Unit 3 Formulas and Equations Caffeine Mr Taglias











- Slides: 50

Unit 3: Formulas and Equations Caffeine Mr. Taglia’s Chemistry Class TNT

Topic Overview Chemistry is much like a language spoken worldwide… It involves a universal system of symbols and formulas used by all chemists. In this unit you will learn how to interpret and write using these symbols and formulas. IUPAC- International Union of Pure and Applied Chemists (created this naming system)

Key Terms Review Element- a substance consisting of a single type of atom that cannot be broken down by chemical means Compound- a substance formed when two or more different atoms chemically combine

Chemical Symbols Each element on the periodic table is assigned a one or two letter chemical symbol Symbols are meant to be a shorthand for abbreviating element names. Usually easy to remember because most letters in symbols correspond to element names Ex) H for Hydrogen or Li for Lithium Some Symbols relate instead to Greek or Latin element names Ex) Na for sodium because Latin word was Natrium * Three letter symbols like Uuq are only temporary for artificially made elements

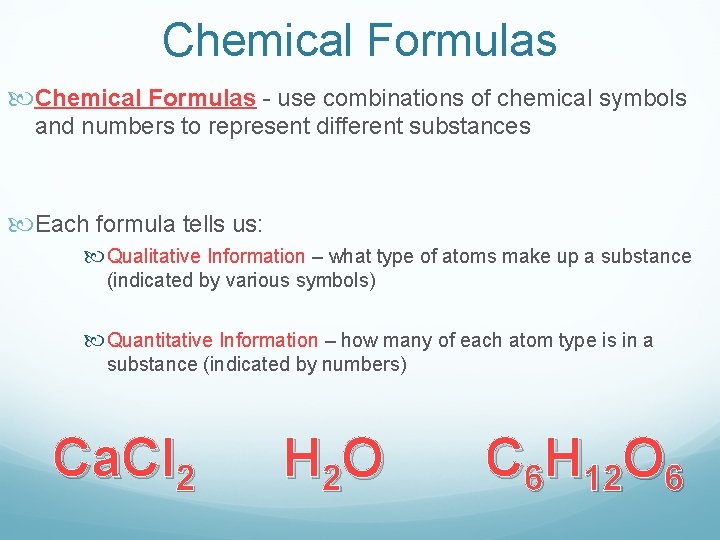
Chemical Formulas - use combinations of chemical symbols and numbers to represent different substances Each formula tells us: Qualitative Information – what type of atoms make up a substance (indicated by various symbols) Quantitative Information – how many of each atom type is in a substance (indicated by numbers) Ca. Cl 2 H 2 O C 6 H 12 O 6

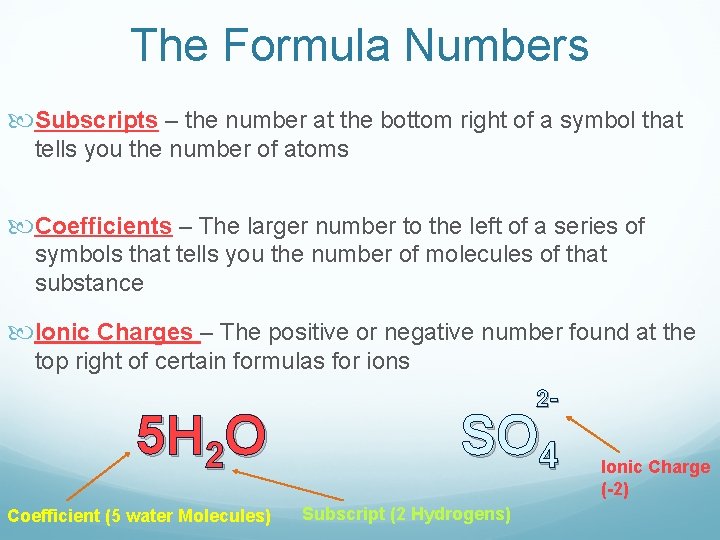
The Formula Numbers Subscripts – the number at the bottom right of a symbol that tells you the number of atoms Coefficients – The larger number to the left of a series of symbols that tells you the number of molecules of that substance Ionic Charges – The positive or negative number found at the top right of certain formulas for ions 5 H 2 O Coefficient (5 water Molecules) 2 - SO 4 Subscript (2 Hydrogens) Ionic Charge (-2)

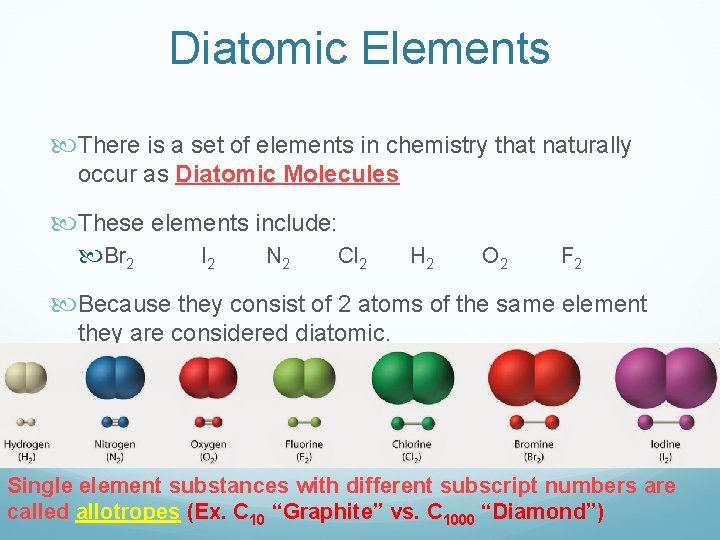
Diatomic Elements There is a set of elements in chemistry that naturally occur as Diatomic Molecules These elements include: Br 2 I 2 N 2 Cl 2 H 2 O 2 F 2 Because they consist of 2 atoms of the same element they are considered diatomic. Single element substances with different subscript numbers are called allotropes (Ex. C 10 “Graphite” vs. C 1000 “Diamond”)

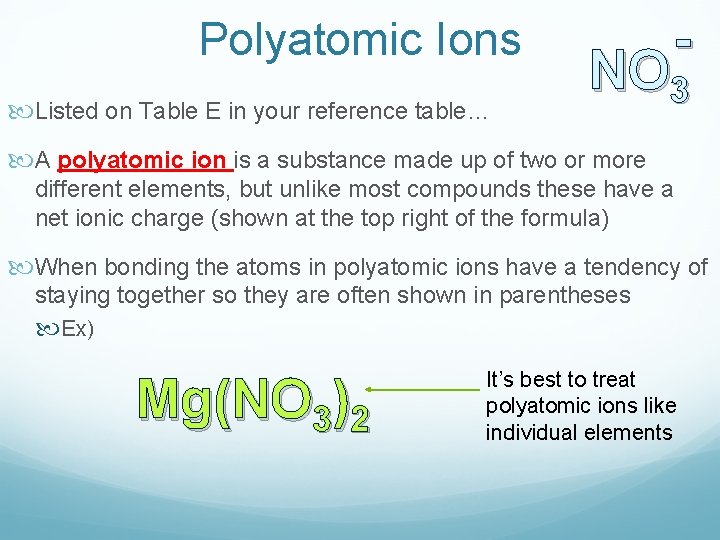
Polyatomic Ions Listed on Table E in your reference table… - NO 3 A polyatomic ion is a substance made up of two or more different elements, but unlike most compounds these have a net ionic charge (shown at the top right of the formula) When bonding the atoms in polyatomic ions have a tendency of staying together so they are often shown in parentheses Ex) Mg(NO 3)2 It’s best to treat polyatomic ions like individual elements

Hydrate Crystals When some compounds form they can trap water crystals in their molecules, this usually happens with salts. These hydrate crystals will be written like water molecules as part of a chemical formula Ex) Ba. Cl 2 2 H 2 O 1 Barium Atom 2 Chlorine Atoms 2 Hydrate Crystals

Types of Chemical Formula 2 types of formula we use in chemistry: Molecular Formula – The arrangement of atoms in a substance with subscripts to show the number of each atom. C 6 H 12 O 6 Empirical Formula – The simplified ratio of different atoms in a molecular formula based on common multiples C H 2 O ***Sometimes the Empirical and Molecular Formulas of a compound are the same (Ex. H 2 O )

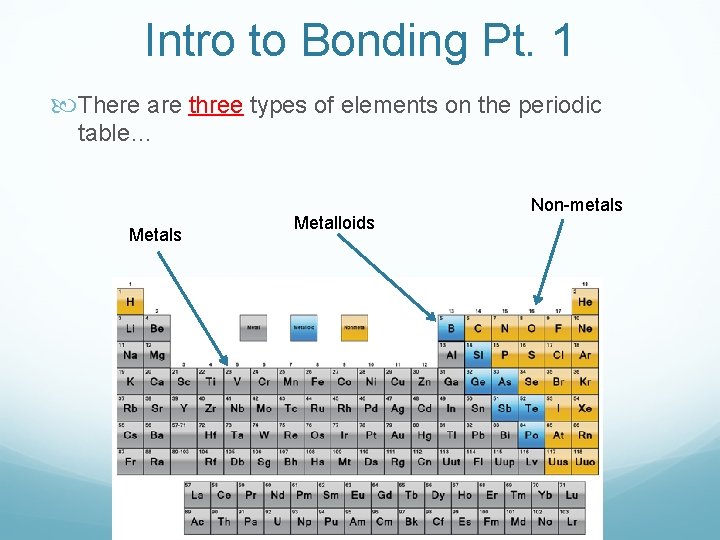
Intro to Bonding Pt. 1 There are three types of elements on the periodic table… Metals Metalloids Non-metals

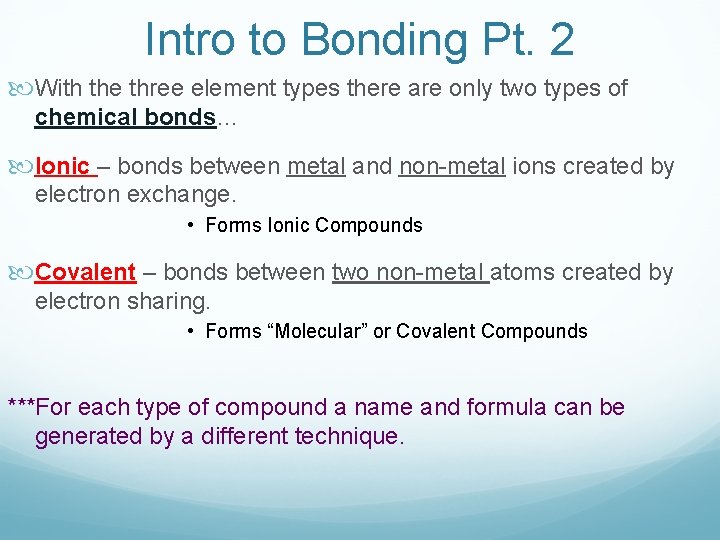
Intro to Bonding Pt. 2 With the three element types there are only two types of chemical bonds… Ionic – bonds between metal and non-metal ions created by electron exchange. • Forms Ionic Compounds Covalent – bonds between two non-metal atoms created by electron sharing. • Forms “Molecular” or Covalent Compounds ***For each type of compound a name and formula can be generated by a different technique.

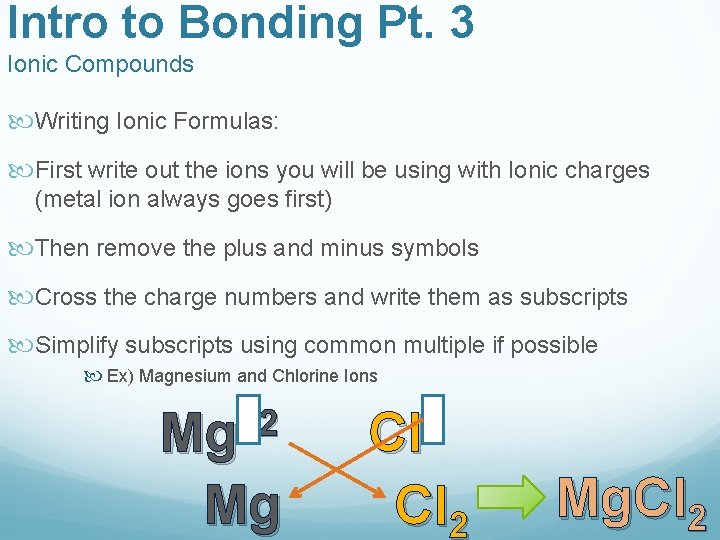
Intro to Bonding Pt. 3 Ionic Compounds Writing Ionic Formulas: First write out the ions you will be using with Ionic charges (metal ion always goes first) Then remove the plus and minus symbols Cross the charge numbers and write them as subscripts Simplify subscripts using common multiple if possible Ex) Magnesium and Chlorine Ions +2 Mg Mg Cl Cl 2 Mg. Cl 2

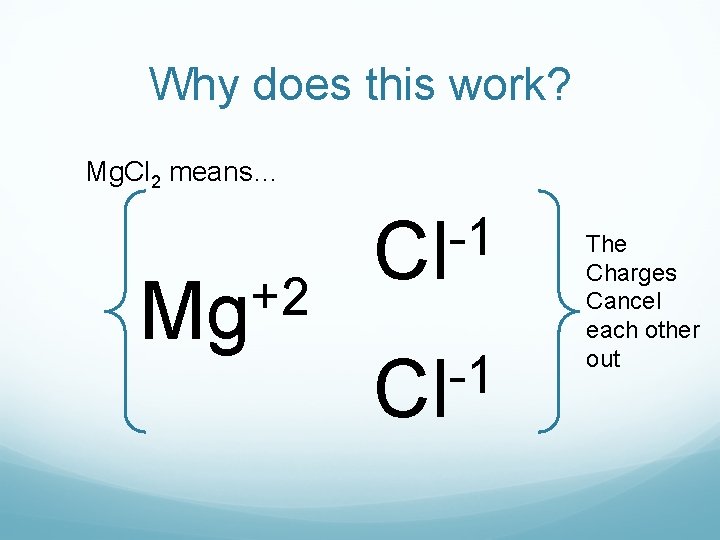
Why does this work? Mg. Cl 2 means… +2 Mg -1 Cl The Charges Cancel each other out

More Examples Ex) Barium and Oxygen Ions Ex) Sodium and Chlorine Ions

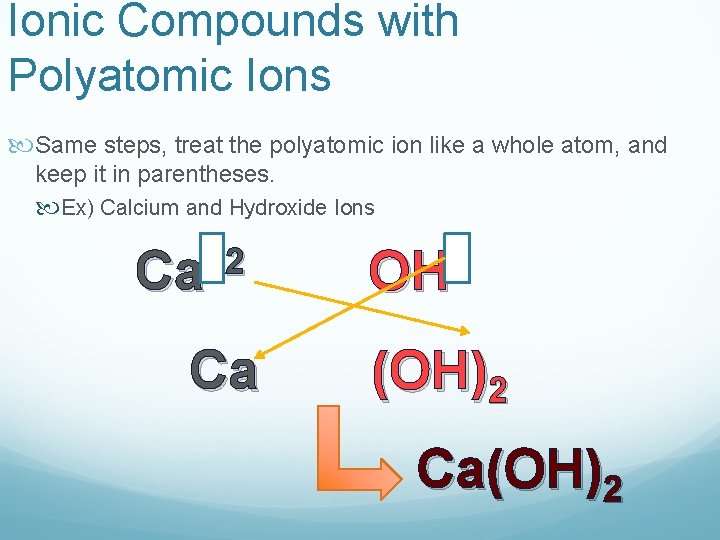
Ionic Compounds with Polyatomic Ions Same steps, treat the polyatomic ion like a whole atom, and keep it in parentheses. Ex) Calcium and Hydroxide Ions +2 Ca Ca OH (OH)2 Ca(OH)2

Intro to Bonding Pt. 3 Ionic Compounds Naming Ionic Compounds: First write the full name of the metal atom Then write the full name of the non-metal atoms, but change the ending to (-ide) For Polyatomic Ions just write the full name of each listed on table E Try These: Li. Cl Ca(OH)2 Al 2(SO 4)3

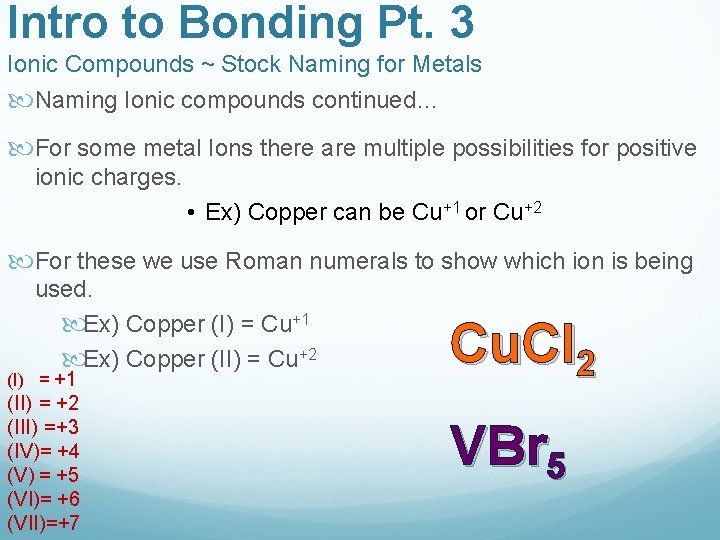
Intro to Bonding Pt. 3 Ionic Compounds ~ Stock Naming for Metals Naming Ionic compounds continued… For some metal Ions there are multiple possibilities for positive ionic charges. • Ex) Copper can be Cu+1 or Cu+2 For these we use Roman numerals to show which ion is being used. Ex) Copper (I) = Cu+1 Ex) Copper (II) = Cu+2 (I) = +1 (II) = +2 (III) =+3 (IV)= +4 (V) = +5 (VI)= +6 (VII)=+7 Cu. Cl 2 VBr 5

More Examples ~ Give the names that go with the following Ionic Compounds * Be sure to include roman numerals for metals with more than one possible ionic charge… Ti. Cl 3 Pb. S Fe 2 O 3 Mn(SO 4)2

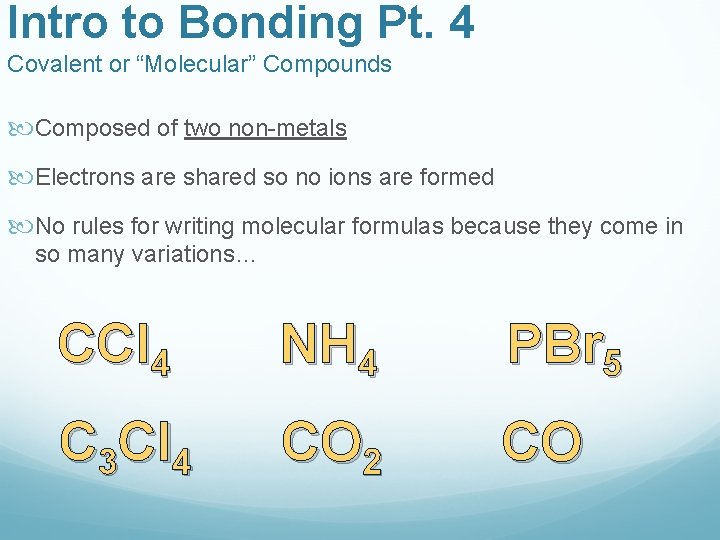
Intro to Bonding Pt. 4 Covalent or “Molecular” Compounds Composed of two non-metals Electrons are shared so no ions are formed No rules for writing molecular formulas because they come in so many variations… CCl 4 NH 4 PBr 5 C 3 Cl 4 CO 2 CO

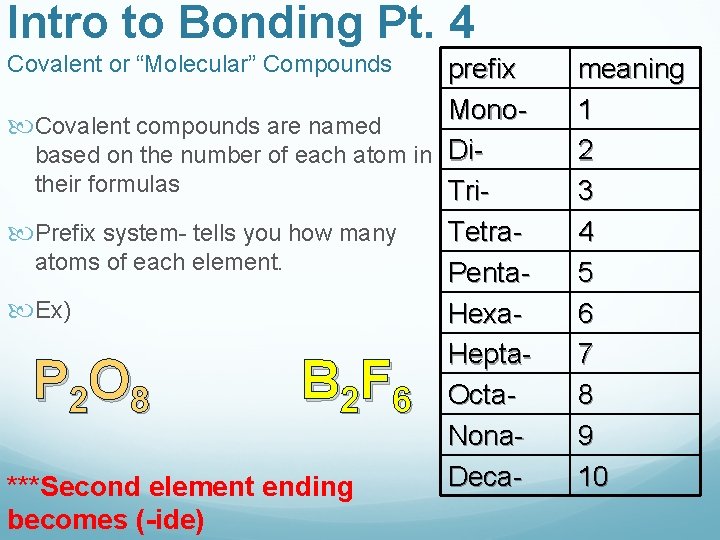
Intro to Bonding Pt. 4 Covalent or “Molecular” Compounds prefix Mono Covalent compounds are named based on the number of each atom in Ditheir formulas Tri Prefix system- tells you how many Tetraatoms of each element. Penta Ex) Hexa. Hepta 2 8 2 6 Octa. Nona. Deca***Second element ending becomes (-ide) PO BF meaning 1 2 3 4 5 6 7 8 9 10

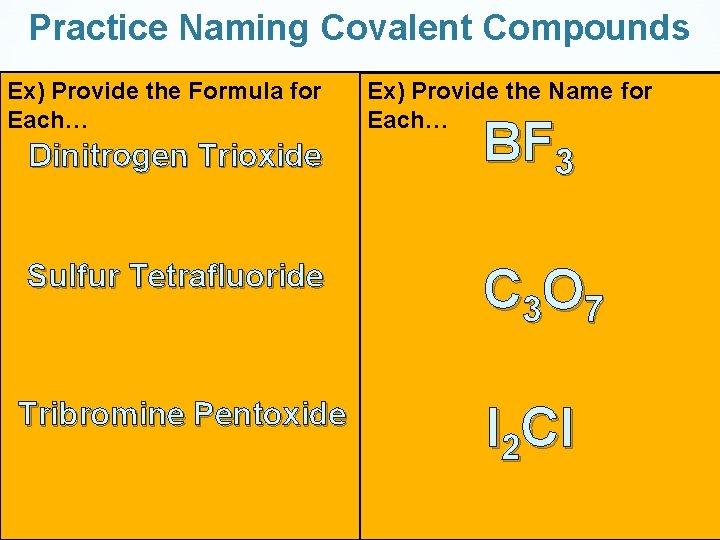
Practice Naming Covalent Compounds Ex) Provide the Formula for Each… Dinitrogen Trioxide Ex) Provide the Name for Each… BF 3 Sulfur Tetrafluoride C 3 O 7 Tribromine Pentoxide I 2 Cl

Key Terms Review Physical Change- When matter changes in appearance but no new substances are formed Chemical Change- A new substance is produced as chemical formulas are rearranged. *We write Chemical Equations to represent chemical changes

Chemical Reactions Pt. 1 ~Chemical Equations Chemical reactions are written as equations with (+) signs used to separate different chemicals. Ex) Word Equation Carbon + Oxygen Carbon Dioxide Ex) Formula Equation *The Arrow is like an equal sign meaning “produces” or “yields” C(s) + O 2(g) CO 2(g) Reactants- are the starting chemicals to the left of the arrow Products- are the end result of the reaction to the right of the arrow.

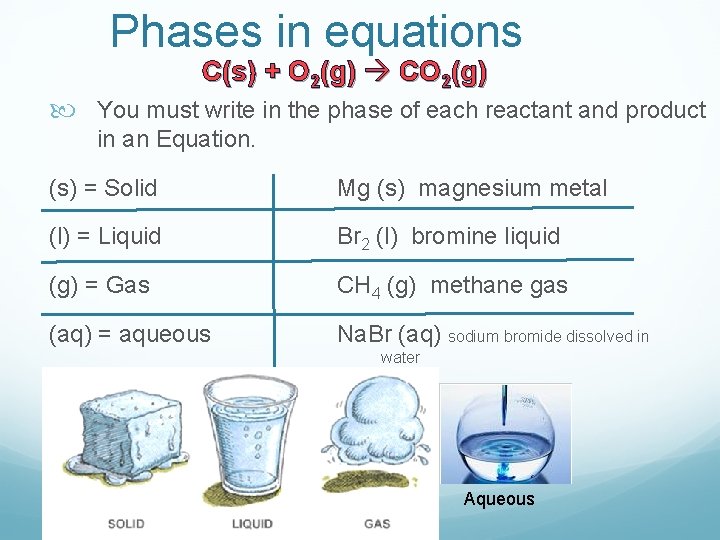
Phases in equations C(s) + O 2(g) CO 2(g) You must write in the phase of each reactant and product in an Equation. (s) = Solid Mg (s) magnesium metal (l) = Liquid Br 2 (l) bromine liquid (g) = Gas CH 4 (g) methane gas (aq) = aqueous Na. Br (aq) sodium bromide dissolved in water Aqueous

Chemical Reactions Pt. 2 ~ Reaction Law All chemical reactions follow… Law of Conservation of Mass – the reactants must have the same amount of atoms as the product. Law of Conservation of Energy – Energy can either be absorbed or released in a reaction based on the change in surrounding temperatures

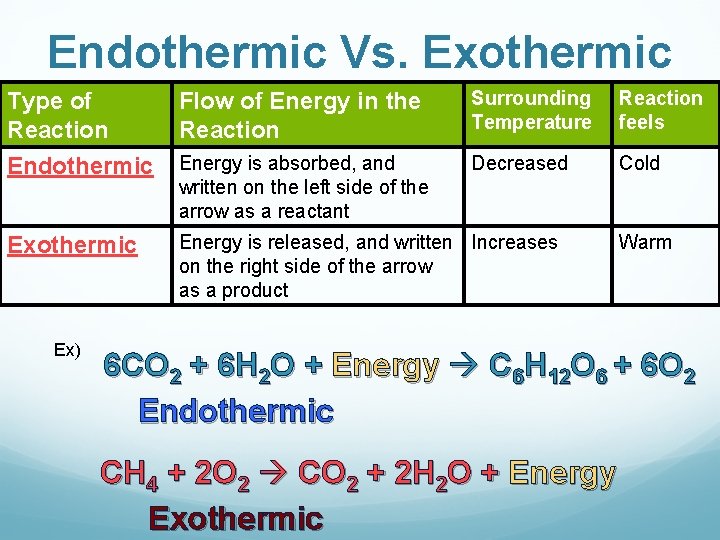
Endothermic Vs. Exothermic Type of Reaction Endothermic Flow of Energy in the Reaction Surrounding Temperature Reaction feels Energy is absorbed, and written on the left side of the arrow as a reactant Decreased Cold Exothermic Energy is released, and written Increases on the right side of the arrow as a product Ex) Warm 6 CO 2 + 6 H 2 O + Energy C 6 H 12 O 6 + 6 O 2 Endothermic CH 4 + 2 O 2 CO 2 + 2 H 2 O + Energy Exothermic

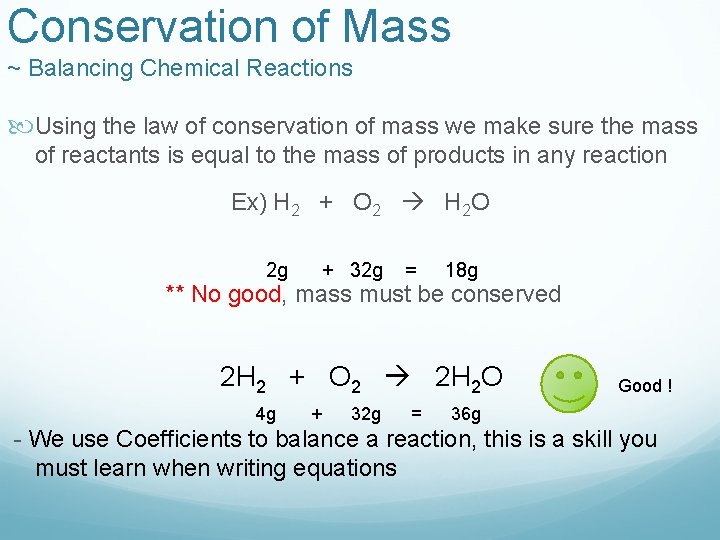
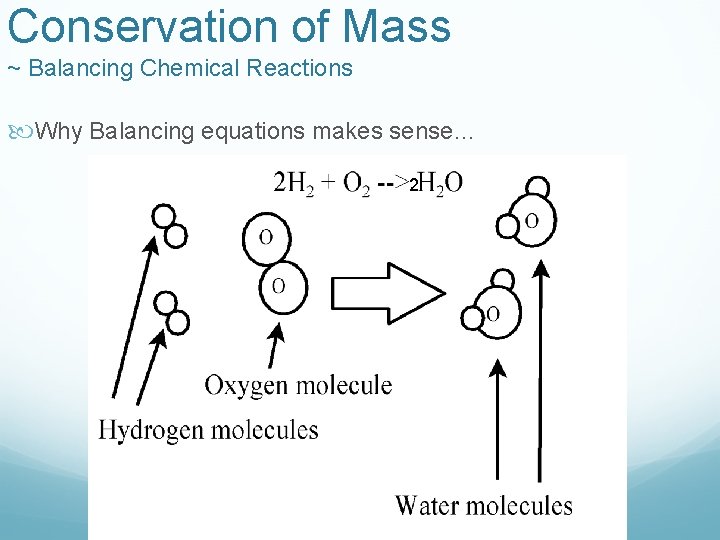
Conservation of Mass ~ Balancing Chemical Reactions Using the law of conservation of mass we make sure the mass of reactants is equal to the mass of products in any reaction Ex) H 2 + O 2 H 2 O 2 g + 32 g = 18 g ** No good, mass must be conserved 2 H 2 + O 2 2 H 2 O 4 g + 32 g = Good ! 36 g - We use Coefficients to balance a reaction, this is a skill you must learn when writing equations

Conservation of Mass ~ Balancing Chemical Reactions Why Balancing equations makes sense… 2

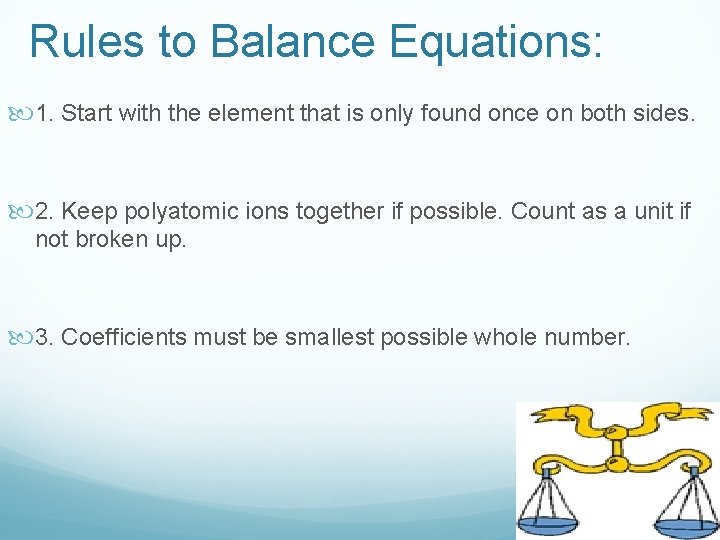
Rules to Balance Equations: 1. Start with the element that is only found once on both sides. 2. Keep polyatomic ions together if possible. Count as a unit if not broken up. 3. Coefficients must be smallest possible whole number.

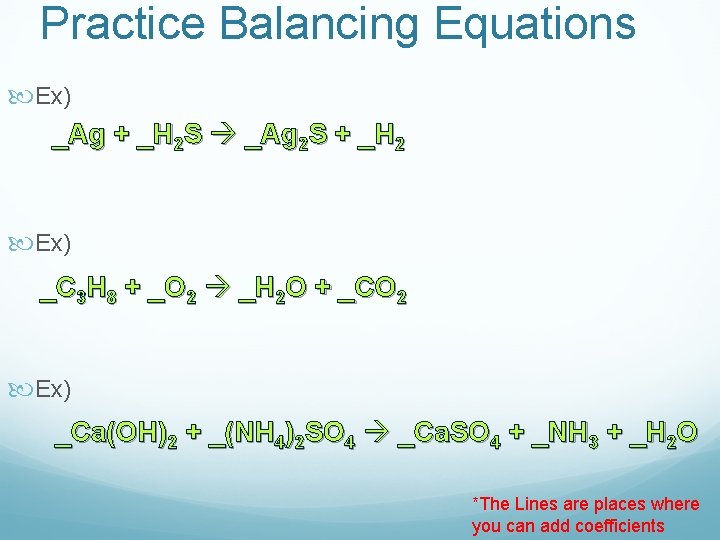
Practice Balancing Equations Ex) _Ag + _H 2 S _Ag 2 S + _H 2 Ex) _C 3 H 8 + _O 2 _H 2 O + _CO 2 Ex) _Ca(OH)2 + _(NH 4)2 SO 4 _Ca. SO 4 + _NH 3 + _H 2 O *The Lines are places where you can add coefficients

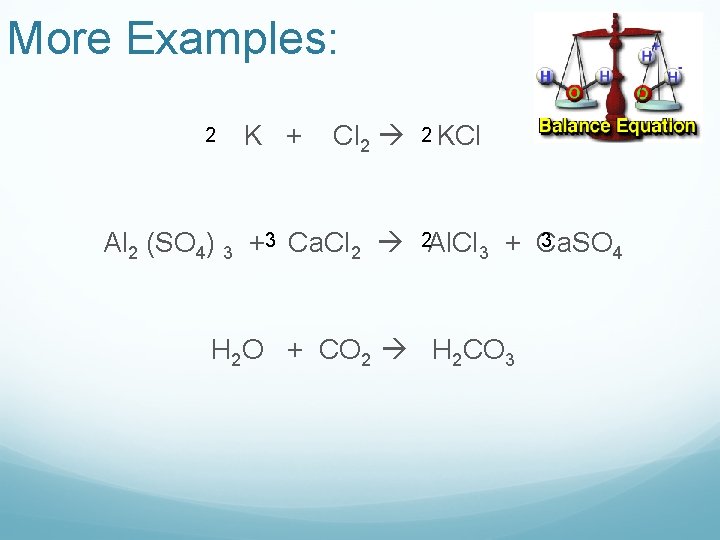
More Examples: 2 K + Cl 2 2 KCl 3 Al 2 (SO 4) 3 +3 Ca. Cl 2 2 Al. Cl 3 + Ca. SO 4 H 2 O + CO 2 H 2 CO 3

Types of Chemical Reactions In basic chemistry all reactions can be classified into 4 main reaction types… • Synthesis Reaction • Decomposition Reaction • Single Replacement Reaction • Double Replacement Reaction Each chemical reaction follows a specific pattern from reactants to products. If you recognize the pattern you will be able to identify the type of reaction.

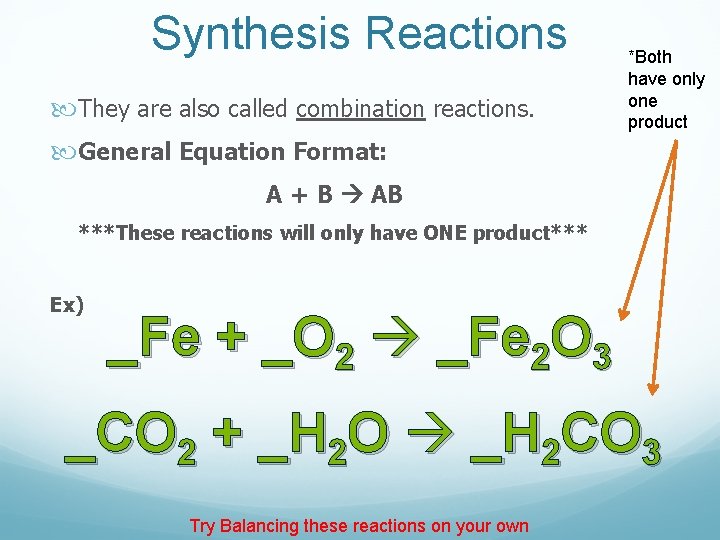
Synthesis Reactions They are also called combination reactions. *Both have only one product General Equation Format: A + B AB ***These reactions will only have ONE product*** Ex) _Fe + _O 2 _Fe 2 O 3 _CO 2 + _H 2 O _H 2 CO 3 Try Balancing these reactions on your own

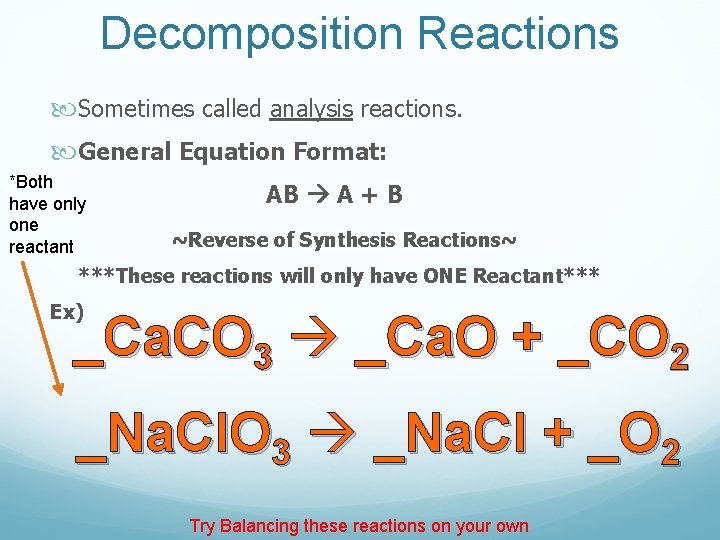
Decomposition Reactions Sometimes called analysis reactions. General Equation Format: *Both have only one reactant AB A + B ~Reverse of Synthesis Reactions~ ***These reactions will only have ONE Reactant*** Ex) _Ca. CO 3 _Ca. O + _CO 2 _Na. Cl. O 3 _Na. Cl + _O 2 Try Balancing these reactions on your own

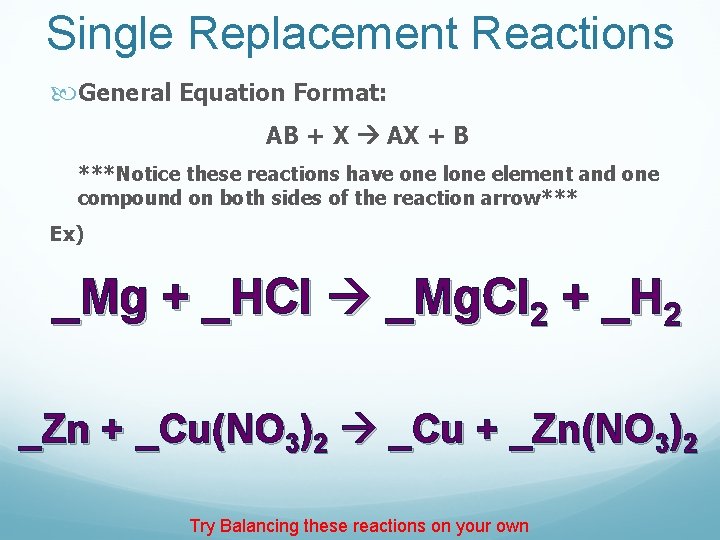
Single Replacement Reactions General Equation Format: AB + X AX + B ***Notice these reactions have one lone element and one compound on both sides of the reaction arrow*** Ex) _Mg + _HCl _Mg. Cl 2 + _H 2 _Zn + _Cu(NO 3)2 _Cu + _Zn(NO 3)2 Try Balancing these reactions on your own

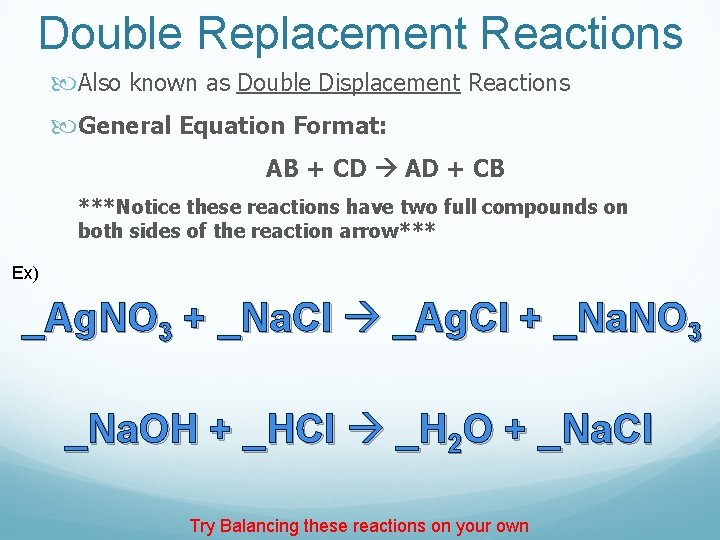
Double Replacement Reactions Also known as Double Displacement Reactions General Equation Format: AB + CD AD + CB ***Notice these reactions have two full compounds on both sides of the reaction arrow*** Ex) _Ag. NO 3 + _Na. Cl _Ag. Cl + _Na. NO 3 _Na. OH + _HCl _H 2 O + _Na. Cl Try Balancing these reactions on your own

Combustion Reactions General Equation Format: C? H? + O 2 CO 2 + H 2 O ***Notice these reactions always produce the same products (H 2 O and CO 2)*** Ex) _CH 4 + _O 2 _CO 2 + _H 2 O _C 3 H 8 + _O 2 _CO 2 + _H 2 O

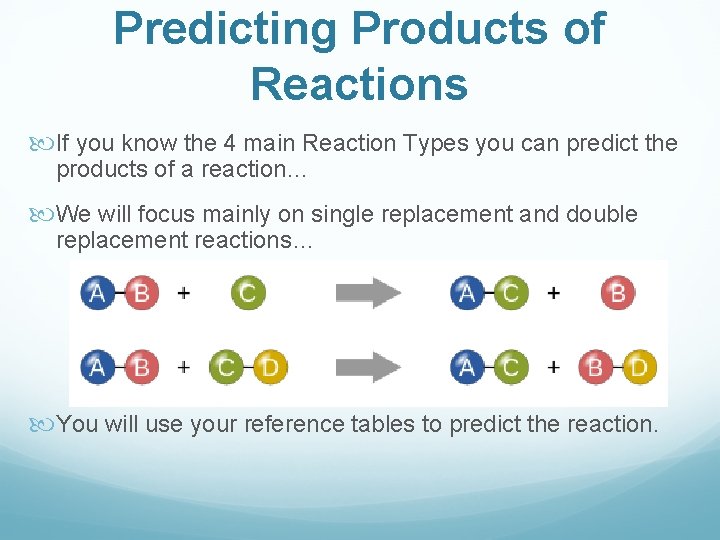
Predicting Products of Reactions If you know the 4 main Reaction Types you can predict the products of a reaction… We will focus mainly on single replacement and double replacement reactions… You will use your reference tables to predict the reaction.

Single Replacement Reactions In a single replacement reaction the lone element will replace the element in compound that has the same ionic charge. Zn + HCl “Zn” forms a positive ion so it will replace “H” the positive ion in HCl. Br 2 + HCl “Br” forms a negative ion so it will replace “Cl” the negative ion in HCl. These reactions don’t always occur “spontaneously”-

Single Replacement Example: Zn + 2 HCl Zn. Cl 2 + H 2 If the lone element is more reactive (active) than the element it’s supposed to replace then the reaction will occur spontaneously If the element is less reactive then the reaction will not occur (non -spontaneous) In the reaction above, Zinc is more reactive than Hydrogen so the Zinc will replace the Hydrogen in the compound to form Zinc Chloride. The Hydrogen would leave the reaction as a gas. (H 2 because it is a Br. INCl. HOF element).

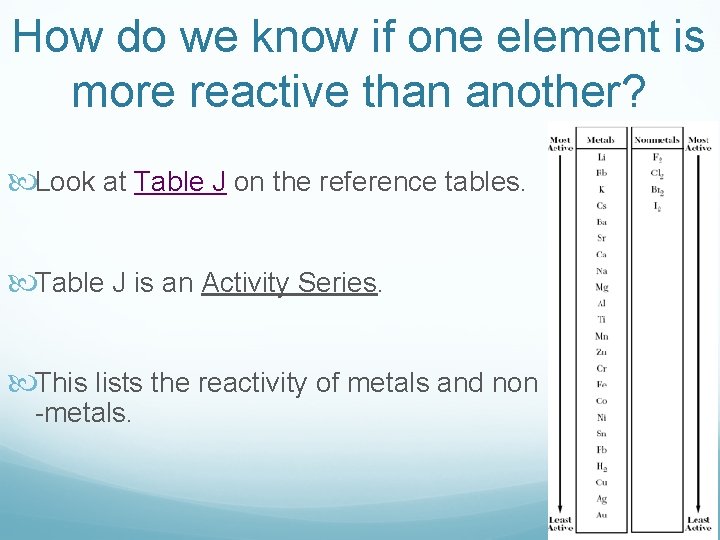
How do we know if one element is more reactive than another? Look at Table J on the reference tables. Table J is an Activity Series. This lists the reactivity of metals and non -metals.

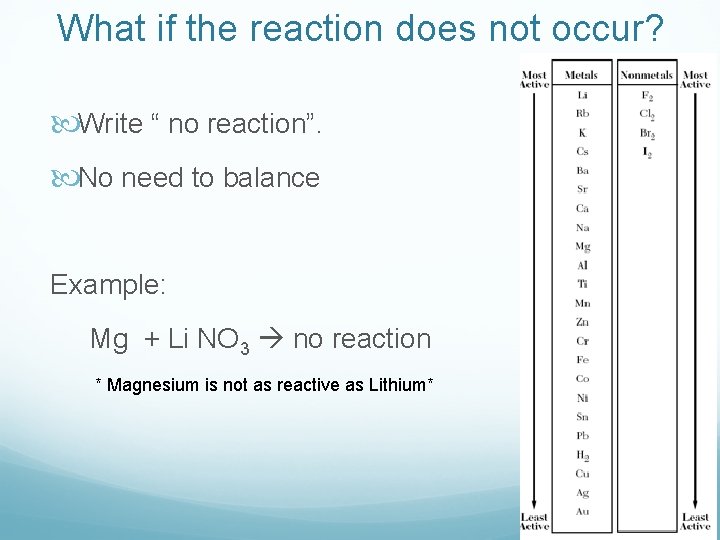
What if the reaction does not occur? Write “ no reaction”. No need to balance Example: Mg + Li NO 3 no reaction * Magnesium is not as reactive as Lithium*

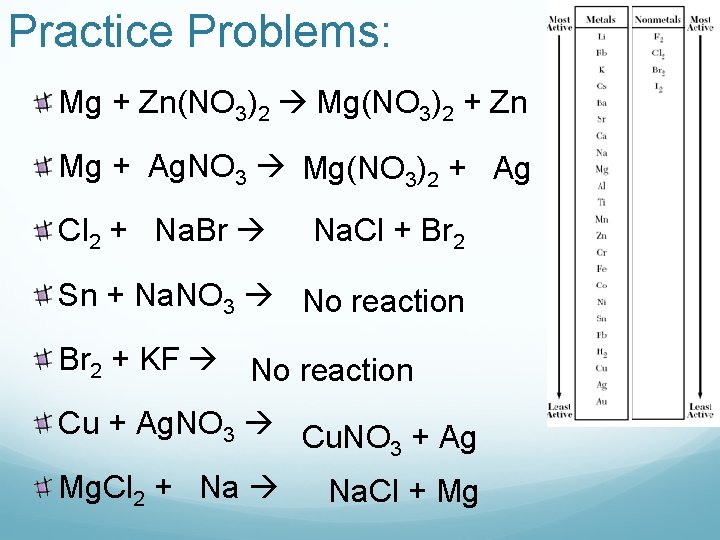
Practice Problems: Mg + Zn(NO 3)2 Mg(NO 3)2 + Zn Mg + Ag. NO 3 Mg(NO 3)2 + Ag Cl 2 + Na. Br Na. Cl + Br 2 Sn + Na. NO 3 No reaction Br 2 + KF No reaction Cu + Ag. NO 3 Cu. NO + Ag 3 Mg. Cl 2 + Na Na. Cl + Mg

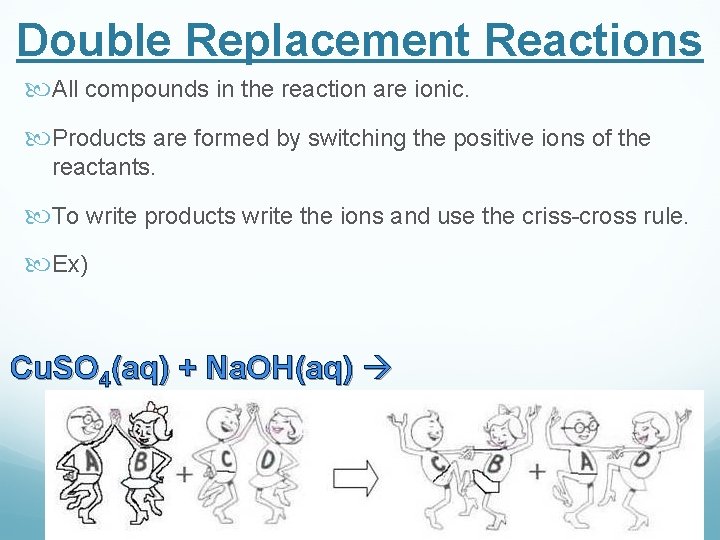
Double Replacement Reactions All compounds in the reaction are ionic. Products are formed by switching the positive ions of the reactants. To write products write the ions and use the criss-cross rule. Ex) Cu. SO 4(aq) + Na. OH(aq)

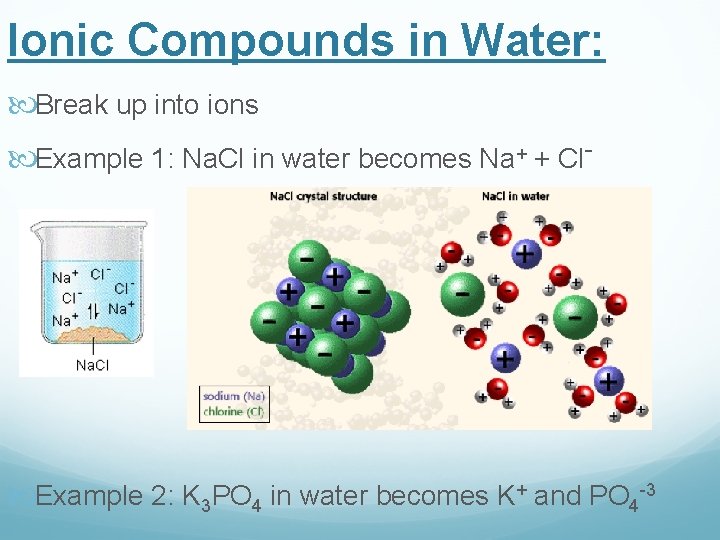
Ionic Compounds in Water: Break up into ions Example 1: Na. Cl in water becomes Na+ + Cl- Example 2: K 3 PO 4 in water becomes K+ and PO 4 -3

How Can we Tell if the DDR Occurs? DDR “go to completion” or occur if a solid, liquid, or gas forms: (s) = use Table F in reference table to find out if the new compounds are “insoluble” or solid (l) = H 2 O (only if water forms “HOH”) (g) = H 2, Cl 2, etc. any diatomic elements **If all products are still (aq) or “aqueous” then no reaction happens. KNO 3 (aq) + Ca. I 2 (aq) KI (aq) + Ca(NO 3)2 (aq) No Reaction

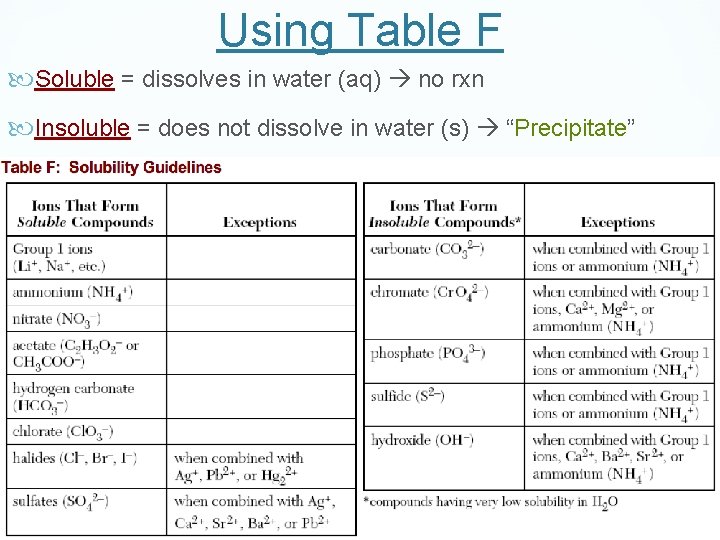
Using Table F Soluble = dissolves in water (aq) no rxn Insoluble = does not dissolve in water (s) “Precipitate”

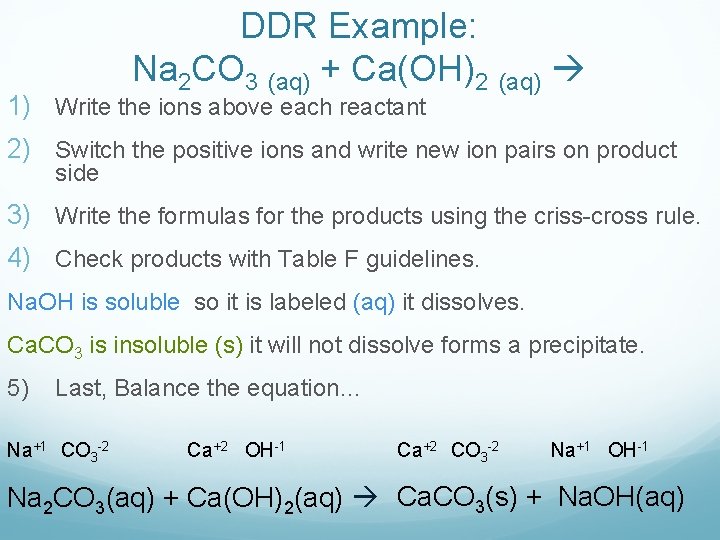
DDR Example: Na 2 CO 3 (aq) + Ca(OH)2 (aq) 1) Write the ions above each reactant 2) Switch the positive ions and write new ion pairs on product side 3) Write the formulas for the products using the criss-cross rule. 4) Check products with Table F guidelines. Na. OH is soluble so it is labeled (aq) it dissolves. Ca. CO 3 is insoluble (s) it will not dissolve forms a precipitate. 5) Last, Balance the equation… Na+1 CO 3 -2 Ca+2 OH-1 Ca+2 CO 3 -2 Na+1 OH-1 Na 2 CO 3(aq) + Ca(OH)2(aq) Ca. CO 3(s) + Na. OH(aq)

Try these… Write products and predict if it “goes to completion” NH 4+1 CO 3 -2 Ca+2 Cl-1 (NH 4)2 CO 3 (aq) + Ca. Cl 2(aq) K+1 NO 3 -1 Ca+2 I-1 KNO 3 (aq) + Ca. I 2 (aq) Na+1 OH-1 H+1 SO 4 -2 Na. OH(aq) + H 2 SO 4 (aq) Cu+2 SO 4 -2 Na+1 OH-1 Cu. SO 4 (aq) + Na. OH (aq)