Unit 10 Chapter 17 Thermodynamics Cartoon courtesy of













- Slides: 65

Unit 10 (Chapter 17): Thermodynamics Cartoon courtesy of Nearing. Zero. net

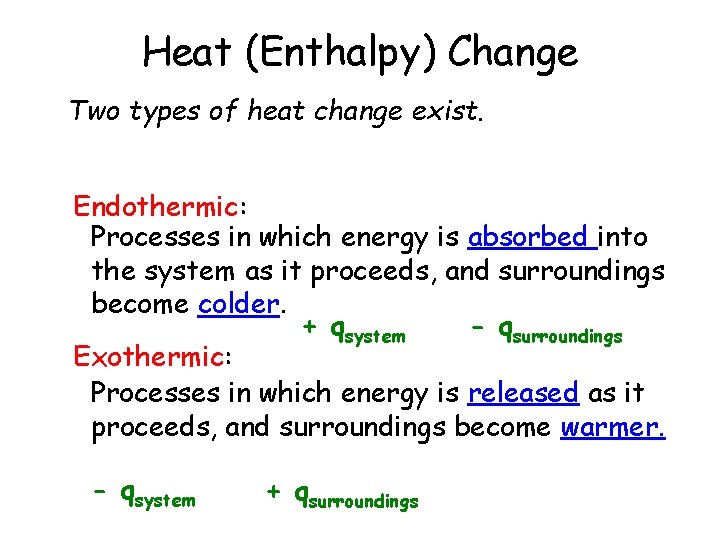
I. Introduction Thermochemistry: Study of heat flow in chemical reactions. Heat (Q) – The energy that transfers from one object to another, because of a temperature difference between them. • flows from warmer cooler object Enthalpy (Δ H) – The heat released or absorbed. • Used interchangeably with “Q”! Joules (J), Kilojoules (k. J), calories (cal), kilocalories (kcal) Food: “C” Energy units: 900 C

Exothermic and Endothermic Processes • In studying heat changes, think of defining these two parts: – the system - the part of the universe on which you focus your attention. – the surroundings - includes everything else in the universe.

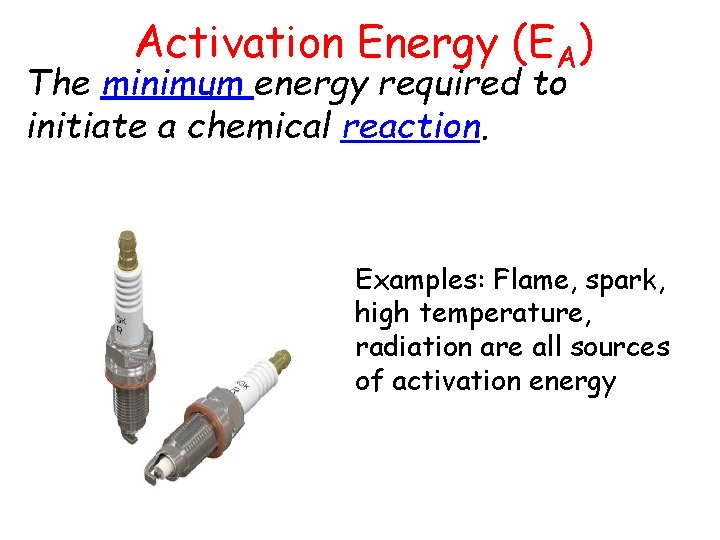
Heat (Enthalpy) Change Two types of heat change exist. Endothermic: Processes in which energy is absorbed into the system as it proceeds, and surroundings become colder. + qsystem - qsurroundings Exothermic: Processes in which energy is released as it proceeds, and surroundings become warmer. - qsystem + qsurroundings

Activation Energy (EA) The minimum energy required to initiate a chemical reaction. Examples: Flame, spark, high temperature, radiation are all sources of activation energy

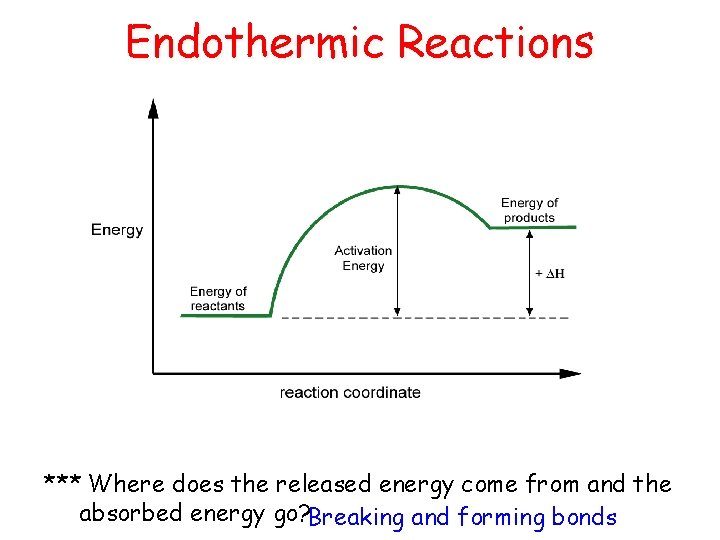
Endothermic Reactions *** Where does the released energy come from and the absorbed energy go? Breaking and forming bonds

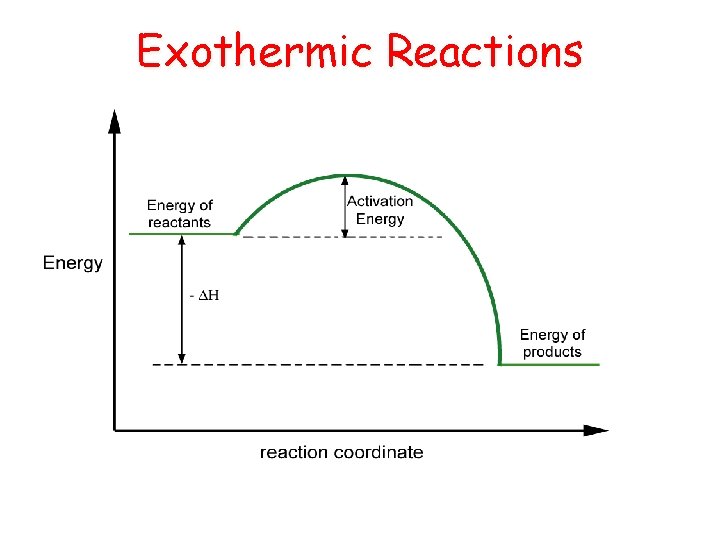
Exothermic Reactions

Exothermic and Endothermic • Fig. 17. 2, page 506 - on the left, the system (the people) gain heat from it’s surroundings (the fire) – this is endothermic • On the right, the system (the body) cools as perspiration evaporates, and heat flows to the surroundings – this is exothermic

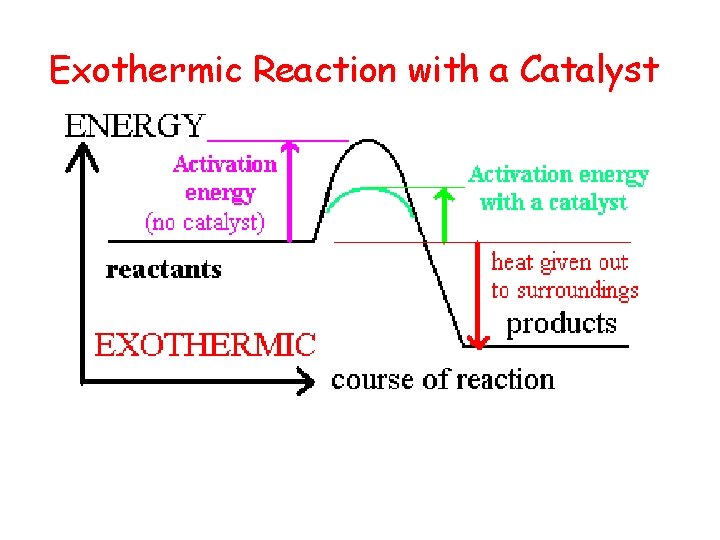
Exothermic and Endothermic • Every reaction has an energy change associated with it. • Exothermic reactions release energy, usually in the form of heat. • Endothermic reactions absorb energy. • Energy is stored in bonds between atoms. 9

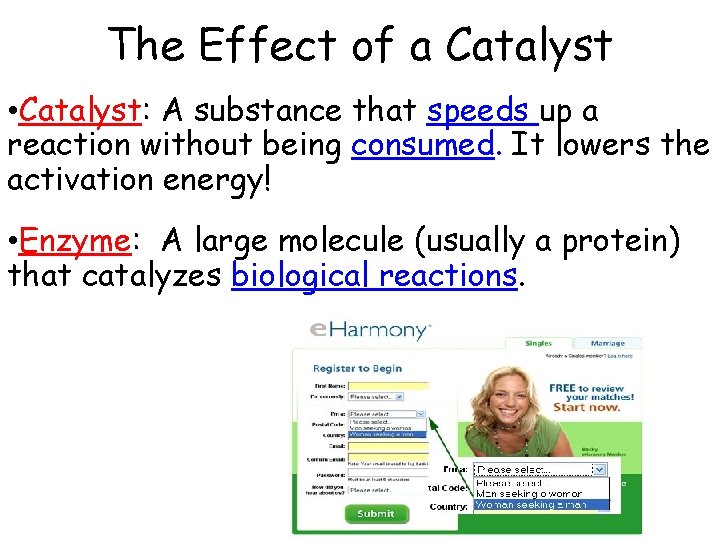
The Effect of a Catalyst • Catalyst: A substance that speeds up a reaction without being consumed. It lowers the activation energy! • Enzyme: A large molecule (usually a protein) that catalyzes biological reactions.

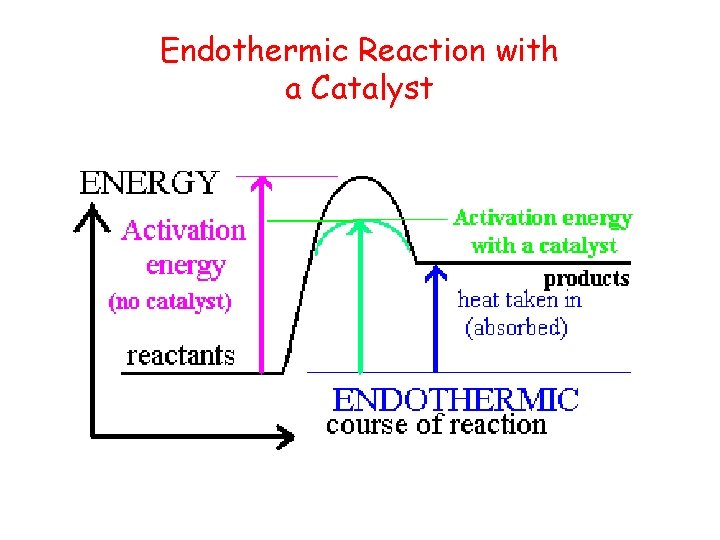
Endothermic Reaction with a Catalyst

Exothermic Reaction with a Catalyst

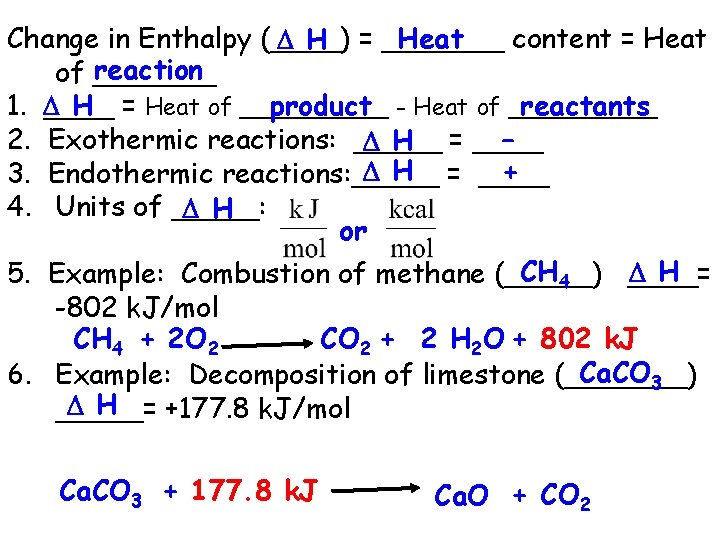
Heat Change in Enthalpy (____) content = Heat H = _______ reaction of _______ H = Heat of _____ product - Heat of _____ reactants 1. ____ 2. Exothermic reactions: _____ H = ____ – H = ____ + 3. Endothermic reactions: _____ 4. Units of _____: H or CH 4 H 5. Example: Combustion of methane (_____) ____= -802 k. J/mol CH 4 + 2 O 2 CO 2 + 2 H 2 O + 802 k. J Ca. CO 3 6. Example: Decomposition of limestone (_______) H _____= +177. 8 k. J/mol Ca. CO 3 + 177. 8 k. J Ca. O + CO 2

H Determination of ______? 3 Techniques 1. Experimentally Formation 2. Using Heats of _____ Hess’s Law 3. Using ______

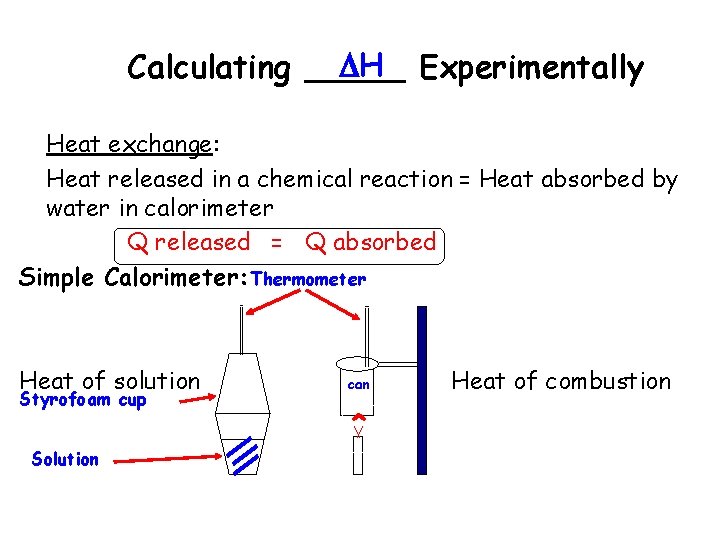
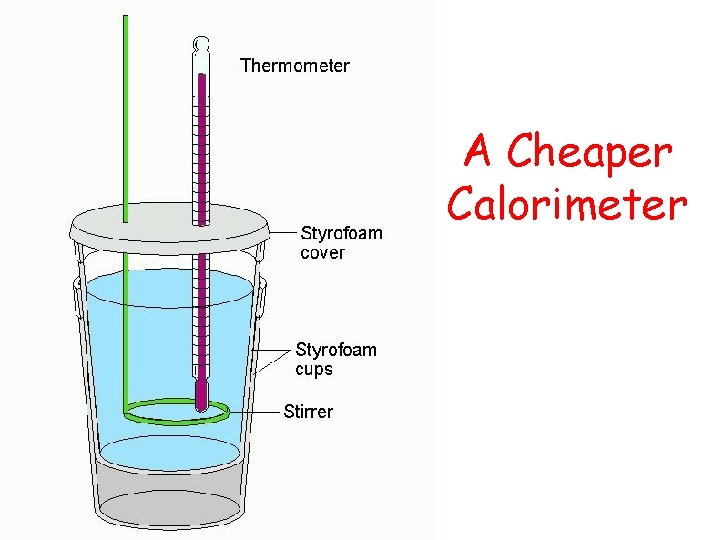
H Experimentally Calculating _____ Heat exchange: Heat released in a chemical reaction = Heat absorbed by water in calorimeter Q released = Q absorbed Simple Calorimeter: Thermometer Heat of solution Styrofoam cup Solution can ^ Heat of combustion

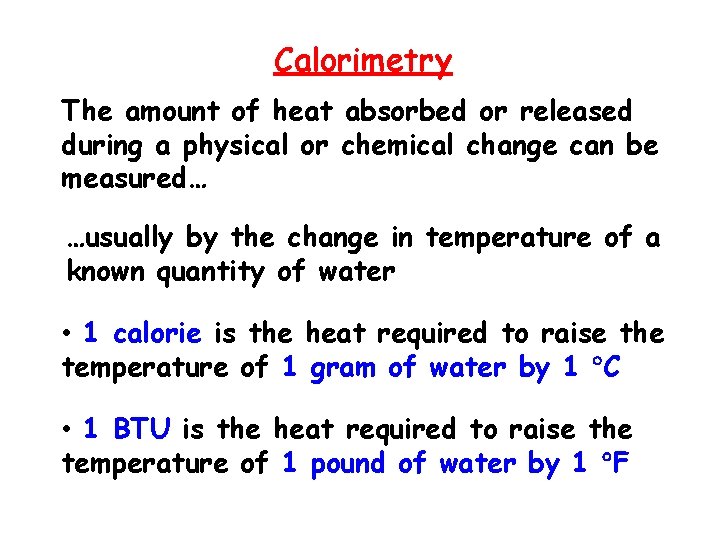
Calorimetry The amount of heat absorbed or released during a physical or chemical change can be measured… …usually by the change in temperature of a known quantity of water • 1 calorie is the heat required to raise the temperature of 1 gram of water by 1 C • 1 BTU is the heat required to raise the temperature of 1 pound of water by 1 F

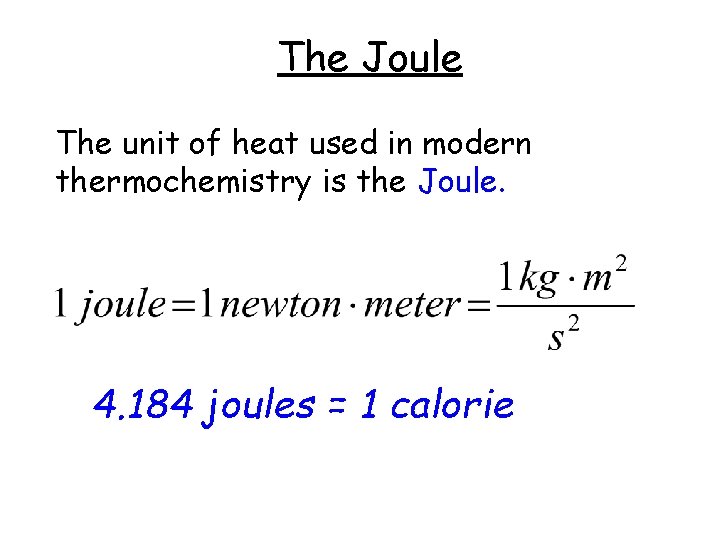
The Joule The unit of heat used in modern thermochemistry is the Joule. 4. 184 joules = 1 calorie

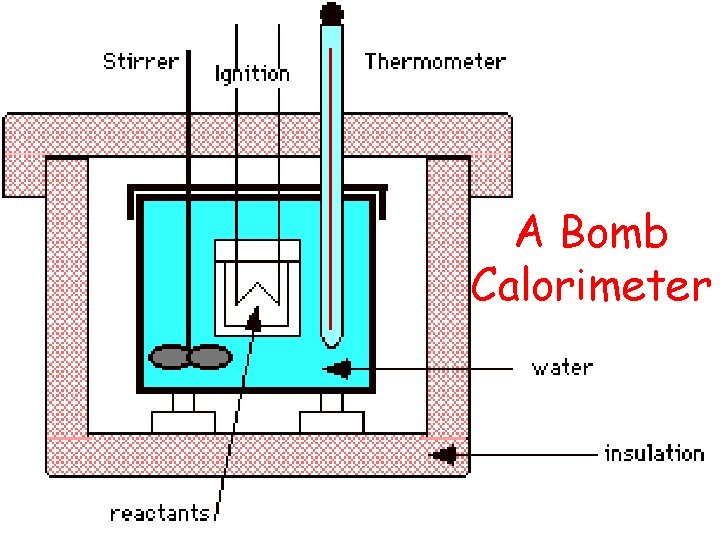
A Bomb Calorimeter

A Cheaper Calorimeter

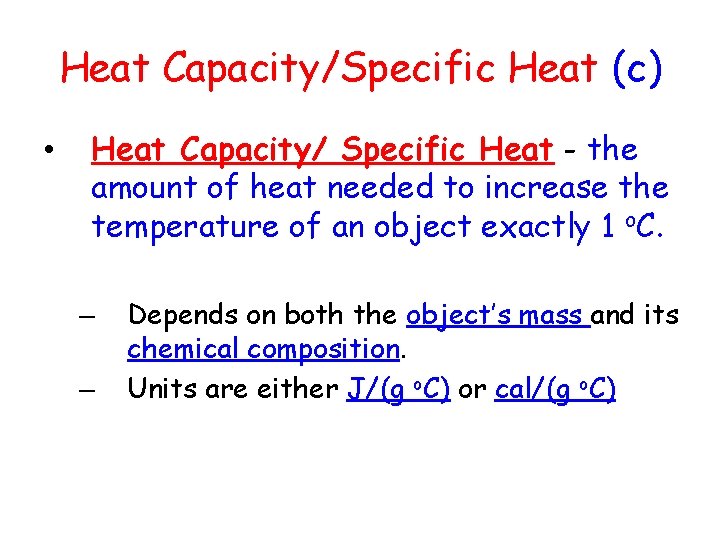
Heat Capacity/Specific Heat (c) • Heat Capacity/ Specific Heat - the amount of heat needed to increase the temperature of an object exactly 1 o. C. – – Depends on both the object’s mass and its chemical composition. Units are either J/(g o. C) or cal/(g o. C)

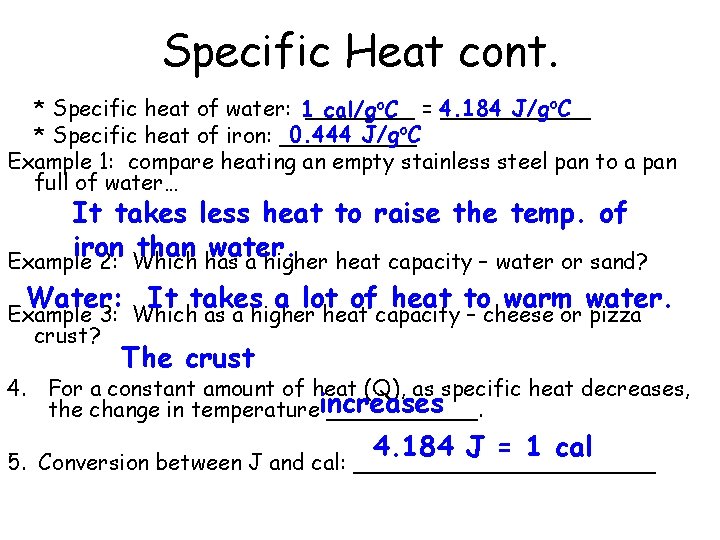
Specific Heat cont. J/go. C * Specific heat of water: 1______ cal/go. C = 4. 184 0. 444 J/go. C * Specific heat of iron: _____ Example 1: compare heating an empty stainless steel pan to a pan full of water… It takes less heat to raise the temp. of iron than water. Example 2: Which has a higher heat capacity – water or sand? Water: It takes a lot of heat to warm water. Example 3: Which as a higher heat capacity – cheese or pizza crust? The crust 4. For a constant amount of heat (Q), as specific heat decreases, the change in temperatureincreases ______. 4. 184 J = 1 cal 5. Conversion between J and cal: ___________

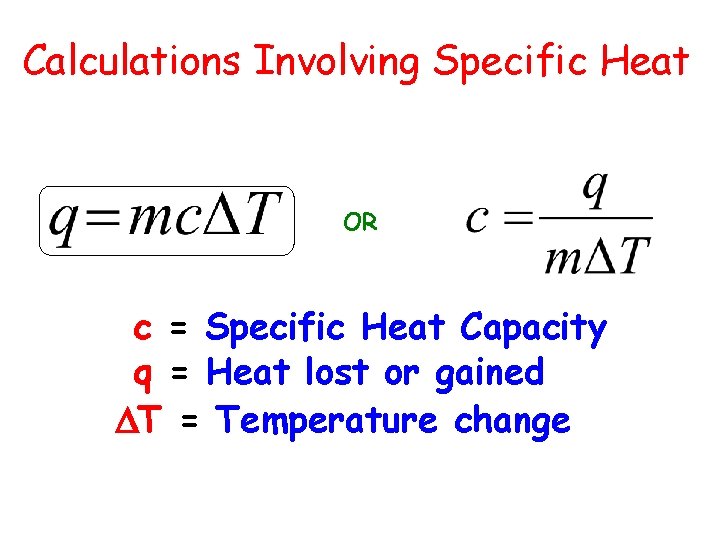
Calculations Involving Specific Heat OR c = Specific Heat Capacity q = Heat lost or gained T = Temperature change

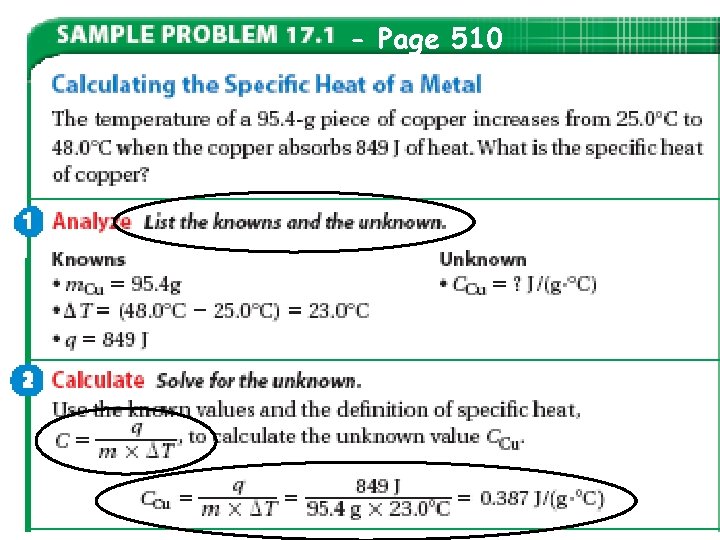
- Page 510

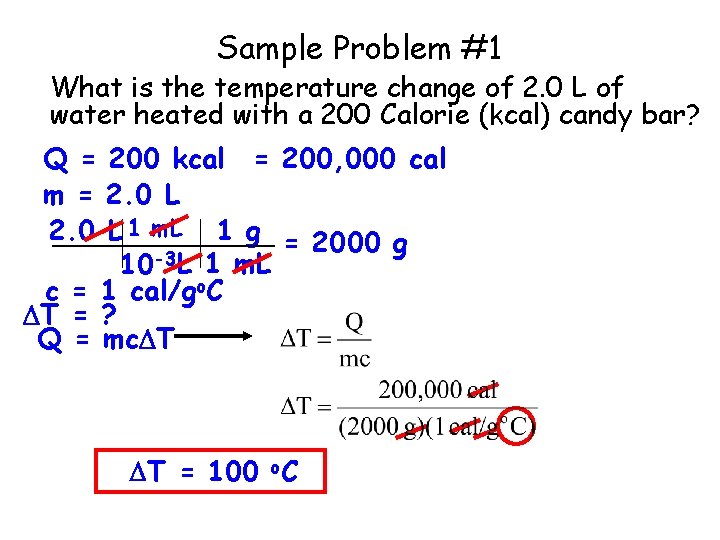
Sample Problem #1 What is the temperature change of 2. 0 L of water heated with a 200 Calorie (kcal) candy bar? Q = 200 kcal = 200, 000 cal m = 2. 0 L 1 m. L 1 g = 2000 g 10 -3 L 1 m. L c = 1 cal/go. C T = ? Q = mc T T = 100 o. C

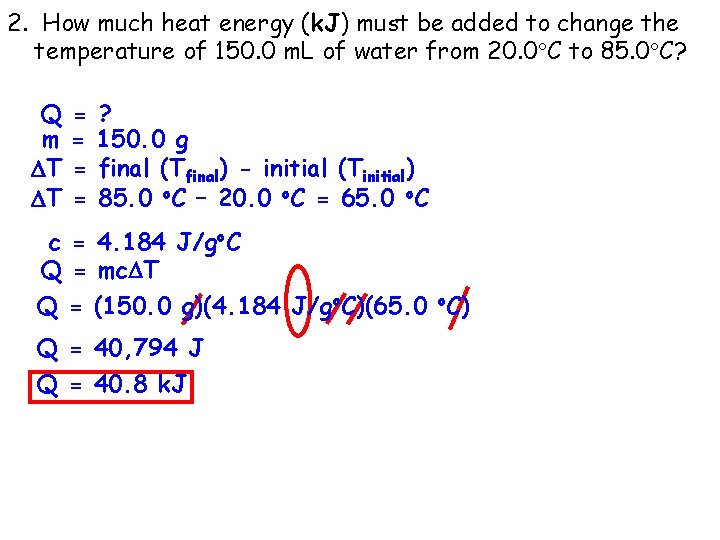
2. How much heat energy (k. J) must be added to change the temperature of 150. 0 m. L of water from 20. 0 C to 85. 0 C? Q m T T = = ? 150. 0 g final (Tfinal) - initial (Tinitial) 85. 0 o. C – 20. 0 o. C = 65. 0 o. C c = 4. 184 J/go. C Q = mc T Q = (150. 0 g)(4. 184 J/go. C)(65. 0 o. C) Q = 40, 794 J Q = 40. 8 k. J

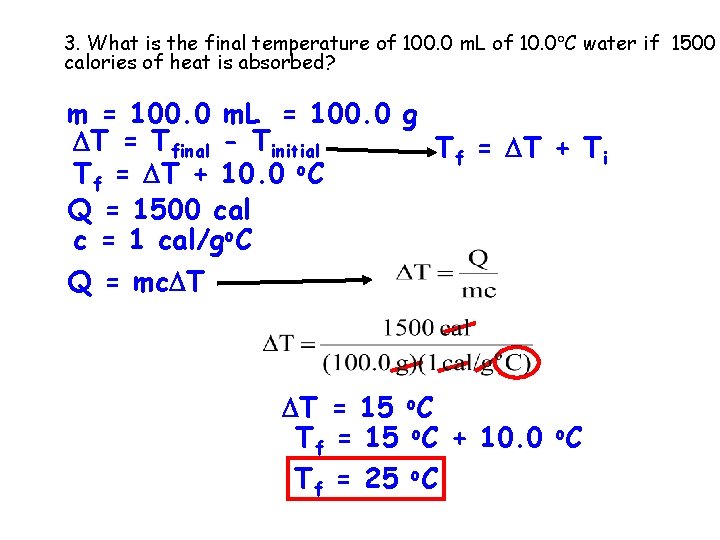
3. What is the final temperature of 100. 0 m. L of 10. 0 C water if 1500 calories of heat is absorbed? m = 100. 0 m. L = 100. 0 g T = Tfinal - Tinitial Tf = T + Ti Tf = T + 10. 0 o. C Q = 1500 cal c = 1 cal/go. C Q = mc T T = 15 o. C Tf = 15 o. C + 10. 0 o. C Tf = 25 o. C

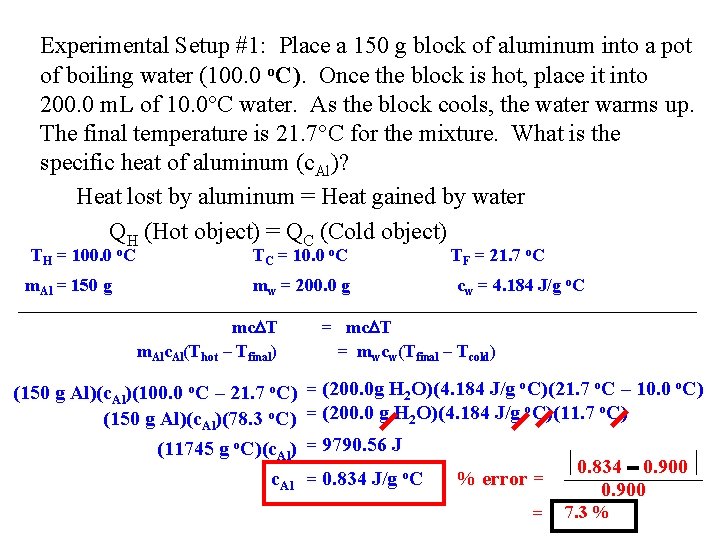
Experimental Setup #1: Place a 150 g block of aluminum into a pot of boiling water (100. 0 o. C). Once the block is hot, place it into 200. 0 m. L of 10. 0 C water. As the block cools, the water warms up. The final temperature is 21. 7 C for the mixture. What is the specific heat of aluminum (c. Al)? Heat lost by aluminum = Heat gained by water QH (Hot object) = QC (Cold object) TH = 100. 0 o. C m. Al = 150 g TC = 10. 0 o. C mw = 200. 0 g mc T m. Alc. Al(Thot – Tfinal) (150 g Al)(c. Al)(100. 0 o. C – 21. 7 o. C) (150 g Al)(c. Al)(78. 3 o. C) (11745 g o. C)(c. Al) c. Al TF = 21. 7 o. C cw = 4. 184 J/g o. C = mc T = mwcw(Tfinal – Tcold) = (200. 0 g H 2 O)(4. 184 J/g o. C)(21. 7 o. C – 10. 0 o. C) = (200. 0 g H 2 O)(4. 184 J/g o. C)(11. 7 o. C) = 9790. 56 J = 0. 834 J/g o. C % error = = 0. 834 0. 900 7. 3 %

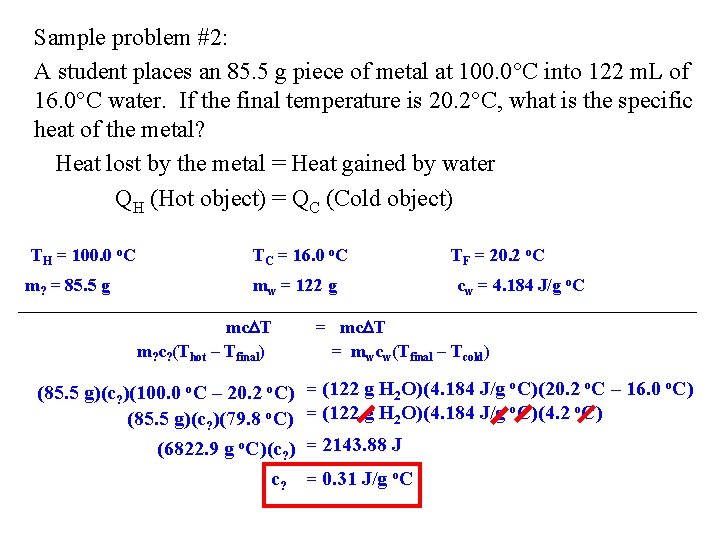
Sample problem #2: A student places an 85. 5 g piece of metal at 100. 0 C into 122 m. L of 16. 0 C water. If the final temperature is 20. 2 C, what is the specific heat of the metal? Heat lost by the metal = Heat gained by water QH (Hot object) = QC (Cold object) TH = 100. 0 o. C m? = 85. 5 g TC = 16. 0 o. C mw = 122 g mc T m? c? (Thot – Tfinal) (85. 5 g)(c? )(100. 0 o. C – 20. 2 o. C) (85. 5 g)(c? )(79. 8 o. C) (6822. 9 g o. C)(c? ) c? TF = 20. 2 o. C cw = 4. 184 J/g o. C = mc T = mwcw(Tfinal – Tcold) = (122 g H 2 O)(4. 184 J/g o. C)(20. 2 o. C – 16. 0 o. C) = (122 g H 2 O)(4. 184 J/g o. C)(4. 2 o. C) = 2143. 88 J = 0. 31 J/g o. C

IQ #1 1) Calorimetry is based on what concepts? 2) How can enthalpy changes be shown in a chemical reaction? 3) How much heat is required is to raise a 150 g piece of aluminum 35°C?

Specific Heat Activity Procedure: 1. Record the mass of your cup (place in volume of water row) and keep the same cup throughout the experiment. 2. Fill the your cup with enough water to cover the metal. Record mass of water (mc). (Do not use too much water!!) 3. Record the temperature of the water (Tc). (Record temp to tenths) 4. Specific heat of water (cc) = 4. 184 J/go. C. 5. Record the temperature of the metal (Th). (Iron, Marble, Aluminum, & Brass). 6. Transfer hot metal to your Styrofoam cup (Splash/Thermometer). 7. Let temperature balance out and record the final temperature (Tf). Remember, this is the final temperature for water and for the metal as well! 8. Dry of the metal and record the mass of the metal (mh). 9. Determine the specific heat of each metal in J/go. C & cal/go. C 10. Determine % Error for Known metal (#1)

Book Work #2 Ans. 12) 1. 46 KJ 13) 146 J

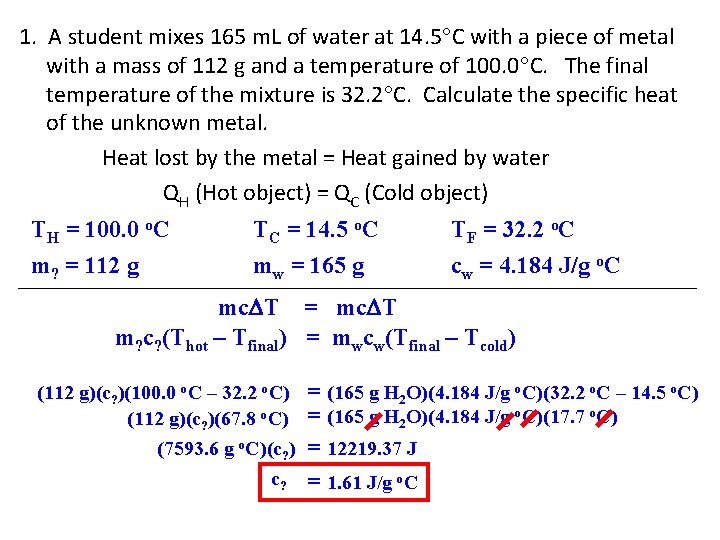
1. A student mixes 165 m. L of water at 14. 5 C with a piece of metal with a mass of 112 g and a temperature of 100. 0 C. The final temperature of the mixture is 32. 2 C. Calculate the specific heat of the unknown metal. Heat lost by the metal = Heat gained by water QH (Hot object) = QC (Cold object) TH = 100. 0 o. C m? = 112 g TC = 14. 5 o. C mw = 165 g TF = 32. 2 o. C cw = 4. 184 J/g o. C mc T = mc T m? c? (Thot – Tfinal) = mwcw(Tfinal – Tcold) (112 g)(c? )(100. 0 o. C – 32. 2 o. C) (112 g)(c? )(67. 8 o. C) (7593. 6 g o. C)(c? ) c? = (165 g H 2 O)(4. 184 J/g o. C)(32. 2 o. C – 14. 5 o. C) = (165 g H 2 O)(4. 184 J/g o. C)(17. 7 o. C) = 12219. 37 J = 1. 61 J/g o. C

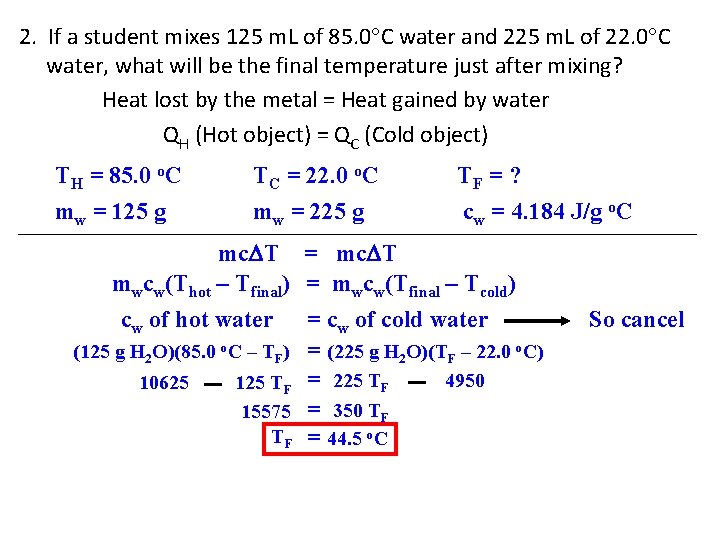
2. If a student mixes 125 m. L of 85. 0 C water and 225 m. L of 22. 0 C water, what will be the final temperature just after mixing? Heat lost by the metal = Heat gained by water QH (Hot object) = QC (Cold object) TH = 85. 0 o. C mw = 125 g TC = 22. 0 o. C mw = 225 g TF = ? cw = 4. 184 J/g o. C mc T = mc T mwcw(Thot – Tfinal) = mwcw(Tfinal – Tcold) cw of hot water = cw of cold water (125 g H 2 O)(85. 0 o. C – TF) = (225 g H 2 O)(TF – 22. 0 o. C) 4950 10625 125 TF = 225 TF 15575 = 350 TF TF = 44. 5 o. C So cancel

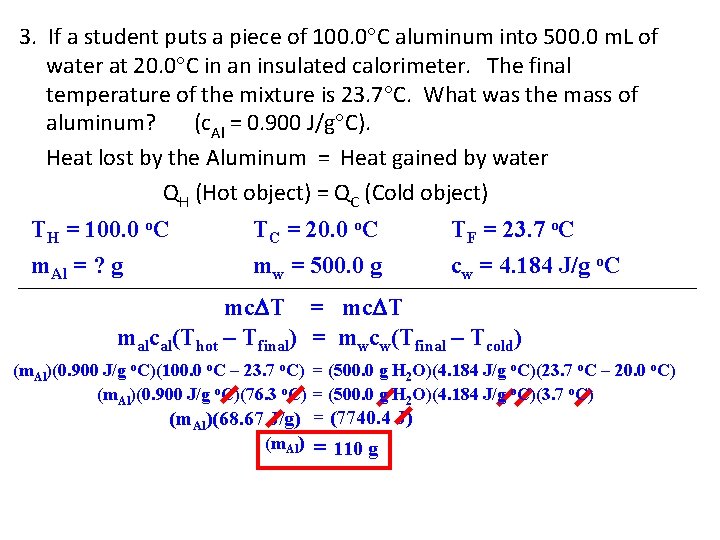
3. If a student puts a piece of 100. 0 C aluminum into 500. 0 m. L of water at 20. 0 C in an insulated calorimeter. The final temperature of the mixture is 23. 7 C. What was the mass of aluminum? (c. Al = 0. 900 J/g C). Heat lost by the Aluminum = Heat gained by water QH (Hot object) = QC (Cold object) TH = 100. 0 o. C m. Al = ? g TC = 20. 0 o. C mw = 500. 0 g TF = 23. 7 o. C cw = 4. 184 J/g o. C mc T = mc T malcal(Thot – Tfinal) = mwcw(Tfinal – Tcold) (m. Al)(0. 900 J/g o. C)(100. 0 o. C – 23. 7 o. C) = (500. 0 g H 2 O)(4. 184 J/g o. C)(23. 7 o. C – 20. 0 o. C) (m. Al)(0. 900 J/g o. C)(76. 3 o. C) = (500. 0 g H 2 O)(4. 184 J/g o. C)(3. 7 o. C) (m. Al)(68. 67 J/g) = (7740. 4 J) (m. Al) = 110 g

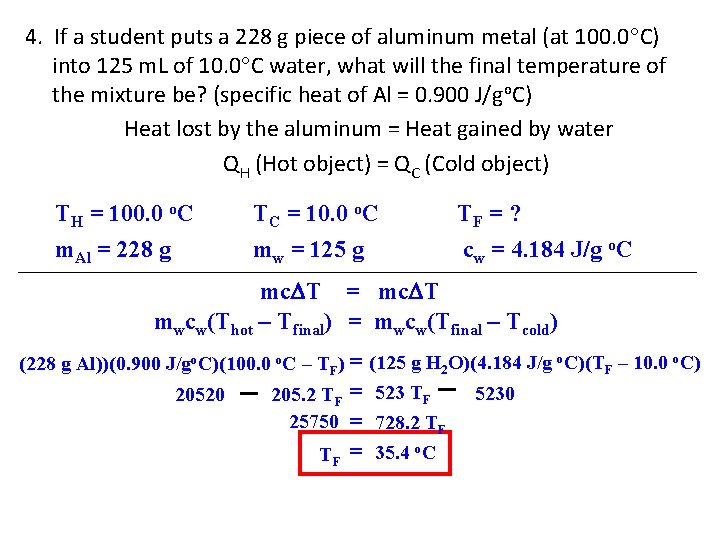
4. If a student puts a 228 g piece of aluminum metal (at 100. 0 C) into 125 m. L of 10. 0 C water, what will the final temperature of the mixture be? (specific heat of Al = 0. 900 J/go. C) Heat lost by the aluminum = Heat gained by water QH (Hot object) = QC (Cold object) TH = 100. 0 o. C m. Al = 228 g TC = 10. 0 o. C mw = 125 g TF = ? cw = 4. 184 J/g o. C mc T = mc T mwcw(Thot – Tfinal) = mwcw(Tfinal – Tcold) (228 g Al))(0. 900 J/go. C)(100. 0 o. C – TF) = (125 g H 2 O)(4. 184 J/g o. C)(TF – 10. 0 o. C) 5230 20520 205. 2 TF = 523 TF 25750 = 728. 2 TF TF = 35. 4 o. C

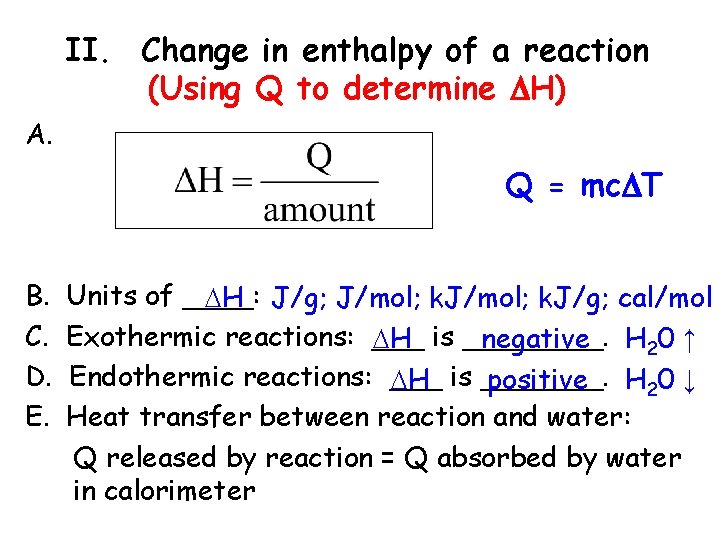
II. Change in enthalpy of a reaction (Using Q to determine H) A. Q = mc T B. C. D. E. Units of ____: H J/g; J/mol; k. J/g; cal/mol Exothermic reactions: ___ H is ____. negative H 20 ↑ Endothermic reactions: ___ H is _______. positive H 20 ↓ Heat transfer between reaction and water: Q released by reaction = Q absorbed by water in calorimeter

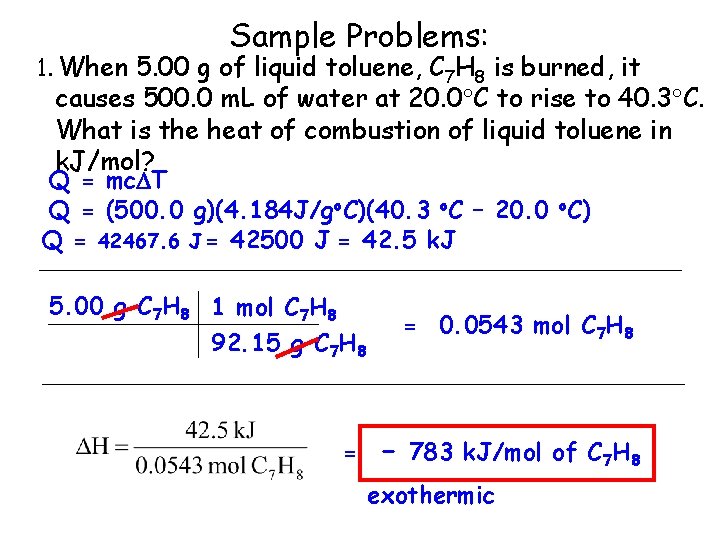
Sample Problems: 1. When 5. 00 g of liquid toluene, C 7 H 8 is burned, it causes 500. 0 m. L of water at 20. 0 C to rise to 40. 3 C. What is the heat of combustion of liquid toluene in k. J/mol? Q = mc T Q = (500. 0 g)(4. 184 J/go. C)(40. 3 o. C – 20. 0 o. C) Q = 42467. 6 J = 42500 J = 42. 5 k. J 5. 00 g C 7 H 8 1 mol C 7 H 8 92. 15 g C 7 H 8 = = 0. 0543 mol C 7 H 8 - 783 k. J/mol of C 7 H 8 exothermic

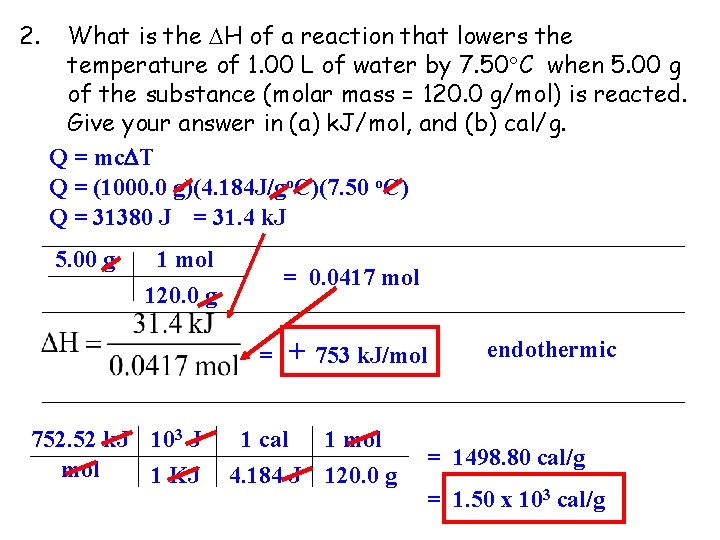
2. What is the H of a reaction that lowers the temperature of 1. 00 L of water by 7. 50 C when 5. 00 g of the substance (molar mass = 120. 0 g/mol) is reacted. Give your answer in (a) k. J/mol, and (b) cal/g. Q = mc T Q = (1000. 0 g)(4. 184 J/go. C)(7. 50 o. C) Q = 31380 J = 31. 4 k. J 5. 00 g 1 mol 120. 0 g = 0. 0417 mol = 752. 52 k. J 103 J mol 1 KJ + 753 k. J/mol 1 cal 4. 184 J 1 mol 120. 0 g endothermic = 1498. 80 cal/g = 1. 50 x 103 cal/g

Experimental Determination of ΔH 1. When a 0. 764 g almond is burned, it heats 225 m. L of water from 14. 8 o. C to 18. 4 o. C. Calculate the heat of combustion of the almond in kcal/g. 2. What is the ∆H of a reaction that lowers the temperature of a liter of water by 7. 50 o. C when 5. 00 g of the substance is dissolved in the water? (Assume the molar mass = 120 g/mol). Give your answer in (a) cal/g; (b) kcal/mol; (c) k. J/mol. 3. A 4. 00 g substance (with molar mass = 80. 0 g/mol) heats 750. 0 m. L from 15. 0 o. C to 22. 0 o. C. Calculate the ∆H in (a) k. J/mol; (b) kcal/mol. 4. When gasoline burns in air, heat is released. If 11. 0 m. L of gasoline burns and the temperature of 10. 0 liters of water increase by 10. 0 o. C, what is the heat of combustion (in k. J/mol)? Assume that gasoline is all octane (C 8 H 18) and has a density of 0. 785 g/m. L.

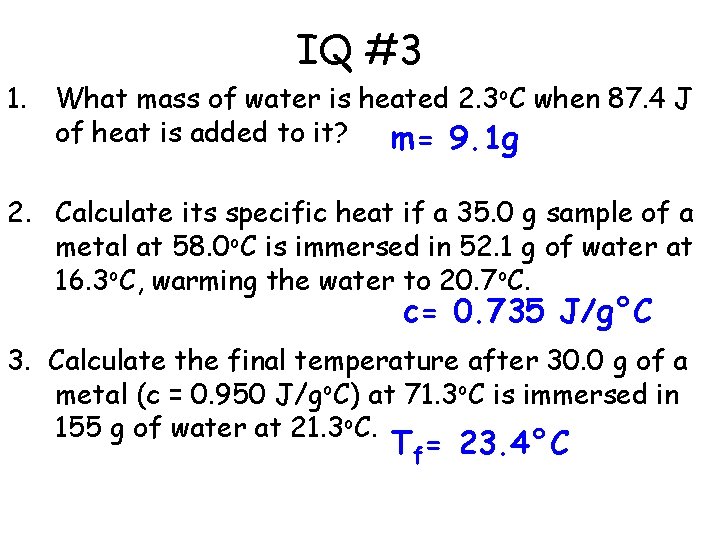
IQ #3 1. What mass of water is heated 2. 3 o. C when 87. 4 J of heat is added to it? m= 9. 1 g 2. Calculate its specific heat if a 35. 0 g sample of a metal at 58. 0 o. C is immersed in 52. 1 g of water at 16. 3 o. C, warming the water to 20. 7 o. C. c= 0. 735 J/g°C 3. Calculate the final temperature after 30. 0 g of a metal (c = 0. 950 J/go. C) at 71. 3 o. C is immersed in 155 g of water at 21. 3 o. C. Tf= 23. 4°C

Section 17. 3 Heat in Changes of State • OBJECTIVES: –Classify the enthalpy change that occurs when a substance melts, freezes, boils, condenses, or dissolves. 41

Section 17. 3 Heat in Changes of State • OBJECTIVES: –Solve for the enthalpy change that occurs when a substance melts, freezes, boils, condenses, or dissolves. 42

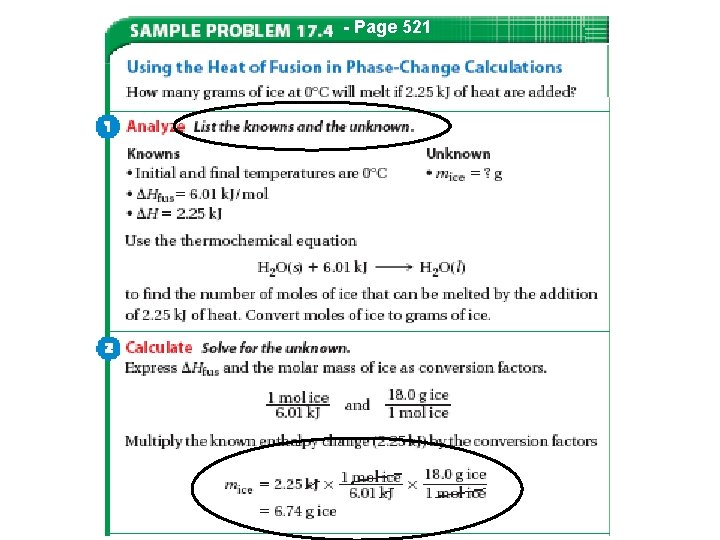
Heat in Changes of State • Molar Heat of Fusion ( Hfus) : the heat absorbed by one mole of a substance in melting from a solid to a liquid – q= mass x Hfus (there is no temperature change) • Molar Heat of Solidification ( Hsolid) : heat lost when one mole of liquid solidifies (or freezes) to a solid – q = mass x Hsolid (no temperature change)

Heat in Changes of State • Heat absorbed by a melting solid is equal to heat lost when a liquid solidifies –Thus, Hfus = - Hsolid • Note Table 17. 3, page 522 44

- Page 521

Heats of Vaporization and Condensation • When liquids absorb heat at their boiling points, they become vapors. • Molar Heat of Vaporization ( Hvap) = the amount of heat necessary to vaporize one mole of a given liquid. • q = mass x Hvap (no temperature change) • Table 17. 3, page 522 46

Heats of Vaporization and Condensation • Condensation is the opposite of vaporization. • Molar Heat of Condensation ( Hcond) = amount of heat released when one mole of vapor condenses to a liquid Hvap = - Hcond 47

Heats of Vaporization and Condensation • Note Figure 17. 10, page 523 • The large values for water Hvap and Hcond are the reason hot vapors such as steam are very dangerous – You can receive a scalding burn from steam when the heat of condensation is released! H 20(g) H 20(l) Hcond = - 40. 7 k. J/mol 48

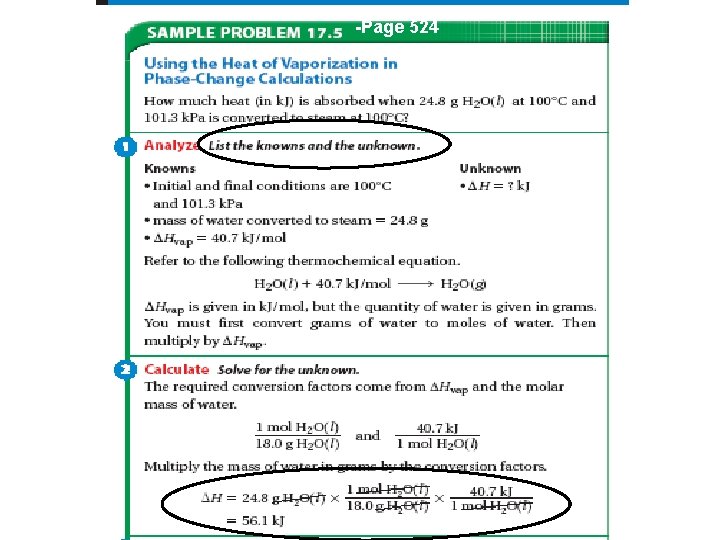
-Page 524

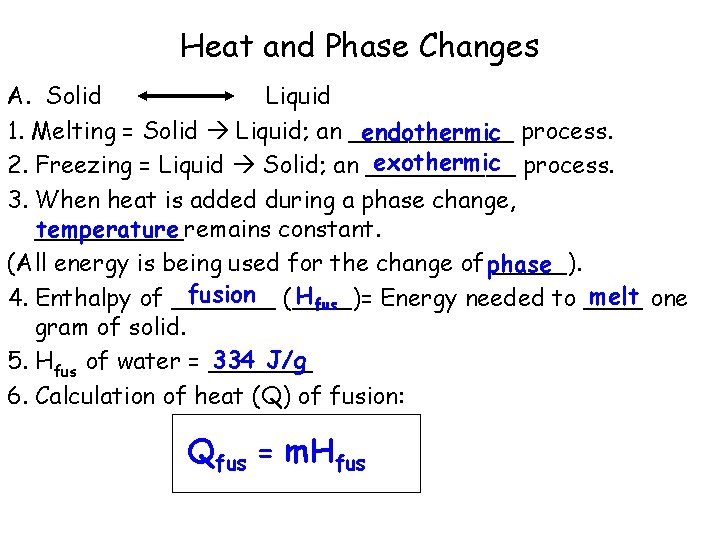
Heat and Phase Changes A. Solid Liquid 1. Melting = Solid Liquid; an ______ endothermic process. exothermic process. 2. Freezing = Liquid Solid; an _____ 3. When heat is added during a phase change, _____remains constant. temperature (All energy is being used for the change of phase _____). fusion (____)= Hfus melt one 4. Enthalpy of _______ Energy needed to ____ gram of solid. 334 J/g 5. Hfus of water = _______ 6. Calculation of heat (Q) of fusion: Qfus = m. Hfus

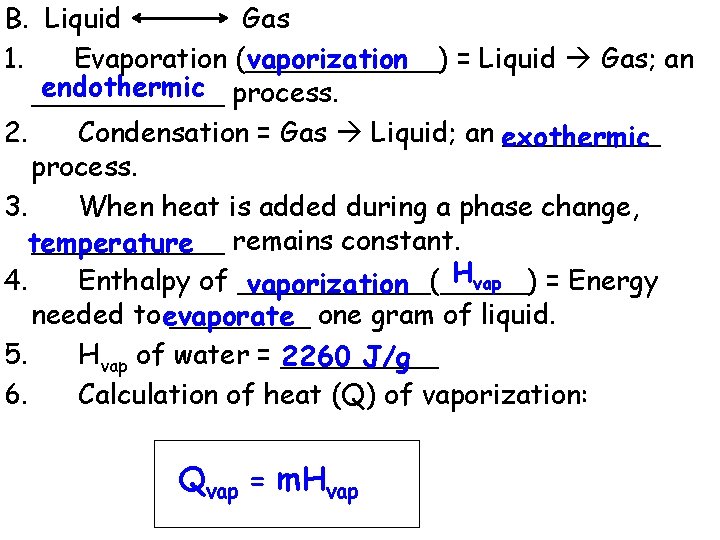
B. Liquid Gas vaporization 1. Evaporation (______) = Liquid Gas; an endothermic process. ______ 2. Condensation = Gas Liquid; an exothermic _____ process. 3. When heat is added during a phase change, ______ remains constant. temperature Hvap 4. Enthalpy of ______(_____) = Energy vaporization needed to evaporate ____ one gram of liquid. 5. Hvap of water = _____ 2260 J/g 6. Calculation of heat (Q) of vaporization: Qvap = m. Hvap

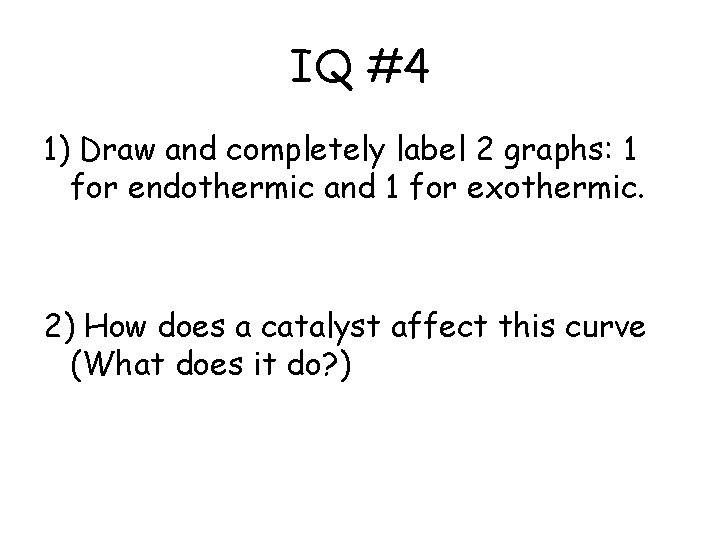
IQ #4 1) Draw and completely label 2 graphs: 1 for endothermic and 1 for exothermic. 2) How does a catalyst affect this curve (What does it do? )

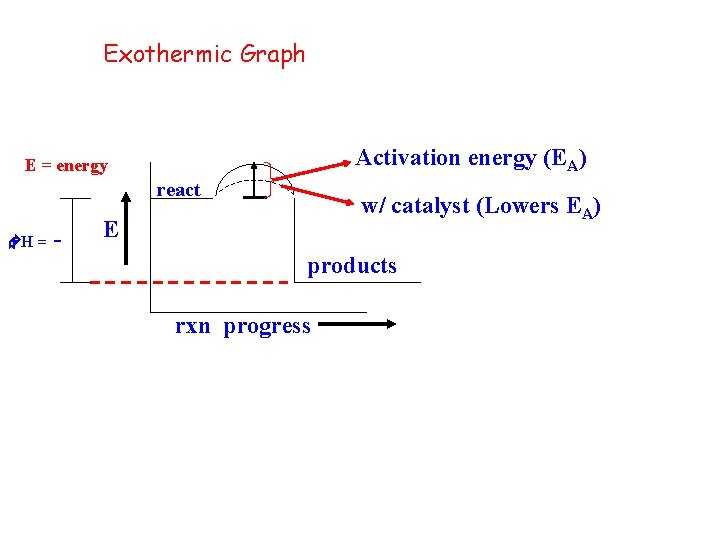
Exothermic Graph Activation energy (EA) E = energy react w/ catalyst (Lowers EA) E H = products rxn progress

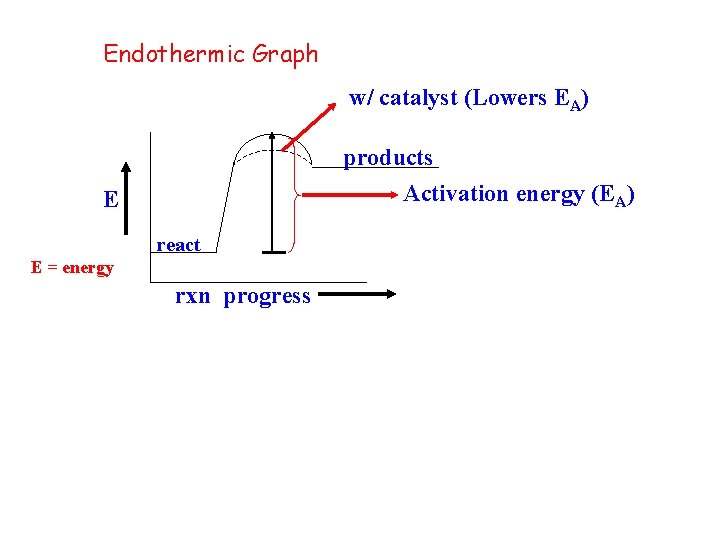
Endothermic Graph w/ catalyst (Lowers EA) products Activation energy (EA) E react E = energy rxn progress

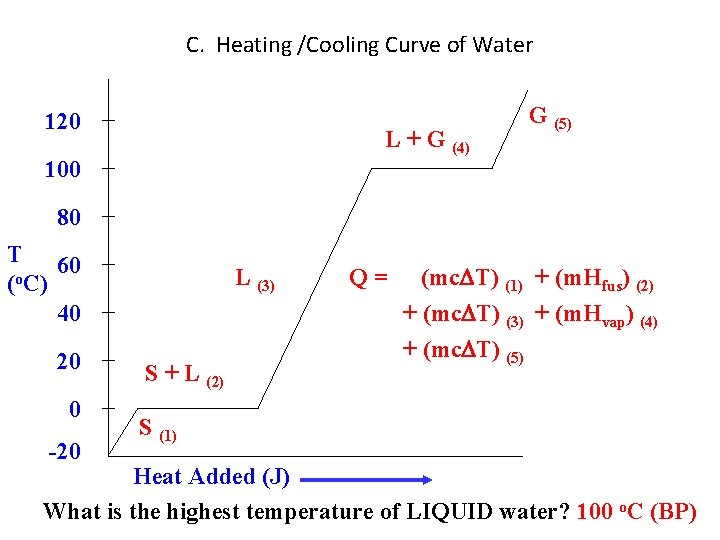
C. Heating /Cooling Curve of Water 120 L + G (4) 100 G (5) 80 T 60 (o. C) 40 20 0 -20 L (3) S + L (2) Q= (mc T) (1) + (m. Hfus) (2) + (mc T) (3) + (m. Hvap) (4) + (mc T) (5) S (1) Heat Added (J) What is the highest temperature of LIQUID water? 100 o. C (BP)

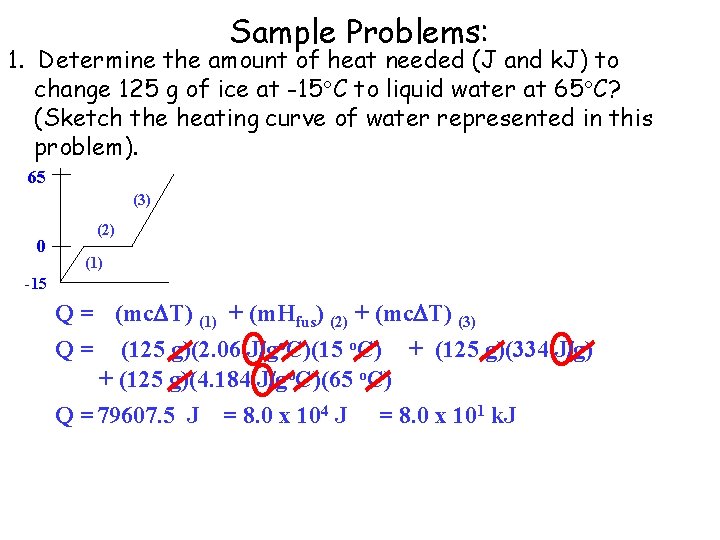
Sample Problems: 1. Determine the amount of heat needed (J and k. J) to change 125 g of ice at -15 C to liquid water at 65 C? (Sketch the heating curve of water represented in this problem). 65 (3) 0 (2) (1) -15 Q = (mc T) (1) + (m. Hfus) (2) + (mc T) (3) Q = (125 g)(2. 06 J/go. C)(15 o. C) + (125 g)(334 J/g) + (125 g)(4. 184 J/go. C)(65 o. C) Q = 79607. 5 J = 8. 0 x 104 J = 8. 0 x 101 k. J

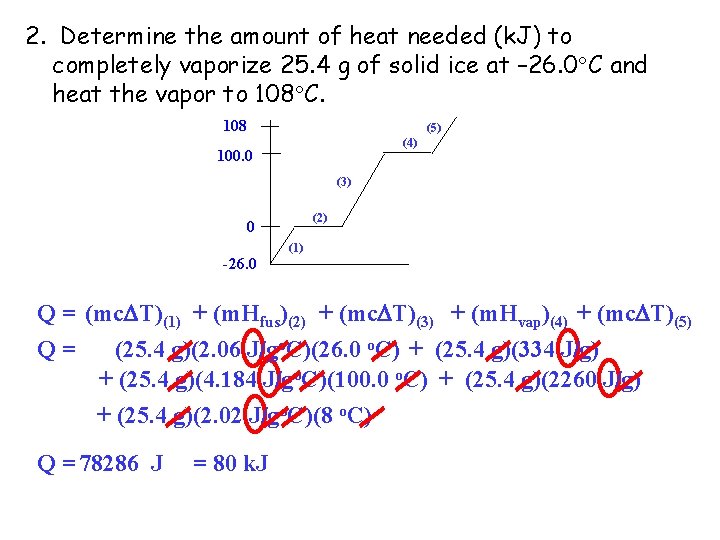
2. Determine the amount of heat needed (k. J) to completely vaporize 25. 4 g of solid ice at – 26. 0 C and heat the vapor to 108 C. 108 (5) (4) 100. 0 (3) (2) 0 (1) -26. 0 Q = (mc T)(1) + (m. Hfus)(2) + (mc T)(3) + (m. Hvap)(4) + (mc T)(5) Q= (25. 4 g)(2. 06 J/go. C)(26. 0 o. C) + (25. 4 g)(334 J/g) + (25. 4 g)(4. 184 J/go. C)(100. 0 o. C) + (25. 4 g)(2260 J/g) + (25. 4 g)(2. 02 J/go. C)(8 o. C) Q = 78286 J = 80 k. J

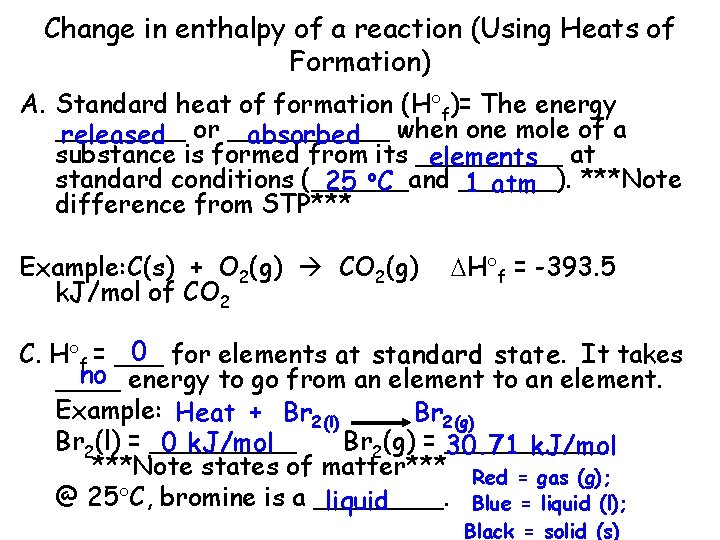
Change in enthalpy of a reaction (Using Heats of Formation) A. Standard heat of formation (H f)= The energy ____ released or _____ absorbed when one mole of a substance is formed from its _____ elements at standard conditions (______and ______). ***Note 25 o. C 1 atm difference from STP*** Example: C(s) + O 2(g) CO 2(g) k. J/mol of CO 2 H f = -393. 5 0 for elements at standard state. It takes C. H f = ___ no energy to go from an element to an element. ____ Example: Heat + Br 2(l) Br 2(g) Br 2(l) = _____ Br 2(g) = _____ 0 k. J/mol 30. 71 k. J/mol ***Note states of matter*** Red = gas (g); @ 25 C, bromine is a ____. liquid Blue = liquid (l); Black = solid (s)

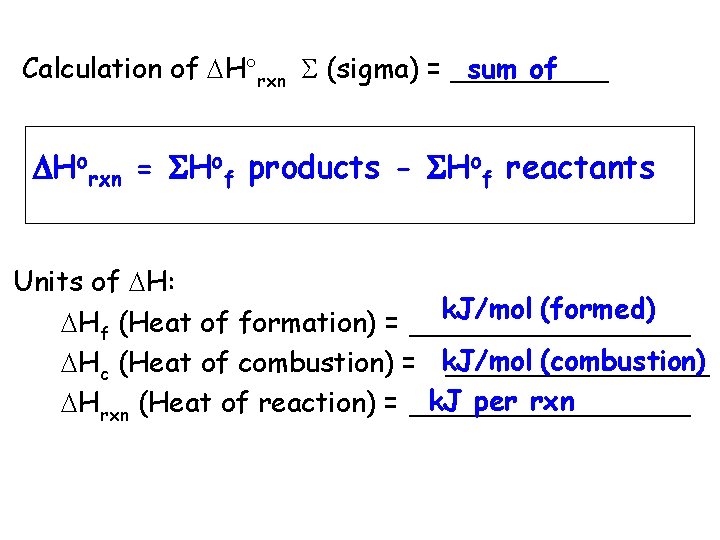
sum of Calculation of H rxn (sigma) = _____ Horxn = Hof products - Hof reactants Units of H: k. J/mol (formed) Hf (Heat of formation) = ________ (combustion) Hc (Heat of combustion) = k. J/mol ________ k. J per rxn Hrxn (Heat of reaction) = ________

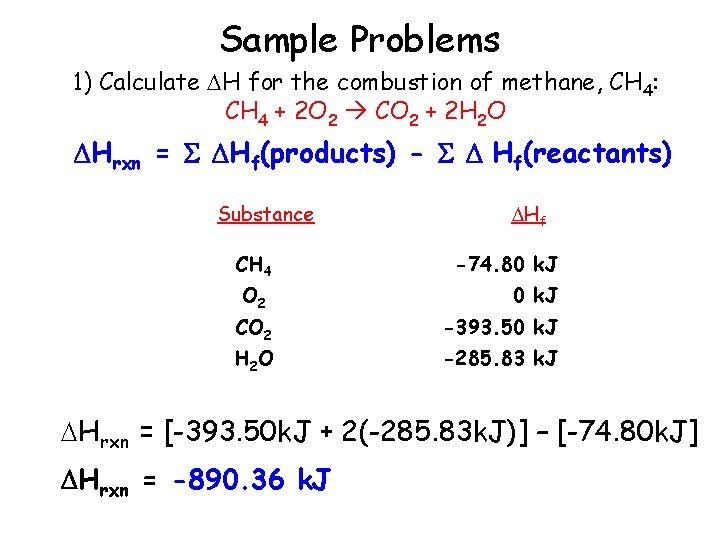
Sample Problems 1) Calculate H for the combustion of methane, CH 4: CH 4 + 2 O 2 CO 2 + 2 H 2 O Hrxn = Hf(products) - Hf(reactants) Substance Hf CH 4 -74. 80 k. J O 2 0 k. J CO 2 H 2 O -393. 50 k. J -285. 83 k. J Hrxn = [-393. 50 k. J + 2(-285. 83 k. J)] – [-74. 80 k. J] Hrxn = -890. 36 k. J

2. Find Hrxn: Cl 2(g) + 2 HBr(g) 2 HCl(g) + Br 2(g) Horxn = Hof products - Hof reactants Horxn = [2(HCl) + (Br 2)] – [(Cl 2) + 2(HBr)] Horxn = [2 mol(-92. 3 k. J/mol) + 1 mol(30. 9 k. J/mol)] – [1 mol( 0 k. J/mol) + 2 mol(-36. 2 k. J/mol)] Horxn = -81. 3 k. J

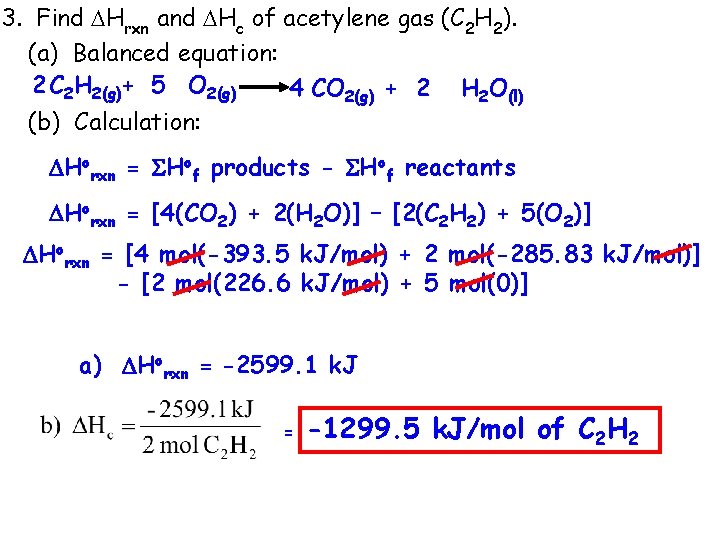
3. Find Hrxn and Hc of acetylene gas (C 2 H 2). (a) Balanced equation: 2 C 2 H 2(g)+ 5 O 2(g) 4 CO 2(g) + 2 H 2 O(l) (b) Calculation: Horxn = Hof products - Hof reactants Horxn = [4(CO 2) + 2(H 2 O)] – [2(C 2 H 2) + 5(O 2)] Horxn = [4 mol(-393. 5 k. J/mol) + 2 mol(-285. 83 k. J/mol)] - [2 mol(226. 6 k. J/mol) + 5 mol(0)] a) Horxn = -2599. 1 k. J = -1299. 5 k. J/mol of C 2 H 2

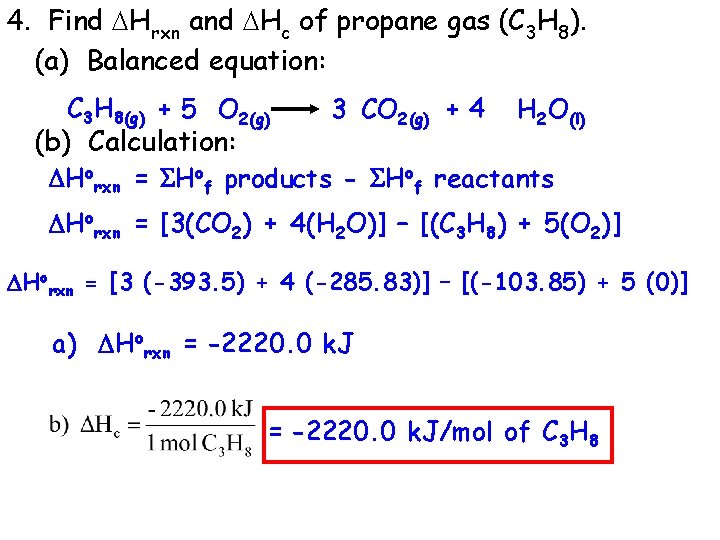
4. Find Hrxn and Hc of propane gas (C 3 H 8). (a) Balanced equation: C 3 H 8(g) + 5 O 2(g) (b) Calculation: 3 CO 2(g) + 4 H 2 O(l) Horxn = Hof products - Hof reactants Horxn = [3(CO 2) + 4(H 2 O)] – [(C 3 H 8) + 5(O 2)] Horxn = [3 (-393. 5) + 4 (-285. 83)] – [(-103. 85) + 5 (0)] a) Horxn = -2220. 0 k. J/mol of C 3 H 8

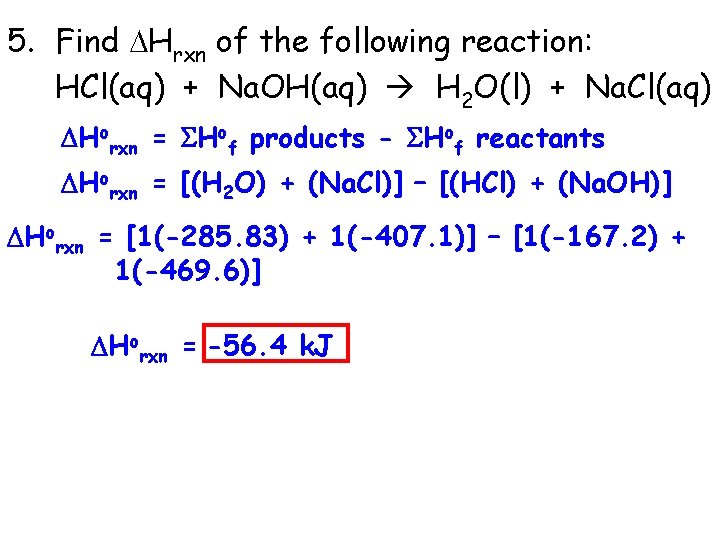
5. Find Hrxn of the following reaction: HCl(aq) + Na. OH(aq) H 2 O(l) + Na. Cl(aq) Horxn = Hof products - Hof reactants Horxn = [(H 2 O) + (Na. Cl)] – [(HCl) + (Na. OH)] Horxn = [1(-285. 83) + 1(-407. 1)] – [1(-167. 2) + 1(-469. 6)] Horxn = -56. 4 k. J

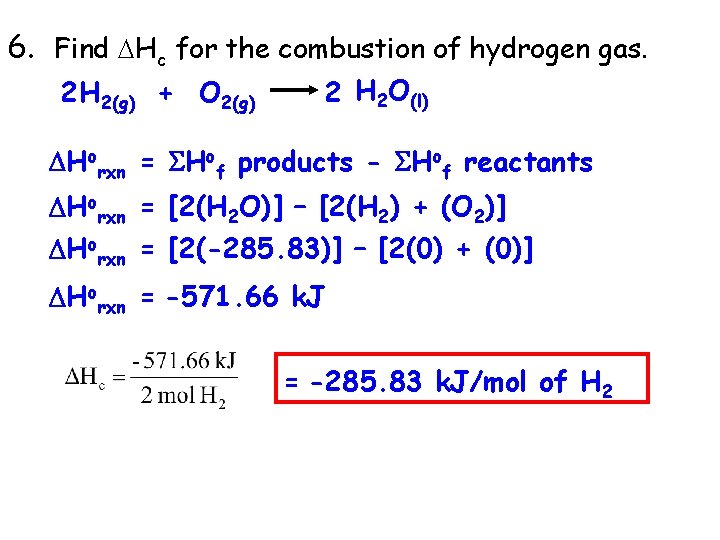
6. Find Hc for the combustion of hydrogen gas. 2 H 2 O(l) 2 H 2(g) + O 2(g) Horxn = Hof products - Hof reactants Horxn = [2(H 2 O)] – [2(H 2) + (O 2)] Horxn = [2(-285. 83)] – [2(0) + (0)] Horxn = -571. 66 k. J = -285. 83 k. J/mol of H 2