Chemistry Topic 3 The Mathematics of Formulas and








- Slides: 15

Chemistry Topic 3 The Mathematics of Formulas and Equations

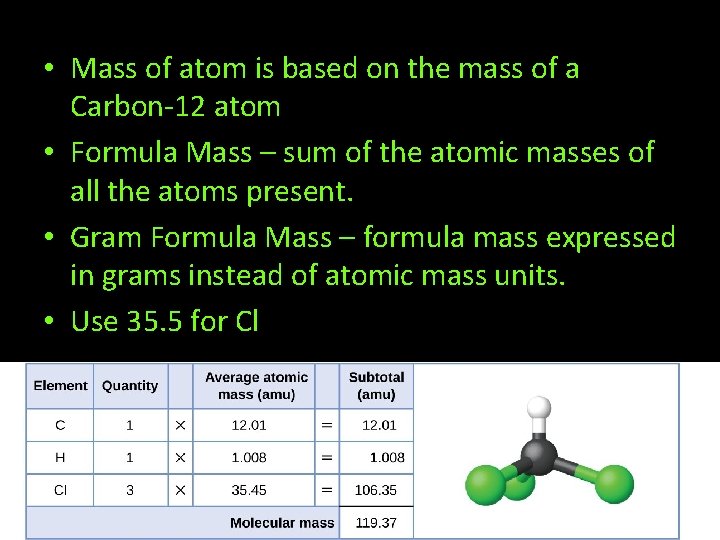
• Mass of atom is based on the mass of a Carbon-12 atom • Formula Mass – sum of the atomic masses of all the atoms present. • Gram Formula Mass – formula mass expressed in grams instead of atomic mass units. • Use 35. 5 for Cl

Find the Gram Formula Masses • • Na O 2 Na. Cl HBr Na. OH Mg 3 N 2 Al 2(SO 4)3

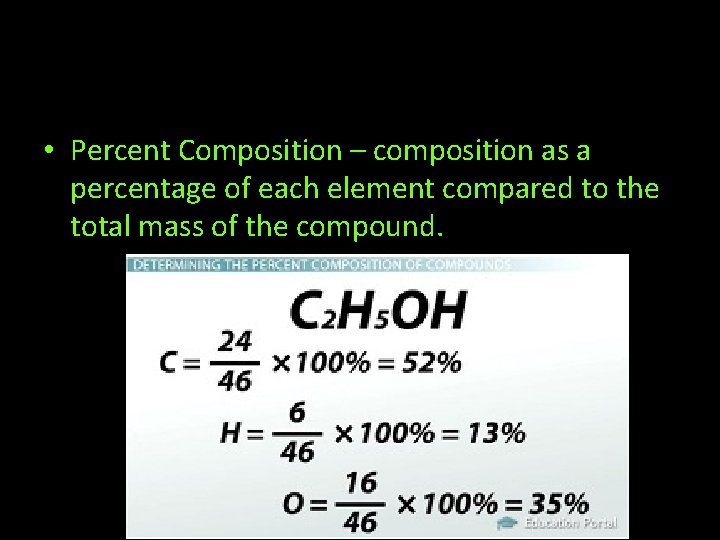
• Percent Composition – composition as a percentage of each element compared to the total mass of the compound.

Find the Percent Composition • • Na O 2 Na. Cl HBr Na. OH Mg 3 N 2 Al 2(SO 4)3

• Hydrates – crystals that contain water. • Anhydrous – substances without water • To calculate % water, treat the water molecule as a single unit.

Calculate % Water • Cu. SO 4∙ 5 H 2 O

11/6 Chemistry Aim: What calculations can we perform with formulas? Obj: SWBAT calculate moles, find molecular formulas, and set up mole ratios Do Now: What is the formula mass of Na. OH? Homework: Worksheet

Moles • Mole = number of atoms of carbon present in 12. 000 g of C-12 • Avogadro’s number = 6. 022 x 1023 – Number of particles in a mole of a substance

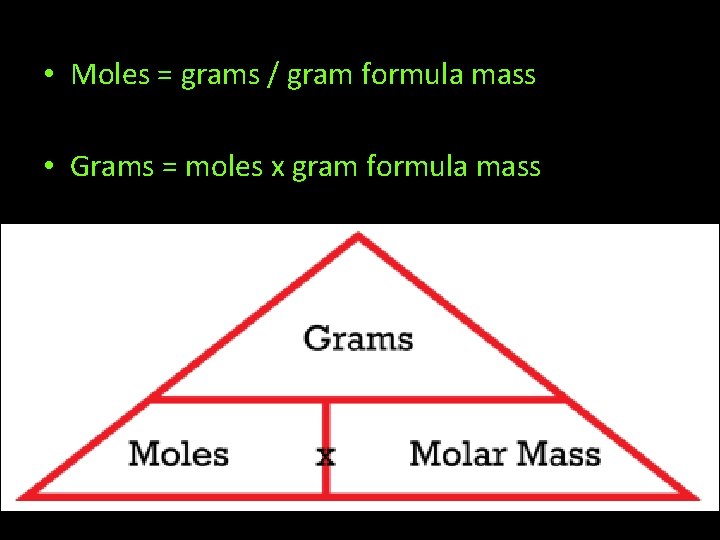
• Moles = grams / gram formula mass • Grams = moles x gram formula mass

• What is the mass in grams of a 8. 4 mole sample of iron? • Convert 0. 45 g of sodium hydroxide, Na. OH to moles. • How many moles of potassium nitrate, KNO 3 are present in a sample with a mass of 85. 2 g. • What is the mass in grams of 0. 94 moles of sodium bicarbonate, Na. HCO 3?

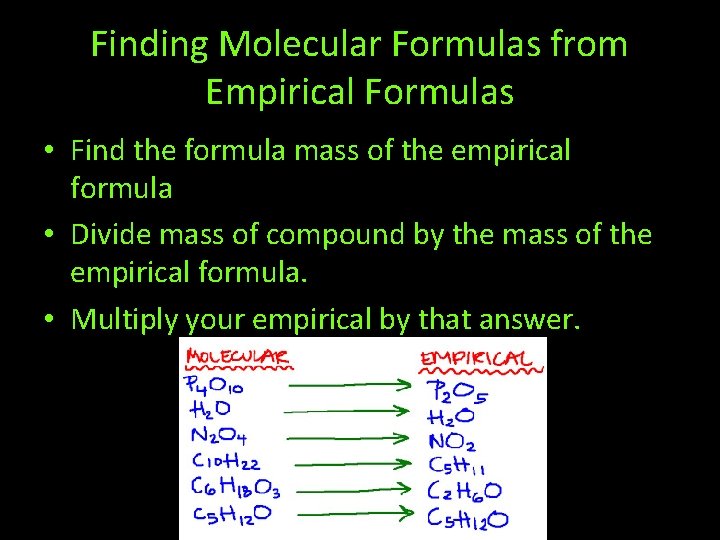
Finding Molecular Formulas from Empirical Formulas • Find the formula mass of the empirical formula • Divide mass of compound by the mass of the empirical formula. • Multiply your empirical by that answer.

• A compound has an empirical formula of CH 2 and a molecular mass of 56 amu. What is its molecular formula? • A compound has an empirical formula of CH and a molecular mass of 78 amu. What is the molecular formula?

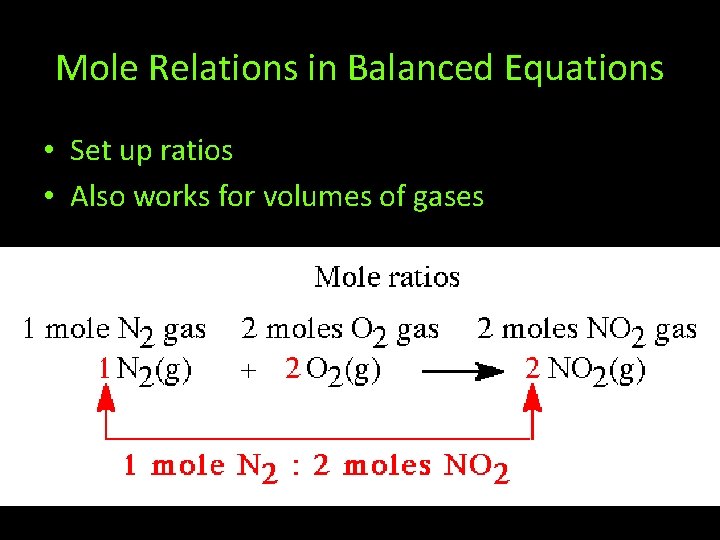
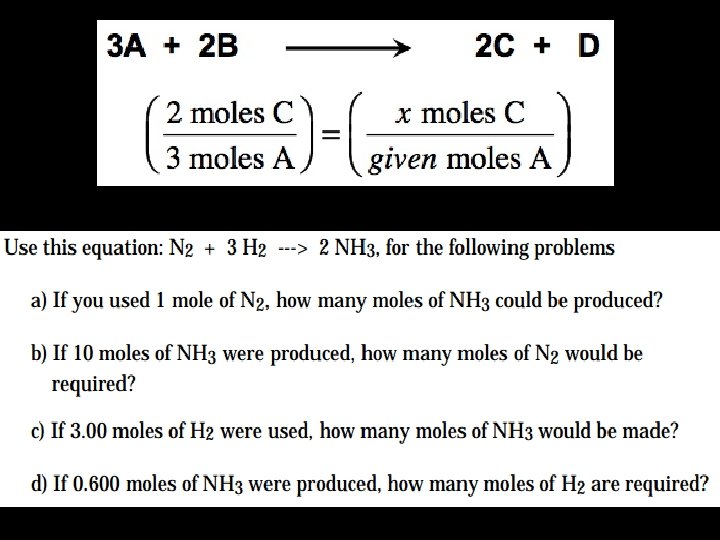
Mole Relations in Balanced Equations • Set up ratios • Also works for volumes of gases

 Topic 3 the mathematics of formulas and equations
Topic 3 the mathematics of formulas and equations Paragraph writing strategies
Paragraph writing strategies Broad topic and specific topic examples
Broad topic and specific topic examples Chapter 9 chemical names and formulas answer key
Chapter 9 chemical names and formulas answer key Organic chemistry formulas
Organic chemistry formulas Science formula list
Science formula list Topic 14 ib chemistry
Topic 14 ib chemistry Topic 11 organic chemistry
Topic 11 organic chemistry Enthalpy of combustion formula ib
Enthalpy of combustion formula ib Topic 2 chemistry
Topic 2 chemistry Ib organic chemistry
Ib organic chemistry Inorganic chemistry vs organic chemistry
Inorganic chemistry vs organic chemistry Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Ng-html
Ng-html Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em