Toxicity and Human Health Inneke Hantoro Toxicity is
















































- Slides: 69

Toxicity and Human Health Inneke Hantoro

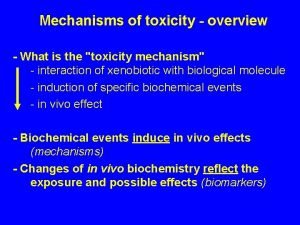
Toxicity is the potential of a chemical to induce an adverse effect in a living organism e. g. , man. How a toxicant enters an organism Toxicity How it interacts with target molecule How organism deals with the insult

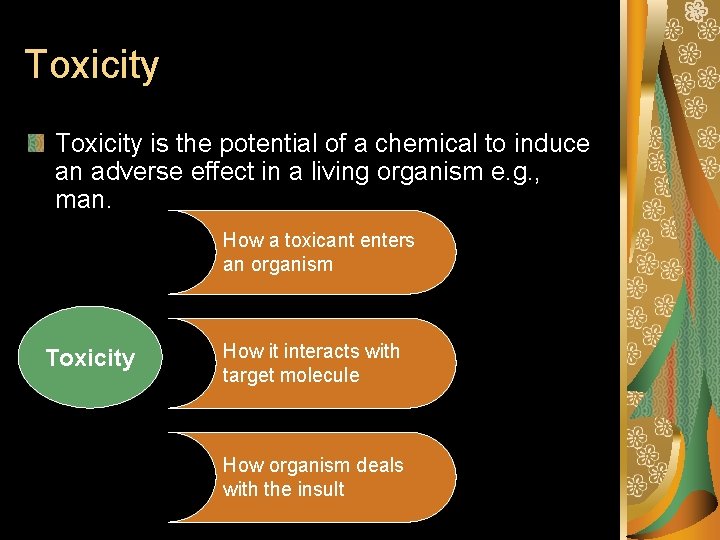
The induction of toxic effects largely depends on the disposition of the substances concerned. Interaction of a substance with a living organism Kinetic Phase absorption, distribution, metabolism, and excretion the fate of substance in the body has a number of defense mechanisms at various levels of the kinetic phase, metabolism & excretion Dynamic Phase interactions of the toxicant within the organism and describes processes at organ, tissue, cellular, and molecular levels

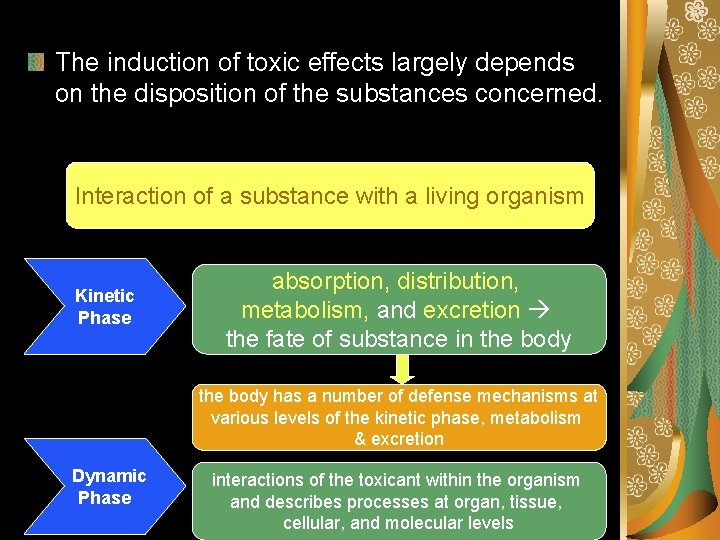
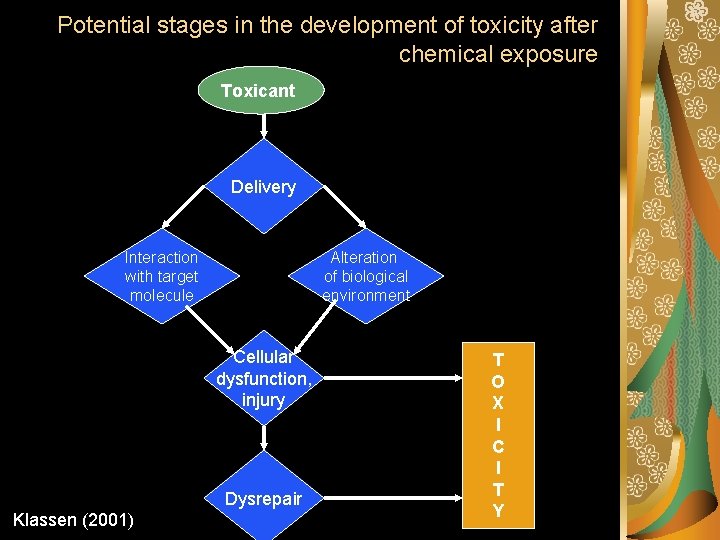
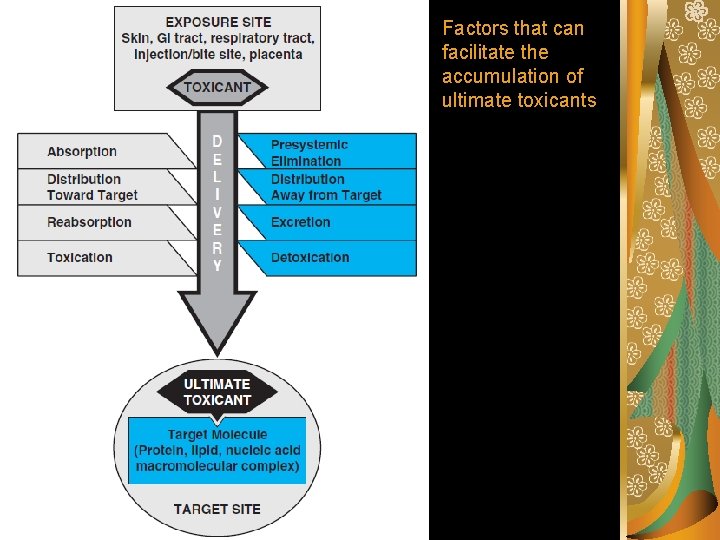
Potential stages in the development of toxicity after chemical exposure Toxicant Delivery Interaction with target molecule Alteration of biological environment Cellular dysfunction, injury Dysrepair Klassen (2001) T O X I C I T Y

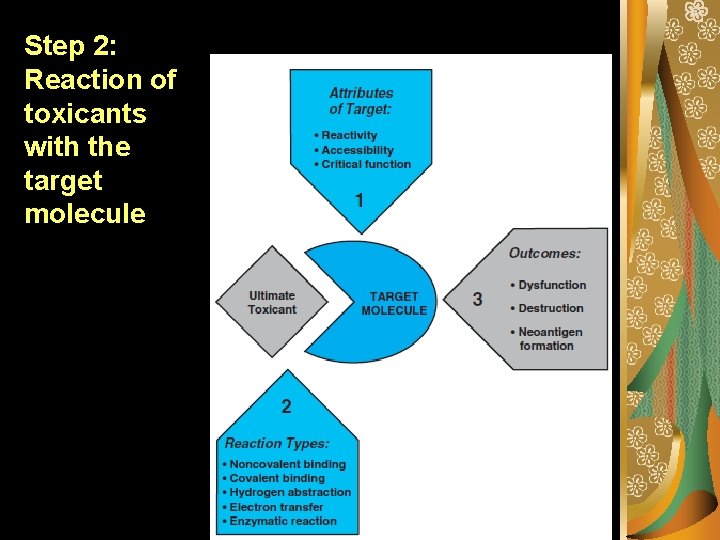
Step 1: Delivery Theoretically, the intensity of a toxic effect depends primarily on the concentration and persistence of the ultimate toxicant at its site of action. The ultimate toxicant is the chemical species that reacts with the endogenous target molecule (e. g. , receptor, enzyme, DNA, protein, lipid) or critically alters the biological (micro) environment, initiating structural and/or functional alterations that result is toxicity.

Factors that can facilitate the accumulation of ultimate toxicants

Absorption is the transfer of a chemical from the site of exposure, usually an external or internal body surface (e. g. , skin, mucosa of the alimentary and respiratory tracts), into the systemic circulation. Presystemic Elimination During transfer from the site of exposure to the systemic circulation, toxicants may be eliminated.

Distribution to and away from the target Mechanisms facilitating distribution to a target: the porosity of the capillary endothelium specialized membrane transport accumulation in cell organelles reversible intracellular binding

Mechanisms Opposing Distribution to a Target Distribution of toxicants to specific sites may be hindered by several processes, including: binding to plasma proteins specialized barriers distribution to storage sites such as adipose tissue association with intracellular binding proteins export from cells

Excretion is the removal of xenobiotics from the blood and their return to the external environment. Reabsorbtion.

Toxication Biotransformation to harmful products is called toxication or metabolic activation. With some xenobiotics, toxication confers physicochemical properties that adversely alter the microenvironment of biological processes or structures. For example, oxalic acid formed from ethylene glycol may cause acidosis and hypocalcaemia as well as obstruction of renal tubules by precipitation as calcium oxalate.

Detoxication Biotransformation that eliminates an ultimate toxicant or prevents its formation is called detoxication.

The absorption of toxicants Process by which the toxicants cross the epithelial cell barriers. Route of absorption: Skin Respiratory Digestive

The absorption of toxicants Absorption through skin, lung or intestinal tissue is followed by passage into the interstitial fluid. Interstitial fluid (15%), intracellular fluid (40%), blood plasma (8%) Toxicants is absorbed & enters the lymph or blood supply and is mobilized to other parts of the body. Toxicant can enter local tissue cells.

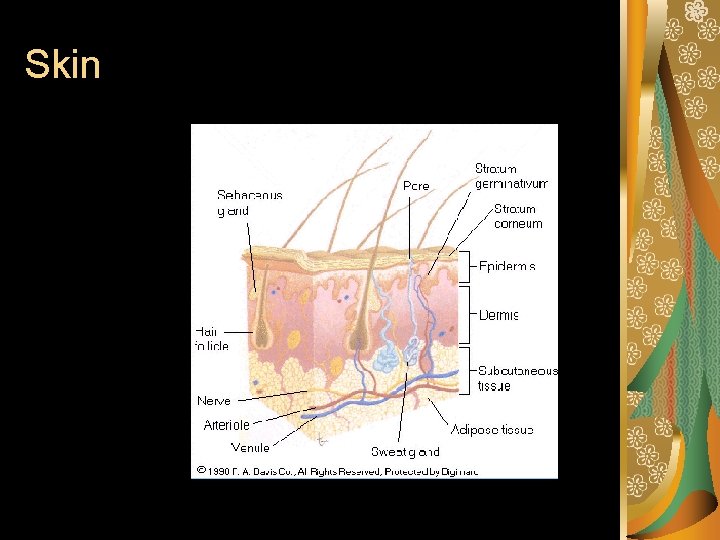
Integumentary System Route Skin, hair, nails, mammary glands. Skin is the largest organ in the body. Epidermis. – Avascular, keratinized stratum corneum, 1520 cells thick, provides most toxicant protection. Dermis. – Highly vascularized; nerve endings, hair follicles, sweat and oil glands. Hypodermis. – Connective and adipose tissue.

Skin

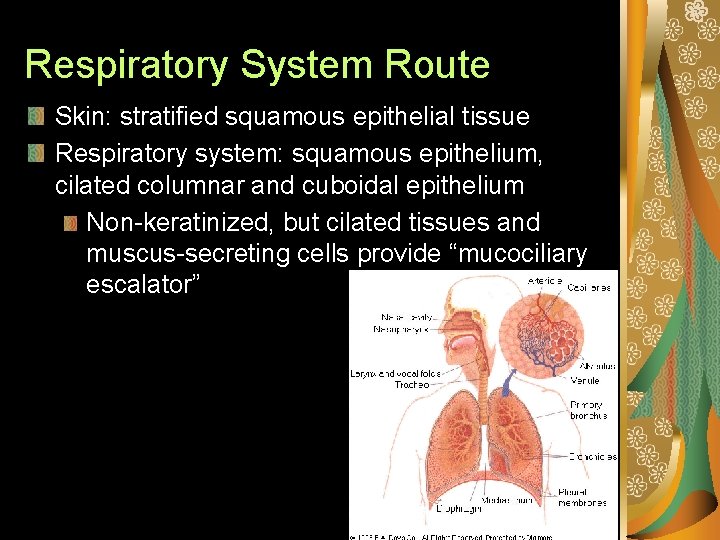
Respiratory System Route Skin: stratified squamous epithelial tissue Respiratory system: squamous epithelium, cilated columnar and cuboidal epithelium Non-keratinized, but cilated tissues and muscus-secreting cells provide “mucociliary escalator”

Nasopharyngeal. – Nostrils, nasopharynx, oropharynx, laryngopharynx. – Hairs and mucus; trap >5 μm particulates. Tracheobronchial. – Trachea, bronchioles; cillial action. – Luminal mucus aerosols and gases. Pulmonary – Alveoli - high surface area gas exchange with cardiovascular system.

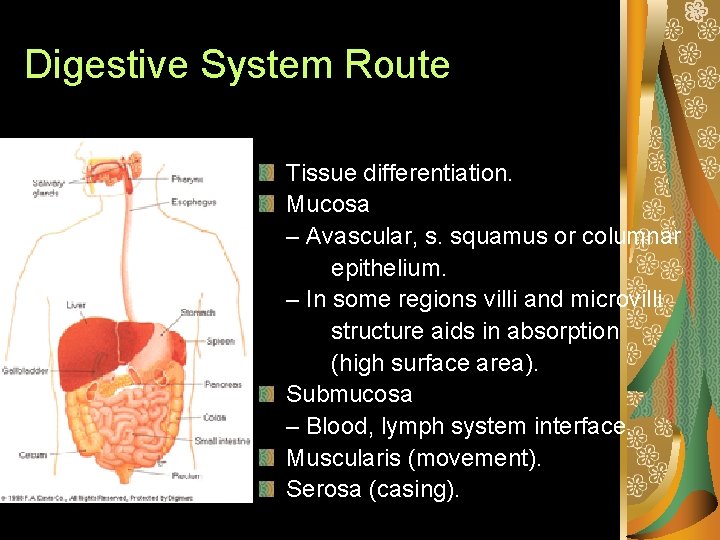
Digestive System Route Mouth, oral cavity, esophagus, stomach, small intestine, rectum, anus. Residence time can determine site of toxicant entry/injury. – Mouth (short); small intestine (long). – Absorption of toxicants can take place anywhere, but much of the tissue structure in the digestion system is specially designed for absorption.

Digestive System Route Tissue differentiation. Mucosa – Avascular, s. squamus or columnar epithelium. – In some regions villi and microvilli structure aids in absorption (high surface area). Submucosa – Blood, lymph system interface. Muscularis (movement). Serosa (casing).

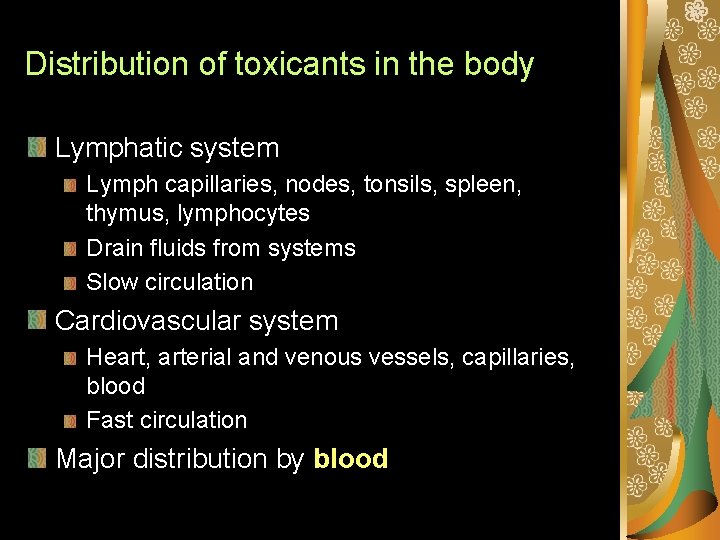
Distribution of toxicants in the body Lymphatic system Lymph capillaries, nodes, tonsils, spleen, thymus, lymphocytes Drain fluids from systems Slow circulation Cardiovascular system Heart, arterial and venous vessels, capillaries, blood Fast circulation Major distribution by blood

In blood system, major toxicant transport medium: Erythrocytes (red blood cell) § Leukocytes (white blood cell) § Platelets (thrombocytes) § Plasma (non-cellular fluid) §

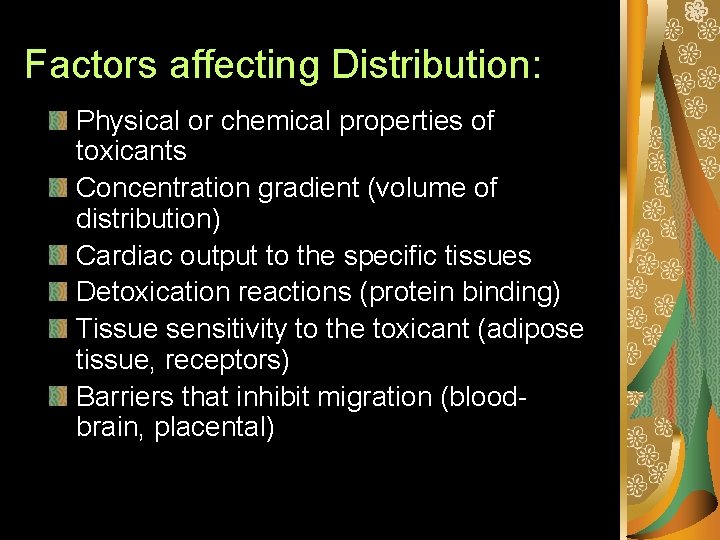
Factors affecting Distribution: Physical or chemical properties of toxicants Concentration gradient (volume of distribution) Cardiac output to the specific tissues Detoxication reactions (protein binding) Tissue sensitivity to the toxicant (adipose tissue, receptors) Barriers that inhibit migration (bloodbrain, placental)

Step 2: Reaction of toxicants with the target molecule

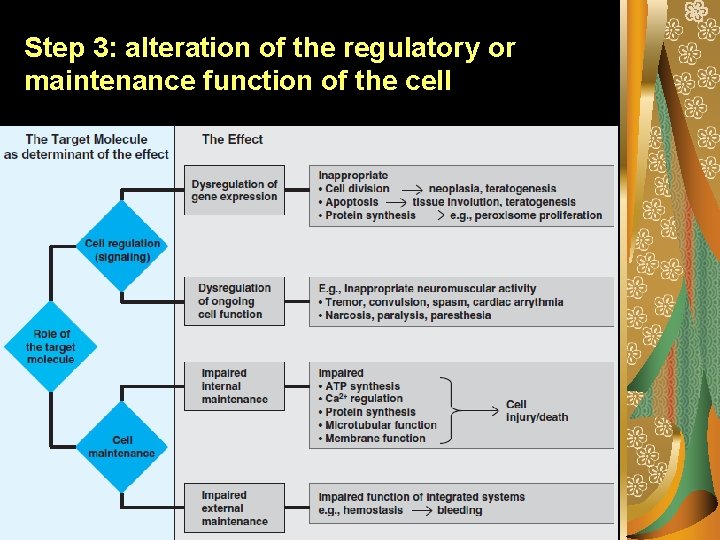
Step 3: alteration of the regulatory or maintenance function of the cell

Storage of toxicants Accumulation of toxicants in specific tissues. Binding to plasma proteins. Albumin most abundant and common binder Storage in bones. Heavy metals, like Pb Storage in liver. Blood flow, biotransformation Storage in the kidneys. Storage in fat. Lipophilic compounds

Target Organ Toxicity Adverse effects or disease states manifested in specific organs in the body High cardiac output = higher exposure Organs each have specialized tissues and cells Differentiated cellular processes and receptors Toxicants and metabolites may have specific reactive pathways

Target Organ Toxicity Toxicants do not affect all organs to the same extent A toxicant may have several sites of action and target organs Multi-toxicant exposure may target the same organ The target organ may not be the site for storage

The main target organs for the systemic toxicity of xenobiotics are: Skin, mucous membrane Lungs Liver, kidney Bone marrow Immune system Nervous system (central & peripheral) Cardiovascular system Reproductive system Muscle and bones

Why an organ or tissue is sensitive to a particular toxicants? The toxicants accumulates preferably in this organ/tissue Inactive pro-toxicants is activated in this organ/ tissue by phase I enzymes in high concentration The repairing system in the tissue is either less-developed or absent to the toxicant This tissue has receptors specific to this toxicant receptors on the cell membrane This tissue has an elevated physiological sensitivity to this toxicant

Variability of toxic response Individual-related (subjective) Living and working environmentrelated (objective)

Factors influencing the intensity of toxic response Age Gender Endocrine situation Nutritional habits Hereditary, previous disease & therapy Etc.

Types of toxic response Local Occurring only at the site of exposure of the organisms to the potentially toxic substance (skin, lungs, digestive tracts) Systemic Revealing itself after distribution of the toxicant via the bloodstream around the affected organism including the target organ or tissue, distinct from the absorption site.

According to the nature of their adverse effect on the target organs, the toxicants can be divided as: (1) Irritants Cause damage to the eyes & mucous membranes, ex: bromine, chlorine, ammonia, etc. Corrosive substances Corrode the skin & mucous membranes Substances that cause toxic pulmonary edema Chlorine, ammonia, nitrogen oxide Blockers of mitochondrial respiratory enzymes Cyanides, salicylic acid, gossypol

According to the nature of their adverse effect on the target organs, the toxicants can be divided as: (2) Inhibitors of thiol enzymes Heavy metals Blockers of Krebs cycle (citrate cycle) fluoroacetates Emetic substances Apromorphine, zinc, copper sulfate Neurotoxicants Cardiotoxicants Selectively damage the heart Ex: cardioglucosides, digitoxin, aconitine, etc.

According to the nature of their adverse effect on the target organs, the toxicants can be divided as: (3) Hepatotoxic substances Damage the liver Carbon tetrachloride, chloroform, etc. Nefrotoxic substances Damage the kidneys Mercury, chlorine, carbon tetrachloride, lead Substances that damage the bone marrow and blood cells Nirobenzene, etc.

According to the nature of their adverse effect on the target organs, the toxicants can be divided as: (4) Asphyxiants Substances that cause a reduction of blood’s ability to bind and transport oxygen Anticoagulants Substances that disturb blood coagulation Dicumarine, heparin, etc. Hemolytic substances Mushroom toxicants, phenyl-hydrazine, saponins, etc. Histamine and antihistaminic compounds

Based on the character of damage of a cell/ an organism, the toxic effects can be grouped as (1): Generally toxic Damage of the organism as a whole Dystrophic Causing the aging cells or tissues Genotoxic Alteration of the genetic material (DNA, RNA) Mutagenic Generation of irreversible changes in the hereditary materials (chromosomes, genes) of an organism

Based on the character of damage of a cell/ an organism, the toxic effects can be grouped as (2): Carcinogenic Genaration of malignant tumors Gonadotropic Harming and inhibiting the development of the germ cells Teratogenic Evoking disorders in the embryonal development of an organism Sensibilizating Making an organism ultrasensitive to this compound, resulting in allergic reactions and diseases

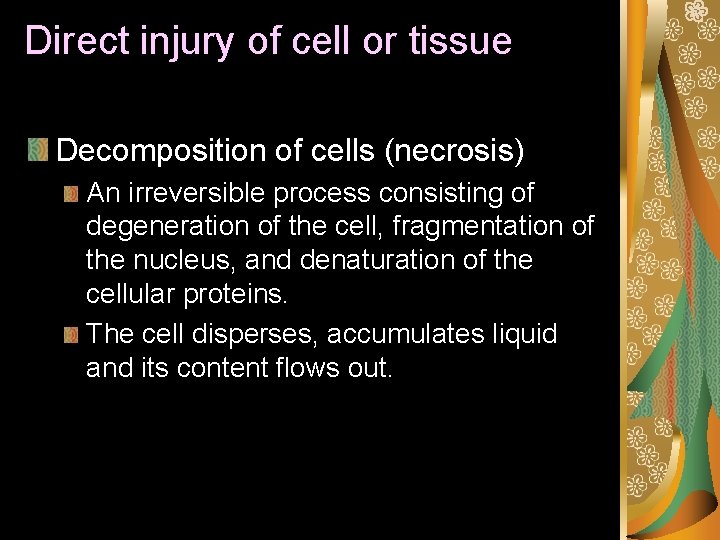
According to the final result, toxic responses can be grouped as: Direct injury of cell or tissue Biochemical damage Neurotoxicity Immunotoxicity Teratogenicity Genetic toxicity Carcinogenicity Endocrine disruption

Direct injury of cell or tissue Decomposition of cells (necrosis) An irreversible process consisting of degeneration of the cell, fragmentation of the nucleus, and denaturation of the cellular proteins. The cell disperses, accumulates liquid and its content flows out.

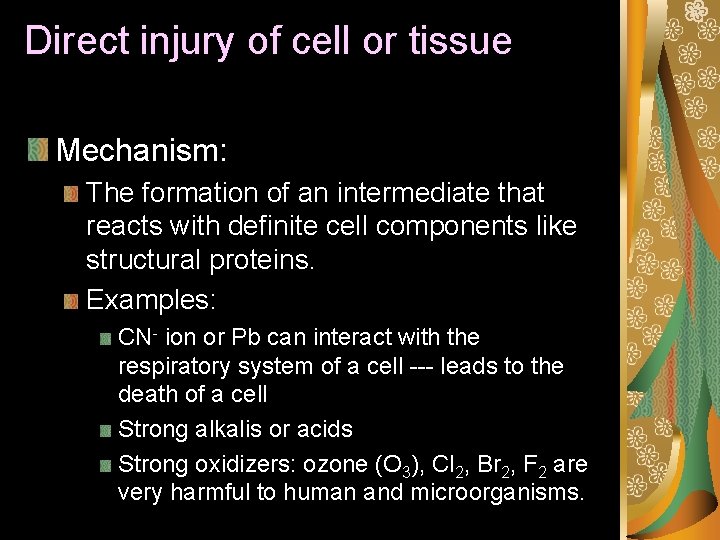
Direct injury of cell or tissue Mechanism: The formation of an intermediate that reacts with definite cell components like structural proteins. Examples: CN- ion or Pb can interact with the respiratory system of a cell --- leads to the death of a cell Strong alkalis or acids Strong oxidizers: ozone (O 3), Cl 2, Br 2, F 2 are very harmful to human and microorganisms.

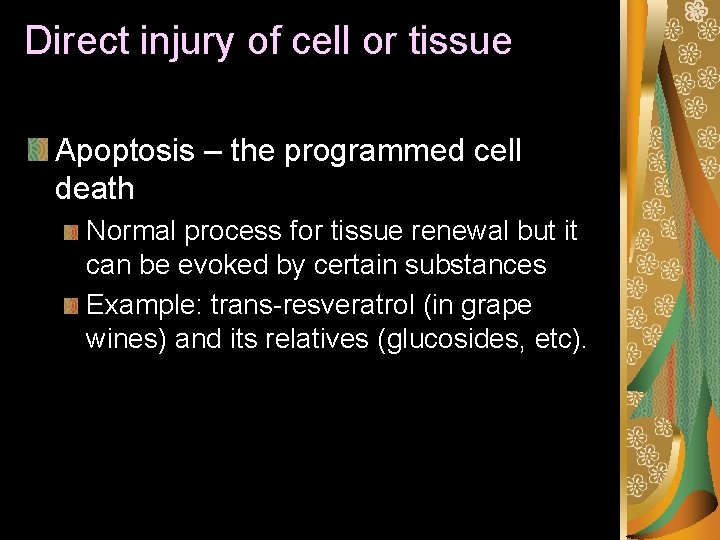
Direct injury of cell or tissue Apoptosis – the programmed cell death Normal process for tissue renewal but it can be evoked by certain substances Example: trans-resveratrol (in grape wines) and its relatives (glucosides, etc).

Biochemical damage Biochemical injury cause: Degeneration of a single cell Influencing vital function of metabolism such as respiration The death of organism: Disruption of cell metabolism Deficiency of several organs

Neurotoxicity Compounds that have a toxic effect on the nervous system: Toxicants of the central nervous system (CNS) Toxicants of the peripheral nervous system (PNS) Toxicants of a combined effect

Neurotoxicity Many toxic compounds can cause serious brain impairment. Based on the mechanism of their effect, toxicants that have undesirable effect to the brain can be grouped: Neurotoxic compounds: These compounds can disturb the function of nervous system Mercury, acrylamide, hexane, CO 2, methyl-n-butylketone.

Neurotoxicity CNS inhibitor: Chlorinated hydrocarbons, benzene, aceton, dietyl eter Psychomimetics: They can disturb psychical activities Mescalin, phenylethylamine derivatives, indole derivaties Compounds that inhibiting the respiration center Narcotics, hydrocarbons

Neurotoxicity Convulsion toxicants Convulsion in central origin Organophosphorus pesticide Toxicants, paralyzing transmission of nerve impulses to the muscle Botulinin Toxicants, paralyzing transmission of nerve impulses in the nerve Tetrodotoxin

Neurotoxicity Neuroparalytic poisons: anticholinesteratic Toxicants, acting with mediators or synaptic poisons: Adrenaline, ephedrine, hydrazines, etc.

Dose – Response & Dose – Effect Relationships

Dose response The intensity of a biological response is proportional to the concentration of the substance in the body fluids of the exposed organism. The concentration of the substance in the body fluids, in turn, is usually proportional to the dose of the substance to which the organism is subjected. As the dose of a substance is increased, the severity of the toxic response will increase until at a high enough dose the substance will be lethal individual dose-response

There will be a range of doses over which the organisms respond in the same way to the test substance. In contrast to the graded individual dose-response, this type of evaluation of toxicity depends on whether or not the test subjects develop a specified response, and is called quantal population response. To specify this group behavior, a plot of percent of individuals that respond in a specified manner against the log of the dose is generated.

Lethal dose 50% (LD 50) A widely used statistical approach for estimating the response of a population to a toxic exposure is the “Effective Dose” or ED. Generally, the mid-point, or 50%, response level is used, giving rise to the “ED 50” value. However, any response level, such as an ED 01, ED 10 or ED 30 could be chosen. Where death is the measured end-point, the ED 50 would be referred to as the Lethal Dose 50 (LD 50).

The TD 50 (toxic dose – such as liver injury) is the statistically determined dose that produced toxicity in 50% of the test organisms. If the toxic response of interest is lethality, then LD 50 is the proper notation.

The Margin of Safety The margin of safety of a substance is the range of doses between the toxic and beneficial effects; to allow for possible differences in the slopes of the effective and toxic dose-response curves, it is computed as follows: Margin of Safety (MS) = LD 1 / ED 99 LD 1 is the 1% lethal dose level and ED 99 is the 99% effective dose level.

Threshold Approaches The threshold (T) represents the dose below which no additional increase in response is observed. NOAEL (No Observed Adverse Effect Level) is the highest dose at which none of the specified toxicity. LOAEL (No Observed Adverse Effect Level) is the lowest dose at which toxicity was produced.

Subchronic exposure can last for different periods of time, but 90 days is the most common test duration. The principal goals of the subchronic study are to establish a NOAEL and to further identify and characterize the specific organ or organs affected by the test compound after repeated administration. One may also obtain a “lowest observed adverse effect level” (LOAEL) as well as the NOAEL for the species tested.

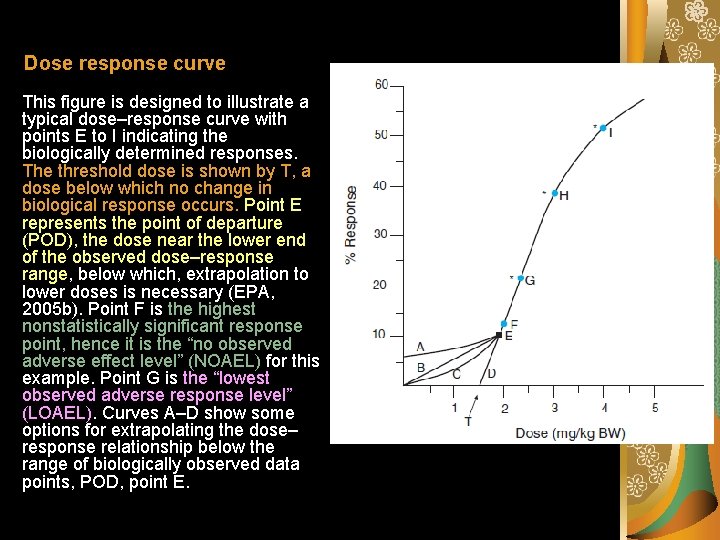
Dose response curve This figure is designed to illustrate a typical dose–response curve with points E to I indicating the biologically determined responses. The threshold dose is shown by T, a dose below which no change in biological response occurs. Point E represents the point of departure (POD), the dose near the lower end of the observed dose–response range, below which, extrapolation to lower doses is necessary (EPA, 2005 b). Point F is the highest nonstatistically significant response point, hence it is the “no observed adverse effect level” (NOAEL) for this example. Point G is the “lowest observed adverse response level” (LOAEL). Curves A–D show some options for extrapolating the dose– response relationship below the range of biologically observed data points, POD, point E.

NOAELs have traditionally served as the basis for risk assessment calculations, such as reference doses or acceptable daily intake (ADI) values. Reference doses (Rf. Ds) or concentrations (Rf. Cs) are estimates of a daily exposure to an agent that is assumed to be without adverse health impact in humans. Rf. D = NOAEL / (UF x MF)

Tolerable daily intake (TDI) Tolerable daily intakes (TDI) can be used to describe intakes for chemicals that are not “acceptable” but are “tolerable” as they are below levels thought to cause adverse health effects. These are calculated in a manner similar to ADI. In principle, dividing by the uncertainty factors allows for interspecies (animal-to-human) and intraspecies (human-to-human) variability with default values of 10 each. An additional uncertainty factor is used to account for experimental inadequacies

If only a LOAEL value is available, then an additional 10 -fold factor commonly is used to arrive at a value more comparable to a NOAEL. For developmental toxicity endpoints, it has been demonstrated that the application of the 10 -fold factor for LOAEL-to-NOAEL conversion is too large. Traditionally, a safety factor of 100 would be used for Rf. D calculations to extrapolate from a wellconducted animal bioassay (10 -fold factor animal to human) and to account for human variability in response (10 -fold factor human-to-human variability).

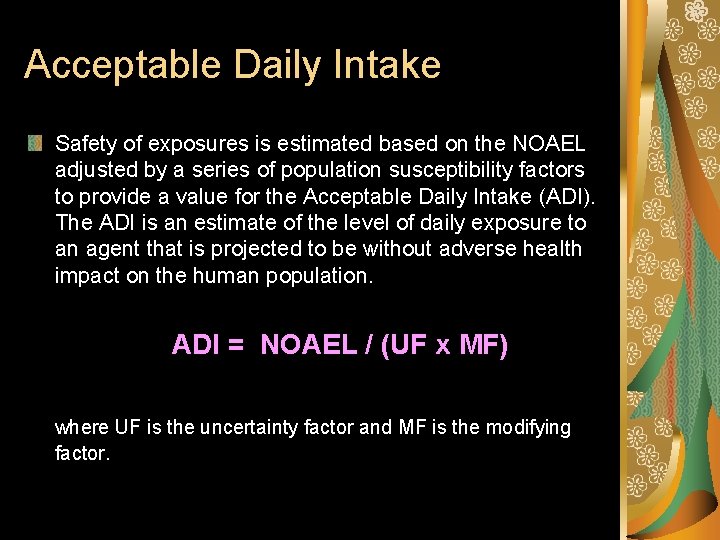
Acceptable Daily Intake Safety of exposures is estimated based on the NOAEL adjusted by a series of population susceptibility factors to provide a value for the Acceptable Daily Intake (ADI). The ADI is an estimate of the level of daily exposure to an agent that is projected to be without adverse health impact on the human population. ADI = NOAEL / (UF x MF) where UF is the uncertainty factor and MF is the modifying factor.

UF and MF provide adjustments to ADI that are presumed to ensure safety by accounting for uncertainty in dose extrapolation, uncertainty in duration extrapolation, differential sensitivities between humans and animals, and differential sensitivities among humans (e. g. , the presumed increased sensitivity for children compared to adults). Thus, for a substance that triggers all four of the uncertainty factors indicated previously, the calculation would be ADI = NOAEL/10, 000.

In some cases, for example, if the metabolism of the substance is known to provide greater sensitivity in the test organism compared to humans, an MF of less than 1 may be applied in the ADI calculation. The ADIs are used by WHO for pesticides and food additives to define “the daily intake of chemical, which during an entire lifetime appears to be without appreciable risk on the basis of all known facts at that time”.

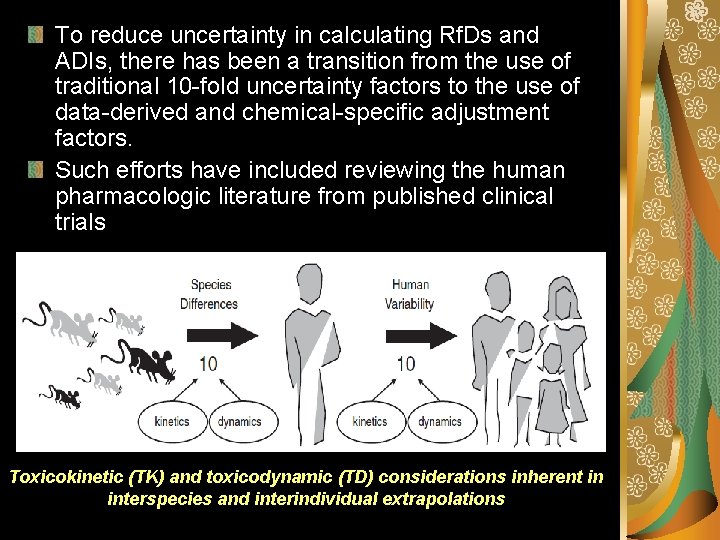
To reduce uncertainty in calculating Rf. Ds and ADIs, there has been a transition from the use of traditional 10 -fold uncertainty factors to the use of data-derived and chemical-specific adjustment factors. Such efforts have included reviewing the human pharmacologic literature from published clinical trials Toxicokinetic (TK) and toxicodynamic (TD) considerations inherent in interspecies and interindividual extrapolations

Benchmark dose lower confidence limit (BMDL) BMD: The dose–response is modeled and the lower confidence bound for a dose at a specified response level [benchmark response (BMR)] is calculated. The BMD is used as an alternative to the NOAEL/LOAEL approach for a more quantitative way of deriving regulatory levels for health effects assumed to have a nonlinear (threshold-like) low dose–response relationship. The BMR is usually specified at 1, 5, or 10%. The BMDx (with x representing the percent benchmark response) is used as an alternative to the NOAEL value for reference dose calculations. Rf. D = BMDx / (UF x MF)

The BMD approach involves modeling the dose–response curve in the range of the observable data, and then using that model to interpolate an estimate of the dose that corresponds to a particular level of response, e. g. , 5 or 10 % for quantal data, or some predefined change in response from controls for continuous data.

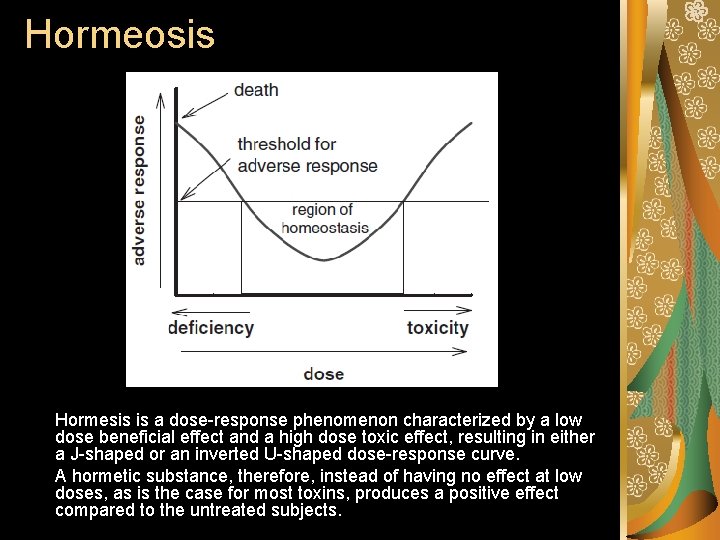
Hormeosis Hormesis is a dose-response phenomenon characterized by a low dose beneficial effect and a high dose toxic effect, resulting in either a J-shaped or an inverted U-shaped dose-response curve. A hormetic substance, therefore, instead of having no effect at low doses, as is the case for most toxins, produces a positive effect compared to the untreated subjects.

Thank You…
 Ve inneke leala
Ve inneke leala Signs and symptoms of oxygen toxicity
Signs and symptoms of oxygen toxicity B6 toxicity symptoms
B6 toxicity symptoms Mcgreafor
Mcgreafor Definition of chronic toxicity
Definition of chronic toxicity Vitamin d toxicity
Vitamin d toxicity Severe preeclampsia criteria
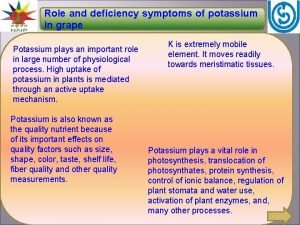
Severe preeclampsia criteria Potassium toxicity symptoms in plants
Potassium toxicity symptoms in plants What is oxygen toxicity
What is oxygen toxicity Organophosphate toxicity
Organophosphate toxicity Hepatic toxicity
Hepatic toxicity Lithium toxicity
Lithium toxicity Thirotoxicosis
Thirotoxicosis Health hazards pictogram
Health hazards pictogram Definition of chronic toxicity
Definition of chronic toxicity Digoxin mechanism
Digoxin mechanism Acetaminophen toxicity stages
Acetaminophen toxicity stages Anticholinergic syndrome
Anticholinergic syndrome Alcohol toxicity
Alcohol toxicity Vitamin c hydroxylation
Vitamin c hydroxylation Cardiotonic drugs
Cardiotonic drugs Vitamin a function
Vitamin a function Treatment of salicylate toxicity
Treatment of salicylate toxicity Zinc toxicity
Zinc toxicity Importance of toxicology
Importance of toxicology Thiamin
Thiamin Lithium toxicity
Lithium toxicity Toxicity index
Toxicity index Acute toxicity
Acute toxicity Rais calculator
Rais calculator Mountains into molehills mass-mole conversions answer key
Mountains into molehills mass-mole conversions answer key Acute toxicity
Acute toxicity Digoxin toxicity mnemonic
Digoxin toxicity mnemonic Risk assessment apes
Risk assessment apes Health and human development unit 1 and 2 notes
Health and human development unit 1 and 2 notes Health and social care component 3
Health and social care component 3 8.3 human needs
8.3 human needs Chapter 8 human needs and human development
Chapter 8 human needs and human development Non human nouns
Non human nouns Milwaukee county health and human services
Milwaukee county health and human services Human capital education and health in economic development
Human capital education and health in economic development Iowa department of health and human services
Iowa department of health and human services Stress health and human flourishing
Stress health and human flourishing Hhd unit 4 aos 2
Hhd unit 4 aos 2 The human body in health and disease chapter 2 answer key
The human body in health and disease chapter 2 answer key The human body in health and disease chapter 2 answer key
The human body in health and disease chapter 2 answer key Adenomalacia is the abnormal hardening of a gland.
Adenomalacia is the abnormal hardening of a gland. Siskiyou county health and human services
Siskiyou county health and human services Milwaukee county human services
Milwaukee county human services Maine department of health and human services
Maine department of health and human services Earmarks of nutrition quackery
Earmarks of nutrition quackery Barnstable county human services
Barnstable county human services Differences between health education and physical education
Differences between health education and physical education Chapter 3 health wellness and health disparities
Chapter 3 health wellness and health disparities Health education and health propaganda
Health education and health propaganda Glencoe health chapter 1 understanding health and wellness
Glencoe health chapter 1 understanding health and wellness Chapter one understanding health and wellness
Chapter one understanding health and wellness Human vs non human bones
Human vs non human bones Human development index definition ap human geography
Human development index definition ap human geography Effect on human health of land pollution
Effect on human health of land pollution Sound pollution effects
Sound pollution effects Health environment definition
Health environment definition To err is human: building a safer health system
To err is human: building a safer health system Health is human rights
Health is human rights Merck human health division
Merck human health division Role and responsibility of occupational health nurse
Role and responsibility of occupational health nurse National programmes related to nutrition
National programmes related to nutrition Louisiana health standards
Louisiana health standards Whole health circle of health
Whole health circle of health Health promotion world health organization
Health promotion world health organization