrupt transmission if outbreaks occur 10/29/2020 Hasten eradication IPV introduction 26

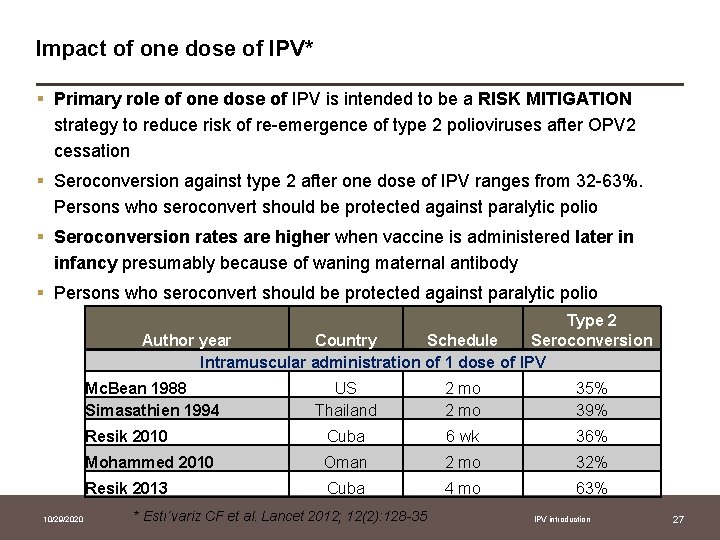
Impact of one dose of IPV* § Primary role of one dose of IPV is intended to be a RISK MITIGATION strategy to reduce risk of re-emergence of type 2 polioviruses after OPV 2 cessation § Seroconversion against type 2 after one dose of IPV ranges from 32 -63%. Persons who seroconvert should be protected against paralytic polio § Seroconversion rates are higher when vaccine is administered later in infancy presumably because of waning maternal antibody § Persons who seroconvert should be protected against paralytic polio Type 2 Author year Country Schedule Seroconversion Intramuscular administration of 1 dose of IPV Mc. Bean 1988 Simasathien 1994 10/29/2020 US Thailand 2 mo 35% 39% Resik 2010 Cuba 6 wk 36% Mohammed 2010 Oman 2 mo 32% Resik 2013 Cuba 4 mo 63% * Estı´variz CF et al. Lancet 2012; 12(2): 128 -35 IPV introduction 27

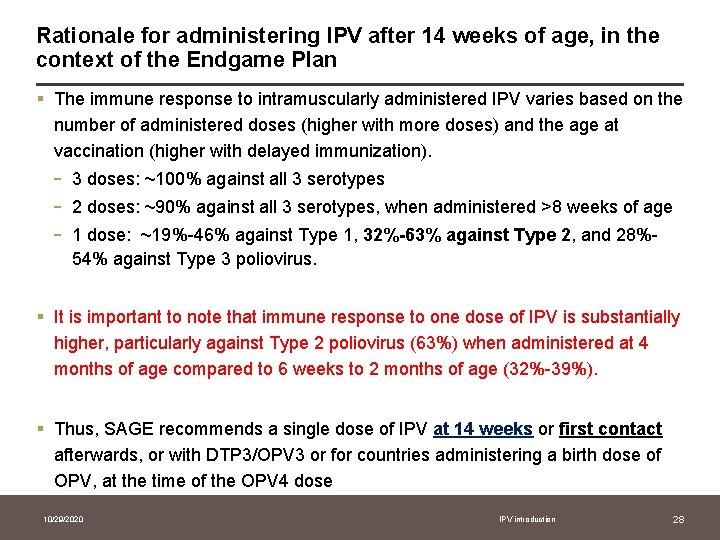
Rationale for administering IPV after 14 weeks of age, in the context of the Endgame Plan § The immune response to intramuscularly administered IPV varies based on the number of administered doses (higher with more doses) and the age at vaccination (higher with delayed immunization). - 3 doses: ~100% against all 3 serotypes 2 doses: ~90% against all 3 serotypes, when administered >8 weeks of age 1 dose: ~19%-46% against Type 1, 32%-63% against Type 2, and 28%54% against Type 3 poliovirus. § It is important to note that immune response to one dose of IPV is substantially higher, particularly against Type 2 poliovirus (63%) when administered at 4 months of age compared to 6 weeks to 2 months of age (32%-39%). § Thus, SAGE recommends a single dose of IPV at 14 weeks or first contact afterwards, or with DTP 3/OPV 3 or for countries administering a birth dose of OPV, at the time of the OPV 4 dose 10/29/2020 IPV introduction 28

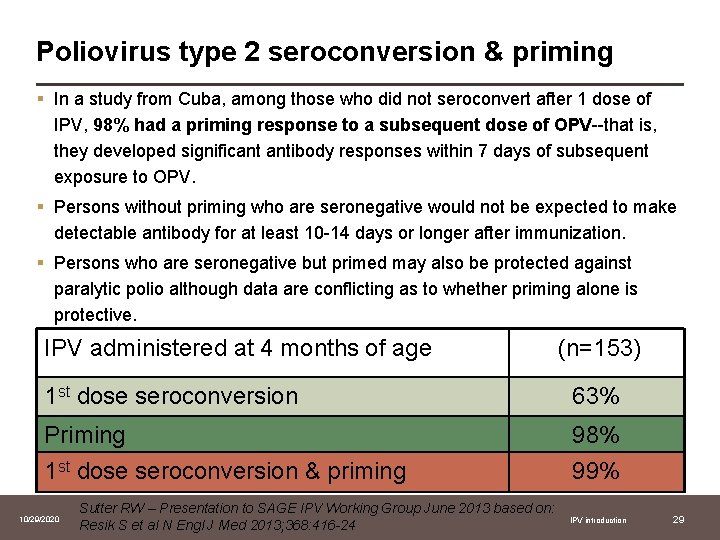
Poliovirus type 2 seroconversion & priming § In a study from Cuba, among those who did not seroconvert after 1 dose of IPV, 98% had a priming response to a subsequent dose of OPV--that is, they developed significant antibody responses within 7 days of subsequent exposure to OPV. § Persons without priming who are seronegative would not be expected to make detectable antibody for at least 10 -14 days or longer after immunization. § Persons who are seronegative but primed may also be protected against paralytic polio although data are conflicting as to whether priming alone is protective. IPV administered at 4 months of age (n=153) 1 st dose seroconversion 63% Priming 1 st dose seroconversion & priming 98% 99% 10/29/2020 Sutter RW – Presentation to SAGE IPV Working Group June 2013 based on: Resik S et al N Engl J Med 2013; 368: 416 -24 IPV introduction 29

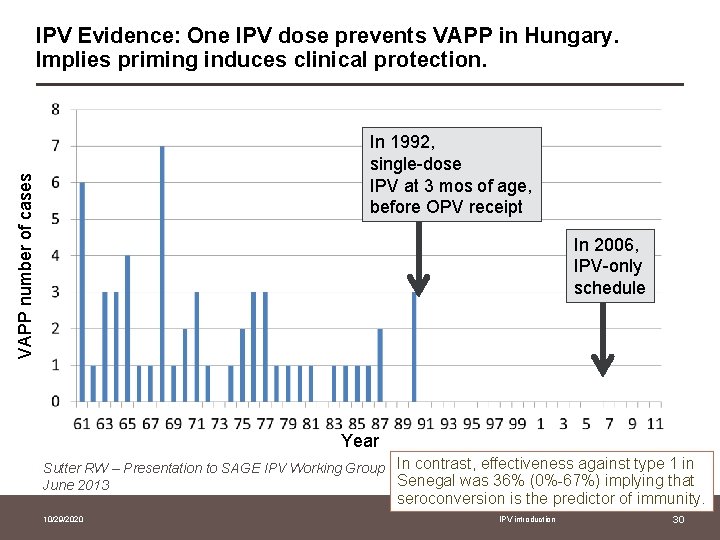
IPV Evidence: One IPV dose prevents VAPP in Hungary. Implies priming induces clinical protection. VAPP number of cases In 1992, single-dose IPV at 3 mos of age, before OPV receipt In 2006, IPV-only schedule Year Sutter RW – Presentation to SAGE IPV Working Group In contrast, effectiveness against type 1 in Senegal was 36% (0%-67%) implying that June 2013 seroconversion is the predictor of immunity. 10/29/2020 IPV introduction 30

Outbreak control with m. OPV 2 in a population which previously received IPV -- REDUCE RISKS Reduce risks IPV 10/29/2020 Interrupt transmission if outbreaks occur Hasten eradication 31

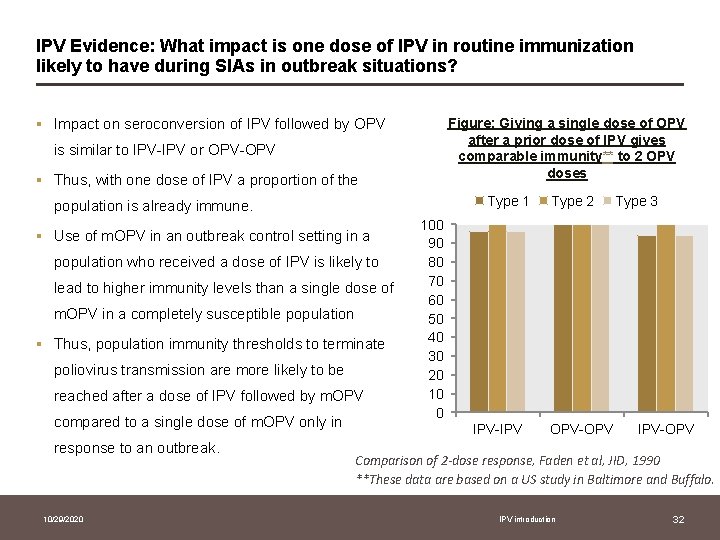
IPV Evidence: What impact is one dose of IPV in routine immunization likely to have during SIAs in outbreak situations? Figure: Giving a single dose of OPV after a prior dose of IPV gives comparable immunity** to 2 OPV doses § Impact on seroconversion of IPV followed by OPV is similar to IPV-IPV or OPV-OPV § Thus, with one dose of IPV a proportion of the Type 1 population is already immune. § Use of m. OPV in an outbreak control setting in a population who received a dose of IPV is likely to lead to higher immunity levels than a single dose of m. OPV in a completely susceptible population § Thus, population immunity thresholds to terminate poliovirus transmission are more likely to be reached after a dose of IPV followed by m. OPV compared to a single dose of m. OPV only in response to an outbreak. 10/29/2020 Type 2 Type 3 100 90 80 70 60 50 40 30 20 10 0 IPV-IPV OPV-OPV IPV-OPV Comparison of 2 -dose response, Faden et al, JID, 1990 **These data are based on a US study in Baltimore and Buffalo. IPV introduction 32

IPV in reducing transmission of polioviruses – Interrupt Transmission Reduce risks IPV 10/29/2020 Interrupt transmission if outbreaks occur Hasten eradication 33

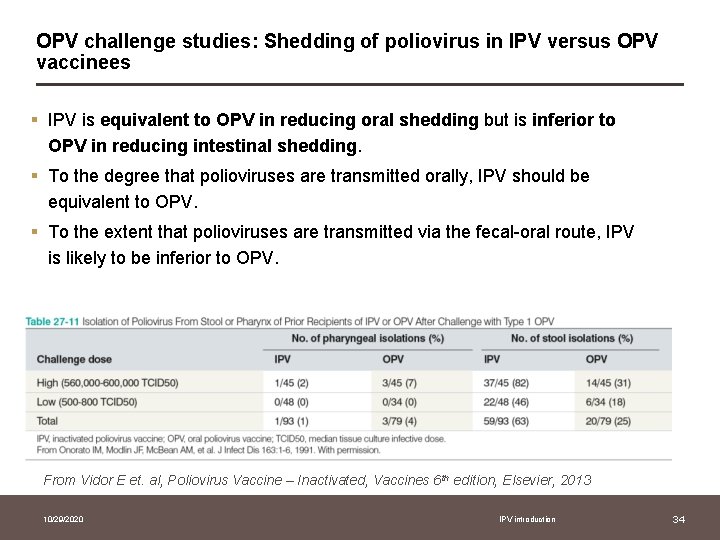
OPV challenge studies: Shedding of poliovirus in IPV versus OPV vaccinees § IPV is equivalent to OPV in reducing oral shedding but is inferior to OPV in reducing intestinal shedding. § To the degree that polioviruses are transmitted orally, IPV should be equivalent to OPV. § To the extent that polioviruses are transmitted via the fecal-oral route, IPV is likely to be inferior to OPV. From Vidor E et. al, Poliovirus Vaccine – Inactivated, Vaccines 6 th edition, Elsevier, 2013 10/29/2020 IPV introduction 34

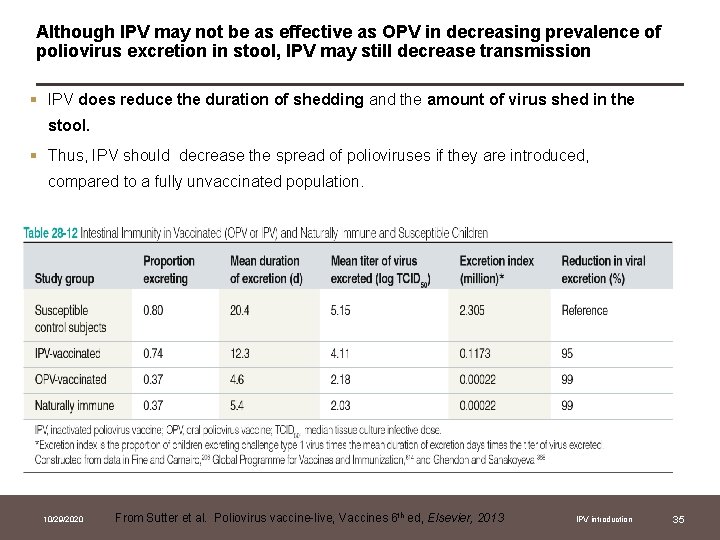
Although IPV may not be as effective as OPV in decreasing prevalence of poliovirus excretion in stool, IPV may still decrease transmission § IPV does reduce the duration of shedding and the amount of virus shed in the stool. § Thus, IPV should decrease the spread of polioviruses if they are introduced, compared to a fully unvaccinated population. 10/29/2020 From Sutter et al. Poliovirus vaccine-live, Vaccines 6 th ed, Elsevier, 2013 IPV introduction 35

IPV in boosting immunity in OPV primed individuals – Hastens Eradication Reduce risks IPV 10/29/2020 Interrupt transmission if outbreaks occur Hasten eradication 36

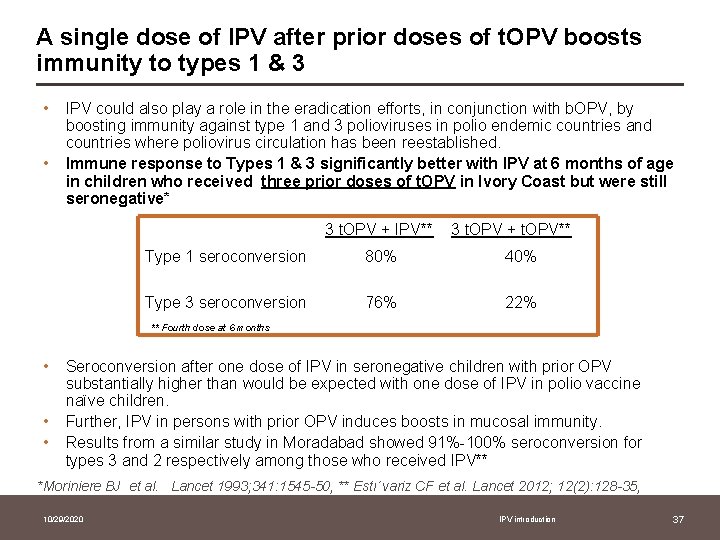
A single dose of IPV after prior doses of t. OPV boosts immunity to types 1 & 3 • • IPV could also play a role in the eradication efforts, in conjunction with b. OPV, by boosting immunity against type 1 and 3 polioviruses in polio endemic countries and countries where poliovirus circulation has been reestablished. Immune response to Types 1 & 3 significantly better with IPV at 6 months of age in children who received three prior doses of t. OPV in Ivory Coast but were still seronegative* 3 t. OPV + IPV** 3 t. OPV + t. OPV** Type 1 seroconversion 80% 40% Type 3 seroconversion 76% 22% ** Fourth dose at 6 months • • • Seroconversion after one dose of IPV in seronegative children with prior OPV substantially higher than would be expected with one dose of IPV in polio vaccine naïve children. Further, IPV in persons with prior OPV induces boosts in mucosal immunity. Results from a similar study in Moradabad showed 91%-100% seroconversion for types 3 and 2 respectively among those who received IPV** *Moriniere BJ et al. Lancet 1993; 341: 1545 -50, ** Estı´variz CF et al. Lancet 2012; 12(2): 128 -35, 10/29/2020 IPV introduction 37

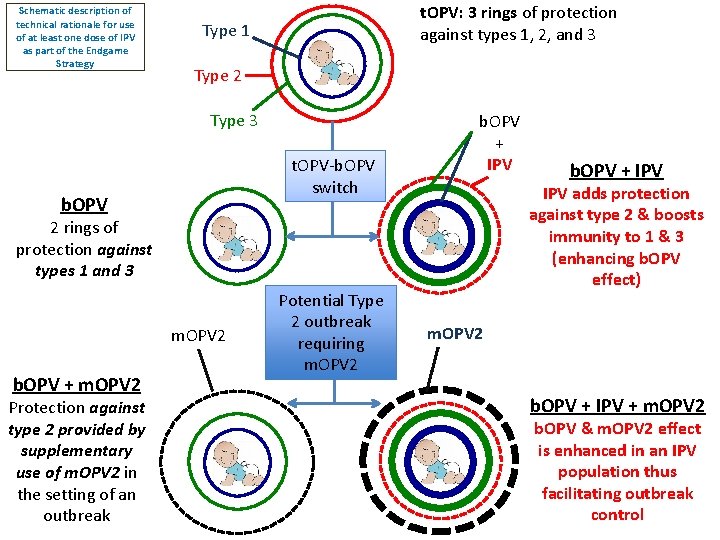
Schematic description of technical rationale for use of at least one dose of IPV as part of the Endgame Strategy t. OPV: 3 rings of protection against types 1, 2, and 3 Type 1 Type 2 Type 3 t. OPV-b. OPV switch b. OPV + IPV adds protection against type 2 & boosts immunity to 1 & 3 (enhancing b. OPV effect) 2 rings of protection against types 1 and 3 m. OPV 2 b. OPV + m. OPV 2 Protection against type 2 provided by supplementary use of m. OPV 2 in the setting of an outbreak Potential Type 2 outbreak requiring m. OPV 2 b. OPV + IPV + m. OPV 2 b. OPV & m. OPV 2 effect is enhanced in an IPV population thus facilitating outbreak control

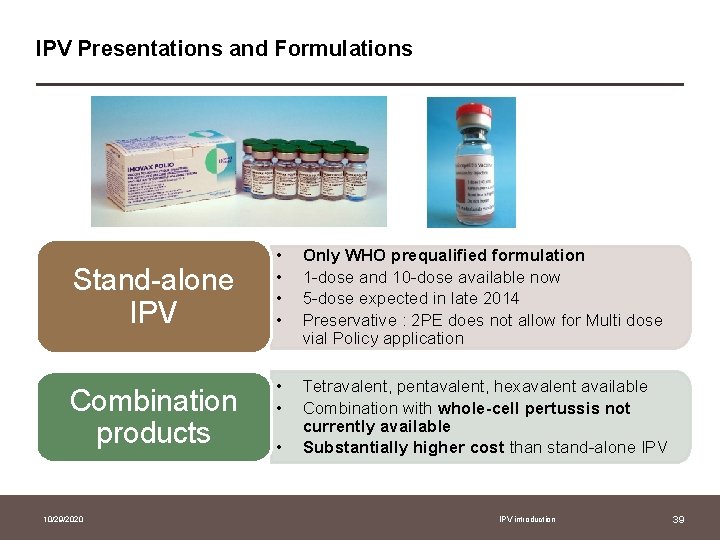
IPV Presentations and Formulations Stand-alone IPV Combination products 10/29/2020 • • Only WHO prequalified formulation 1 -dose and 10 -dose available now 5 -dose expected in late 2014 Preservative : 2 PE does not allow for Multi dose vial Policy application • • Tetravalent, pentavalent, hexavalent available Combination with whole-cell pertussis not currently available Substantially higher cost than stand-alone IPV • IPV introduction 39

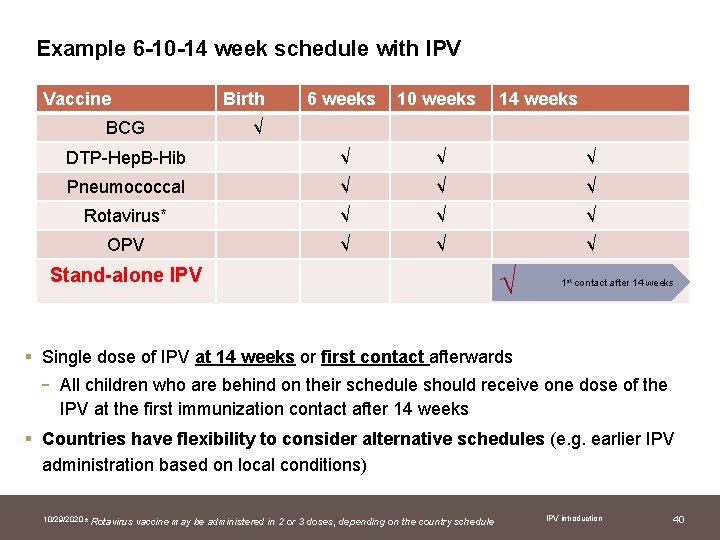
Example 6 -10 -14 week schedule with IPV Vaccine 6 weeks 10 weeks DTP-Hep. B-Hib √ √ √ Pneumococcal √ √ √ Rotavirus* √ √ √ OPV √ √ √ BCG Birth 14 weeks √ Stand-alone IPV √ 1 st contact after 14 weeks § Single dose of IPV at 14 weeks or first contact afterwards - All children who are behind on their schedule should receive one dose of the IPV at the first immunization contact after 14 weeks § Countries have flexibility to consider alternative schedules (e. g. earlier IPV administration based on local conditions) 10/29/2020 * Rotavirus vaccine may be administered in 2 or 3 doses, depending on the country schedule IPV introduction 40

Administration of Inactivated Polio Vaccine (IPV) § IPV is administered by intramuscular injection (IM) or subcutaneously (SQ) in a dose of 0. 5 ml into the outer part of the thigh § When given at the same visit, IPV and other injectable vaccines should be given at different injection sites at least 2 cm apart - For example, if IPV, Pentavalent vaccine, and Pneumococcal vaccine are to be given during the same visit, IPV and Pneumococcal should be in one thigh 2 cm apart and the Pentavalent vaccine (more reactogenic) in the other thigh § IPV should not be mixed with other vaccines in the same vial or syringe § IPV can be administered to prematurely born infants and children with immunodeficiencies (e. g. , HIV, congenital or acquired immunodeficiency, sickle cell disease) 10/29/2020 IPV introduction 41

Multiple Injections: Acceptability and Safety § Recently, more low and middle income countries have begun using multiple vaccine injections with the addition of pneumococcal vaccine and IPV § Substantial evidence has reinforced the well-established record of safety and acceptance of multiple injections from countries using multiple injections* - For example, US infants often receive 3 or more injections during each of the primary series vaccination visits § Giving a child several vaccinations during the same visit offers three major advantages: - Immunizing children as soon as possible provides protection during the vulnerable early months of their lives. - Giving several vaccinations at the same time means parents and caregivers do not need to make as many vaccination visits. 1 - It means that health care providers are able to more efficiently provide and deliver other health services by reducing the time they need to spend providing vaccinations. 10/29/2020 *http: //www. cdc. gov/vaccinesafety/Vaccines/multiplevaccines. html *http: //www. cmaj. ca/content/182/18/E 843. full IPV introduction 42

Specific notes on the recommended IPV schedule* § IPV does not replace ANY of the OPV doses – that is, IPV will be given in addition to OPV and OPV will continue to be used per current practice for now § IPV is recommended for routine immunization programmes and not campaigns because injections are difficult to accommodate in campaigns against polio § The higher the IPV coverage the better, but even low coverage will provide direct benefit to those vaccinated and greatly facilitate building population immunity in an emergency response *from 7 th Meeting of the SAGE Polio Working Group, October 18 -19, 2013 10/29/2020 IPV introduction 43

Planned use of IPV: IPV Rationale Summary § IPV induces immunity in a proportion of children which will protect them against polio caused by vaccine viruses (VAPP and c. VDPVs) and polio caused by wild poliovirus - IPV should lower risk of re-emergence of type 2 polioviruses - IPV will boost immunity to types 1 and 3 which should hasten eradication of types 1 and 3 wild polioviruses and reduce polio disease caused by types 1 and 3 c. VDPVs - IPV by inducing immunity to type 2 will facilitate outbreak control with m. OPV 2 should type 2 viruses be reintroduced IPV in conjunction with b. OPV will decrease the number of cases of VAPP caused by types 1 and 3 − A proportion of the population will already be immune resulting in a higher level of immunity after a dose of m. OPV 2 in outbreak control than after a dose of m. OPV 2 to contain an outbreak in a completely susceptible population § The higher the IPV coverage the better, but even low coverage will provide direct benefit to those vaccinated and greatly facilitate building population immunity in an emergency response 10/29/2020 IPV introduction 44