How Much Caffeine Is in My Soft Drink















- Slides: 22

+ How Much Caffeine Is in My Soft Drink? The Determination of Caffeine Content in Soft Drinks Using Gas Chromatography/Mass Spectrometry (GC/MS)

+ How Caffeine Is Ingested n Certain soft drinks, such as Pepsi, Coke, Mountain Dew) n Certain teas n Chocolate, including hot chocolate drinks n Coffee n Over-the-counter stimulants that help you stay awake, such as No. Doz, Vivarin, Caffedrine, and others Source: “Caffeine overdose: Medline. Plus Medical Encyclopedia” http: //www. nlm. nih. gov/medlineplus/ency/article/002579. htm

+ Effects of Caffeine on Humans Symptoms in adults may include: n n n n Breathing trouble Changes in alertness Confusion Convulsions Diarrhea Dizziness Fever Hallucinations n n n n Increased thirst Irregular heartbeat Muscle twitching Rapid heartbeat Sleeping trouble Urination - increased Vomiting Symptoms in babies may include: n n Muscles are very tense, then very relaxed Nausea Rapid, deep breathing Rapid heartbeat n n n Shock Tremors Vomiting Source: “Caffeine overdose: Medline. Plus Medical Encyclopedia” http: //www. nlm. nih. gov/medlineplus/ency/article/002579. htm

http: //commons. wikimedia. org/wiki/File: Main_symptoms_of_ Caffeine_overdose. png Source: Main symptoms of Caffeine overdose (See also Wikipedia: Caffeine#Caffeine_intoxication). References: Caffeine (Systemic). Medline. Plus (2000 -05 -25). Archived from the original on 2007 -02 -23. Retrieved on 2006 -08 -12. +

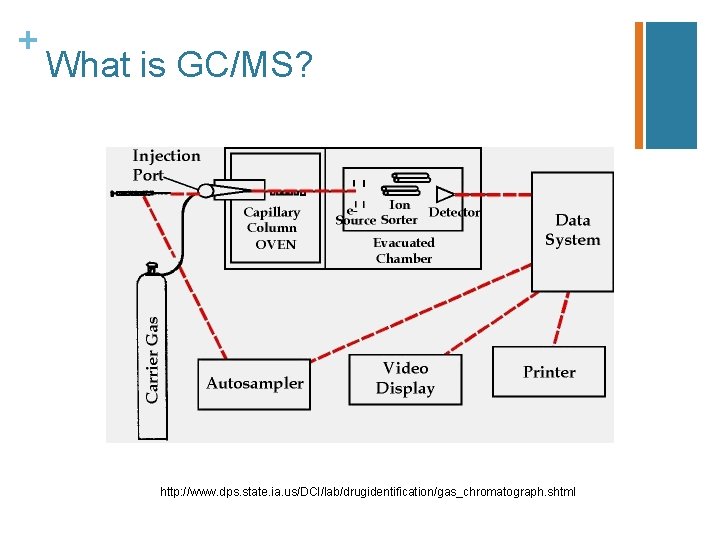
+ What do the letters GC/MS stand for? n. Gas n. Chromatography n. Mass n. Spectrometry

+ What is GC/MS used for? n If you want to identify a compound, you take a solid sample and dissolve it in a solvent. The gas chromatography machine vaporizes the mixture into gas phase. The gas particles are then sent through a mass spectrometer, which separates ions by their mass-tocharge (m/Z) ratios. n Basically, you can analyze a very small sample of a compound and identify it using GC/MS. n It is used to identify pesticides, drugs, accelerants (like in arson investigations), etc.

+ What is GC/MS? http: //www. dps. state. ia. us/DCI/lab/drugidentification/gas_chromatograph. shtml

GC/MS + Courtesy of: http: //qb 3. berkeley. edu/msf/images/GCMS_500. jpg Access GC/MS machine at U. C. Berkeley n Open

+ Electron Impact (EI) Technique GC/MS machine at U. C. Berkeley Mass Spectrometry Facility Your soft drink samples will be analyzed using EI on this machine.

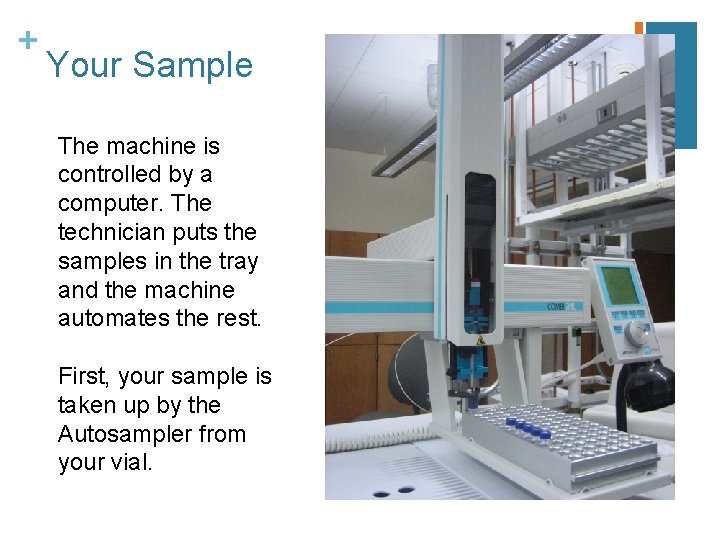
+ Your Sample The machine is controlled by a computer. The technician puts the samples in the tray and the machine automates the rest. First, your sample is taken up by the Autosampler from your vial.

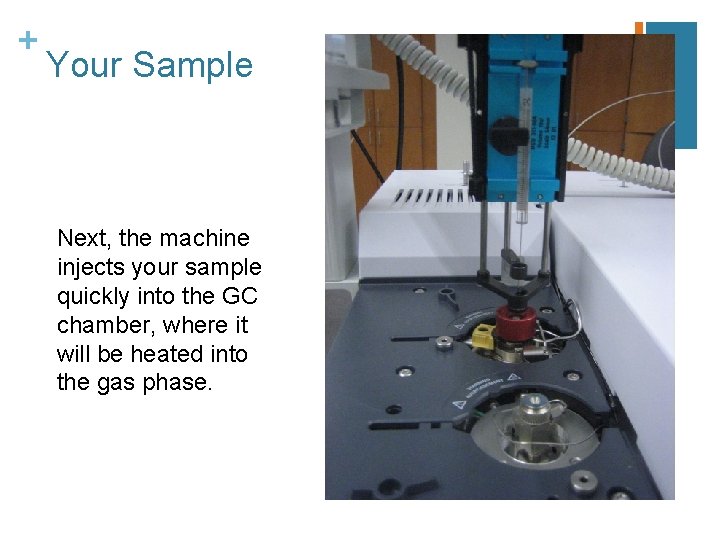
+ Your Sample Next, the machine injects your sample quickly into the GC chamber, where it will be heated into the gas phase.

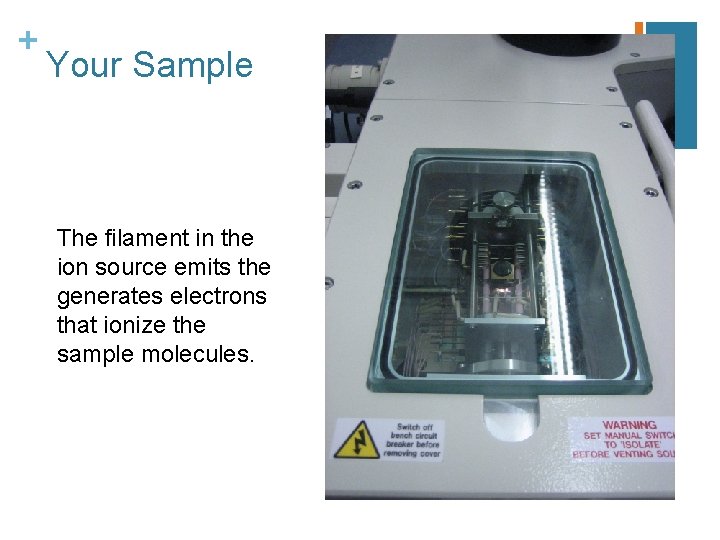
+ Your Sample The filament in the ion source emits the generates electrons that ionize the sample molecules.

+ Concepts that Will Help with the Separation Techniques n density n solubility n polarity

+ The Separation Process ① Combine 1 m. L of your soft drink, 100 µL of deuterated caffeine, and 0. 15 g of sodium carbonate into a capped test tube. Shake gently to dissolve all of the sodium carbonate. ② Pipet 500 µL of rubbing alcohol into the test tube. Cap and invert gently twice, venting to relieve pressure as needed. Let test tube stand for 5 minutes. ③ Withdraw the top isopropanol layer from the test tube and keep extracts in a beaker.

+ The Separation Process ④ Repeat washings twice more: adding 500 µL of rubbing alcohol, inverting, waiting 5 minutes, and withdrawing the upper isopropanol layer each time. ⑤ Combine all isopropanol extracts into the same beaker. Add about 0. 10 g sodium sulfate to the extracts to absorb any leftover water. ⑥ Withdraw 1 m. L of the isopropanol extracts (being careful not to take up any sodium sulfate) and pipet into a GC/MS vial for analysis.

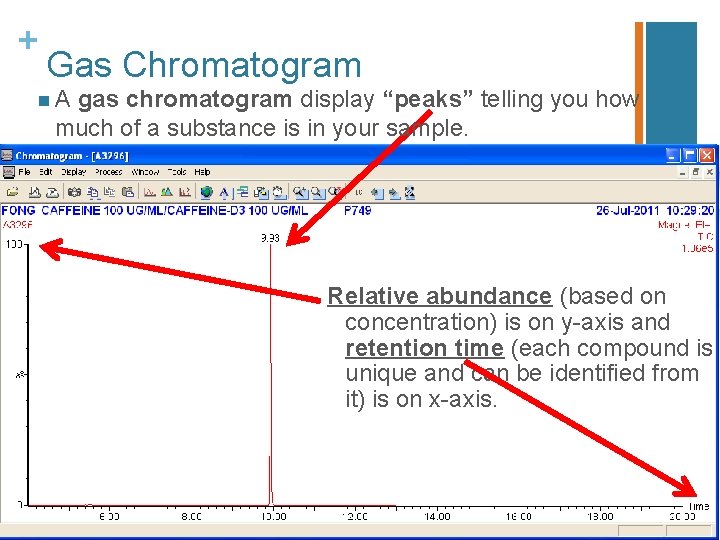
+ Gas Chromatogram n. A gas chromatogram shows the retention time, or how long it takes for a compound separates from a sample. n The gas chromatogram also shows relative abundance, or how much of the compound is present in the sample. Abundance is based on the concentration; so the higher the concentration, the higher the abundance reading of an ion of the compound. The GC/MS machine can usually detect a minimum concentration of 1 ppb, depending on detection method. n Settings most often can be changed according to temperature and time. n See the next slide for the gas chromatogram for caffeine.

+ Gas Chromatogram n. A gas chromatogram display “peaks” telling you how much of a substance is in your sample. Relative abundance (based on concentration) is on y-axis and retention time (each compound is unique and can be identified from it) is on x-axis.

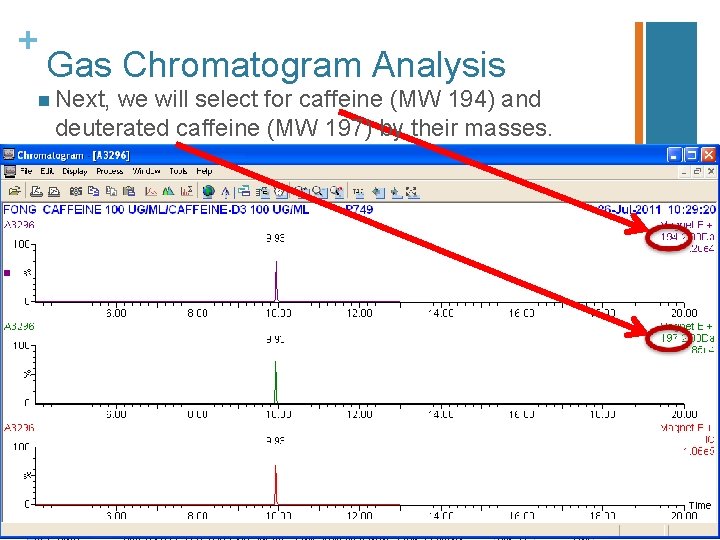
+ Gas Chromatogram Analysis n Next, we will select for caffeine (MW 194) and deuterated caffeine (MW 197) by their masses.

+ Gas Chromatogram Analysis n Finally, we integrate (find area) each of the peaks. We will use the areas to calculate caffeine content.

+ Bibliography n "Caffeine Content of Drinks - How Much Caffeine in Coffee, Soft Drinks, Tea - Amount of Caffeine. " wilstar. com. Wilstar. n. d. Web. 13 June 2011. n "Caffeine Content of Drinks. " energyfiend. com. Energy Fiend, n. d. Web. 20 June 2011. n "Caffeine Lab Pre/Post-Lab Activities. " departments. oxy. edu/tops/. Occidental College. 10 Apr. 2006. Web. 14 June 2011. n "Caffeine from Soda Preparation. " studyblue. com. Study Blue. n. d. Web. 14 June 2011. n "Caffeine in the diet: Medline. Plus Medical Encyclopedia. " nlm. nih. gov. Medline Plus. 23 June 2011. Web. 23 June 2011. n "Caffeine overdose: Medline. Plus Medical Encyclopedia. " nlm. nih. gov. Medline Plus. 23 June 2011. Web. 23 June 2011. n Hill, Devon W. , Mc. Sharry, Brian T. , and Trzupek, Larry S. "Quantitative Analysis by Isotopic Dilution Using Mass Spectroscopy: The Determination of Caffeine by GC-MS. " Journal of Chemical Education 65. 10 (1988): 907 -910. Print. n Horning, Marjorie G. , Boucher, E. Ann, Stafford, Michele, and Horning, Evan C. . "A rapid procedure for the isolation of drugs and drug metabolites from plasma. " Clinica Chimica Act, 37. 3 (1972): 381386. Web. n Laswick, Patty Hall and Laswick, John A. "Caffeine and benzoic acid in soft drinks. " Journal of Chemical Education 49. 10 (1972): 708. Print. n Yang, Min J. , Orton, Maureen L. , and Pawliszyn, Janusz. "Quantitative Determination of Caffeine in Beverages Using a Combined SPME-GC/MS Method. " Journal of Chemical Education 74. 9 (1997): 1130. Print.

+ Special thanks to… n Industry Initiatives for Science and Math Education (IISME) Santa Clara, CA n Syn. BERC —University of California, Berkeley n National Science Foundation (NSF) n Drs. Ulla Andersen, Zhongrui Zhou, Rita Nichiporuk, Tony Iavarone — Mass Spectrometry Facility, University of California, Berkeley n Dante Valdez — College of Chemistry, University of California, Berkeley

+ Credits n Jonathan C. Fong — AP Chemistry Teacher, Lowell High School, San Francisco, CA n Dr. Ulla N. Andersen — Facility Manager, QB 3/Mass Spectrometry Facility, University of California, Berkeley n Evan R. Williams — Professor of Chemistry, University of California, Berkeley