MILK Food Material Science 201213 Inneke Hantoro Definition





































- Slides: 44

MILK Food Material Science 2012/13 Inneke Hantoro

Definition The normal secretion of the mammary glands of all mammals (Potter & Hotchiss, 1996). n Milk is a complete food for the new born. n High density of nutritious components. n

MILK COMPOSITION & STRUCTURE

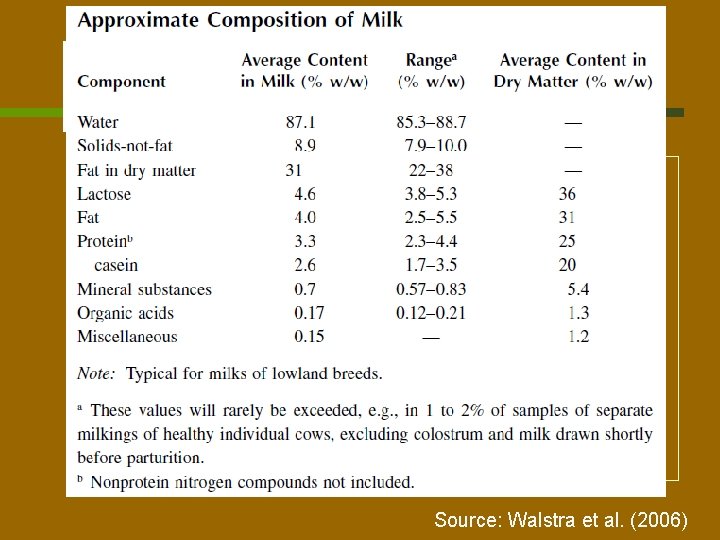
The average composition of milk Source: Walstra et al. (2006)

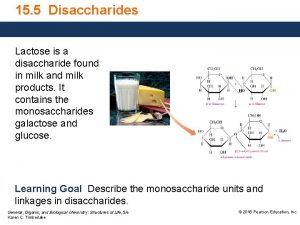
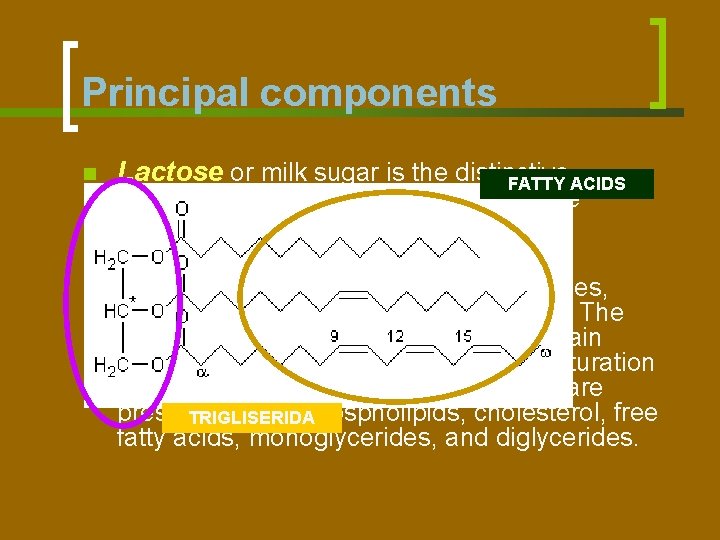
Principal components n Lactose or milk sugar is the distinctive FATTY ACIDS n The fat is largely made up of triglycerides, carbohydrate of milk. It is a disaccharide composed of glucose and galactose. constituting a very complicated mixture. The component fatty acids vary widely in chain length (2 to 20 carbon atoms) and in saturation (0 to 4 double bonds). Other lipids that are present include phospholipids, cholesterol, free TRIGLISERIDA fatty acids, monoglycerides, and diglycerides.

Principal components Protein n About four fifths of the protein consists of casein, actually a mixture of four proteins: αS 1 -, αS 2 -, β-, and κ-casein. The caseins are typical for milk. The remainder consists, for the most part, of the milk serum proteins, the main one being βlactoglobulin. Moreover, milk contains numerous minor proteins, including a wide range of enzymes.

Principal components n n n The mineral substances — primarily K, Na, Ca, Mg, Cl, and phosphate — are not equivalent to the salts. Milk contains numerous other elements in trace quantities. The salts are only partly ionized. The organic acids occur largely as ions or as salts; citrate is the principle one. Furthermore, milk has many miscellaneous components, often in trace amounts.

SERU M Water Carbohydrates lactose glucose others Minerals Ca, bound Ca, ions Mg K Na Cl phosphate sulfate bicarbonate Trace elements Zn/Fe/Cu and many others Organic acids citrate formate acetate lactate oxalate others Gases oxygen nitrogen Lipids glycerides fatty acids phospholipids cerebrosides sterols Vitamins B vitamines ascorbic acid Proteins casein -lactoglobuline -lactalbumine serum albumin immunoglobulines proteose pepton NPN peptides amino acids urea ammonia Enzymes acid phosphatase peroxidases many others Phosphoric esthers Others


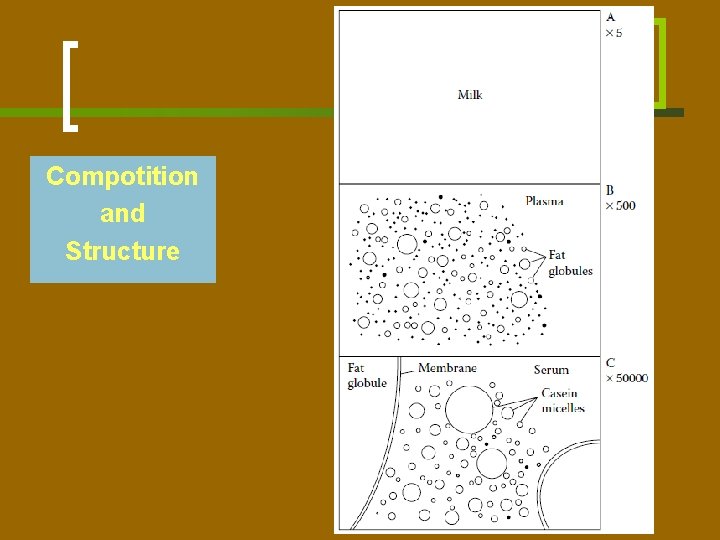
Compotition and Structure

(A) Uniform liquid. However, the liquid is turbid and thus cannot be homogeneous. (B) Spherical droplets, consisting of fat. These globules float in a liquid (plasma). (C) The plasma contains proteinaceous particles, which are casein micelles. The remaining liquid (serum) is still opalescent, so it must contain other particles. The fat globules have a thin outer layer (membrane) of different constitution.

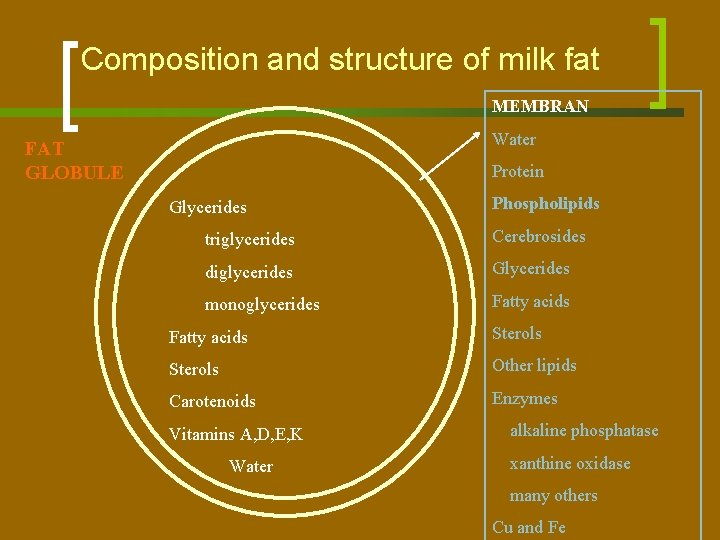
Fat Globules n n n The surface layer or membrane of the fat globule is not an adsorption layer of one single substance but consists of many components; its structure is complicated. The dry mass of the membrane is about 2. 5% of that of the fat. A small part of the lipids of milk is found outside the fat globules. At temperatures below 35°C, part of the fat in the globules can crystallize. Milk minus fat globules is called milk plasma, i. e. , the liquid in which the fat globules float.

Composition and structure of milk fat MEMBRAN Water FAT GLOBULE Protein Glycerides Phospholipids triglycerides Cerebrosides diglycerides Glycerides monoglycerides Fatty acids Sterols Other lipids Carotenoids Enzymes Vitamins A, D, E, K Water alkaline phosphatase xanthine oxidase many others Cu and Fe

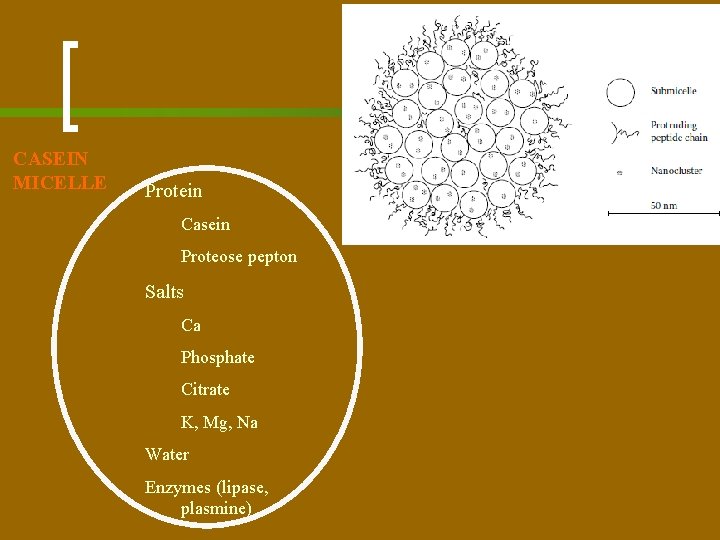
Casein Micelles n n n Casein micelles consist of water, protein, and salts. The protein is casein. Casein is present as a caseinate, which means that it binds cations, primarily calcium and magnesium. The other salts in the micelles occur as a calcium phosphate, varying somewhat in composition and also containing a small amount of citrate. This is often called colloidal phosphate. The whole may be called calciumcaseinate/calcium-phosphate complex.

Casein Micelles n n n The casein micelles are just ‘small particles. ’ The micelles have an open structure and, accordingly, contain much water, a few grams per gram of casein. Milk serum, i. e. , the liquid in which the micelles are dispersed, is milk minus fat globules and casein micelles.

CASEIN MICELLE Protein Casein Proteose pepton Salts Ca Phosphate Citrate K, Mg, Na Water Enzymes (lipase, plasmine)

Other Milk Constituents n n Serum proteins are largely present in milk in molecular form or as very small aggregates. Lipoprotein particles, sometimes called milk microsomes, vary in quantity and shape. Presumably, they consist of remnants of mammary secretory cell membranes. Few definitive data on lipoprotein particles have been published.

Other Milk Constituents n Cells, i. e. , leukocytes, are always present in milk. They account for about 0. 01% of the volume of milk of healthy cows. Of course, the cells contain all cytoplasmic components such as enzymes. They are rich in catalase.

Other milk constituents LEUKOCYTE LIPOPROTEIN PARTICLE Many enzymes Lipids e. g. katalase Protein Nucleic acids Enzymes Water

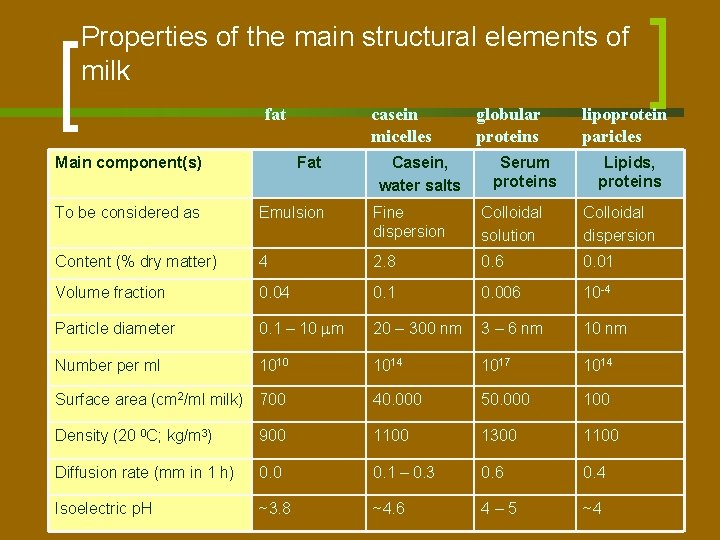
Properties of the main structural elements of milk fat casein micelles Casein, water salts globular proteins Serum proteins lipoprotein paricles Main component(s) Fat Lipids, proteins To be considered as Emulsion Fine dispersion Colloidal solution Colloidal dispersion Content (% dry matter) 4 2. 8 0. 6 0. 01 Volume fraction 0. 04 0. 1 0. 006 10 -4 Particle diameter 0. 1 – 10 m 20 – 300 nm 3 – 6 nm 10 nm Number per ml 1010 1014 1017 1014 Surface area (cm 2/ml milk) 700 40. 000 50. 000 100 Density (20 0 C; kg/m 3) 900 1100 1300 1100 Diffusion rate (mm in 1 h) 0. 0 0. 1 – 0. 3 0. 6 0. 4 Isoelectric p. H ~3. 8 ~4. 6 4– 5 ~4

Colostrum n Colostrum is the secretion produced over the first few days after parturition. The components of colostrum are synthesised in the mammary gland over several days prior to parturition. n Colostrum is rich in special nutrients for the newborn. n Colostrum contains more mineral salts and protein and less ash than later milk. Ca, Na, Mg, P, and chloride are higher in colostrum but K is lower. n The most remarkable difference between colostrum and milk is the high concentration of immunoglobulins (Ig’s) in colostrum. Ig’s are related to passive immunity against gut pathogens.

Colostrum n Colostrum has a higher level of -carotene, imparting an intense yellow colour, and a high level of somatic cells. n Recently there has been a lot of commercial interest in colostrum because of its elevated levels of bioactives, especially growth factors, and there is a wide range of literature supporting the health benefits of colostrum n Colostrum is 10 times more expensive than milk powder.

MILK ATTRIBUTES

Milk quality n Factors that determine the quality of fresh milk (standard indicators) are: ¡ Total solid contents, including protein (min. 2. 7%), fat (min. 3%), solid non fat (min. 8%). Raw milk is purchased by weight, but processed milk is sold by volume. ¡ Freezing point ¡ Density

Milk quality n Some factors can influence the quality of milk, including: ¡ Feed ¡ Genetic ¡ Climate ¡ The health status of cattle ¡ Milking process and storage ¡ Post harvest handling

Emulsion n Milk proteins have excellent emulsifying properties. Milk is categorized as o/w emulsion, since the oil part is dispersed in the water. Milk proteins, both caseinates and whey proteins, are surface active, they are absorbed rapidly to the oil-water interface, forming stable emulsions.

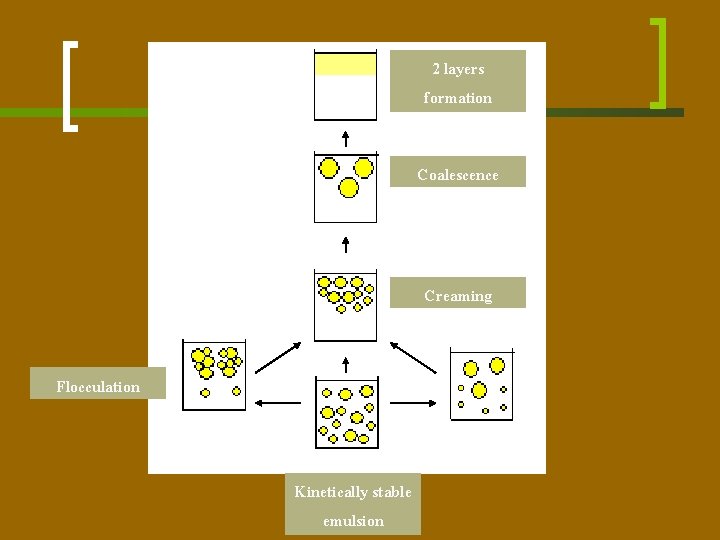
Emulsion n The primary processes leading to emulsion instability are: ¡ Creaming – refers to the gravitational separation of emulsified droplets to form a densely packed phase without change in droplet size. ¡ Flocculation – denotes the aggregation of droplets via interactions between adsorbed proteins. ¡ Coalescence – an increase in droplet size, gradually results in separation of the oil and aqueous phases.

2 layers formation Coalescence Creaming Flocculation Kinetically stable emulsion

Creaming n Since the specific gravity of lipids and skim milk is 0. 9 and 1. 036, respectively, the fat globules in milk held under quiescent conditions will rise to the surface under the influence of gravity, a process referred to as creaming. n The rapid rate of creaming is due to the strong tendency of the fat globules to cluster due to the effect of indigenous immunoglobulin M which precipitates onto the fat globules when milk is cooled (cryoglobulins).

Creaming n Large globules rise faster than smaller ones, collide with them and form aggregates. The clusters of globules rise rapidly and therefore the creaming process is accelerated as the globules rise and clump. n Creaming is inhibited by reduction of the fat globule size by homogenisation. The milk fat globules are reduced in size by pumping at very high pressure (up to 400 bar) through a small slit. The size reduction results in an increase in specific surface area.

Whipping & Foaming n n As milk proteins are surface active, they have the ability to adsorb to the air-water interface during foam formation. Foams are most commonly formed by mechanically dispersing air into a solution containing surface-active agents. A rapid diffusion of the protein to the air-water interface to reduce surface tension, followed by partial unfolding of the protein is essential for the formation of protein-based foams.

Whipping & Foaming n n Caseinates generally give higher foam overruns but produce less stable foams than whey protein concentrates (WPC). The foaming properties are influenced by many factors, including: ¡ protein concentration, ¡ level of denaturation, ¡ ionic strength, ¡ preheat treatment and ¡ presence of lipids.

Fresh Milk Deterioration n Milk can deteriorate fast since milk contains high nutrient contents such as carbohydrate, fat and protein which required by bacteria to grow. Moreover, p. H of milk is close to neutral p. H. This is very suitable for the growth of microorganisms. Lastly, since most of microorganism (mesophilic and psychotrophic bacteria) can grow very well at room temperature, fresh milk stored in room temperature is susceptible to microbial deterioration.

Fresh Milk Deterioration n n Many of the psychrotrophic bacteria isolated from milk produce extracellular enzymes that degrade milk fat and protein (proteolysis and lypolysis). Bacterial lipase causes serious degradation of milk fat. Beside microbial degradation, fresh milk also susceptible to enzymatic degradation. Raw milk has an abundance of lipoprotein lipase, an enzyme that will rapidly hydrolyse milk fat to free fatty acids (FFAs). Some of these FFAs have low organoleptic thresholds and produce odors and flavors (rancid, bitter, soapy or unclean).

The Changes of Milk Flavor n n n Deterioration of milk flavor can be caused by degradation milk fat and protein. Rancidity is a common indicator of the forming of undesirable flavor. Factors stimulating the off-flavor in fresh milk: ¡ ¡ ¡ Light Ion metals Transferred from cow to milk Microorganisms Enzymatic reactions

UHT vs Pasteurized Milk n n n Generally, there are two heat treatment given to fresh milk, i. e. pasteurization and sterilization using ultra high temperature (UHT). Pasteurization is done at 63 o. C for 30 min or 7275 o. C for 15 -20 s (high temperature short time HTST). Pasteurization is used mostly to kill Gramnegative psychrotrophs bacteria, but only has little effect on extracellular degradative enzymes. While UHT is done at 135 - 140 o. C for a few seconds. It can kill both pathogen and spoilage microorganisms. The most heat resistant pathogenic spore – C. botulinum and some enzymes also can be inactivated.

UHT vs Pasteurized Milk n n UHT products are commonly stored at room (ambient) temperature and good quality products should be microbiologically stable. Nevertheless, chemical reactions and physical changes will take place which will change the quality of the product. These include oxidation reactions, Maillard browning and chemical & physical changes which may give rise to agethickening and gelation.

UHT vs Pasteurized Milk n n In pasteurization, thermoduric bacteria and spore forming bacteria can survive. Bacillus cereus spores are relevant here, being the main pathogen which will survive pasteurization and grow at low temperature. It will certainly cause spoilage in heattreated milk. Enzymes in raw milk may give rise to problems in pasteurized milk. For example, indigenous lipases may give rise to soapy off-flavors. However, it is unlikely that bacterial lipases and proteases, which are very heat resistant, will cause problems in pasteurized milks because of their relatively short shelf-life and refrigerated storage conditions.

Milk Coagulation n n Desirable coagulation of milk can be seen in dairy products processing such as cheese, yoghurt, etc. Undesirable coagulation occur in liquid milk. It can caused by lactic acid (produced by bacteria) --- the reduction of p. H or by physical separation (due to density difference) such as creaming, flocculation or coalescence --- see emulsion chapter).

Milk Coagulation n n Milk protein, such as whey protein and casein have important role in coagulation. The example of desirable coagulation: ¡ Acidification forms the basis of production of all fermented milks. The gels of fermented milks, such as yoghurt, are formed by acidification of milk. As the p. H is reduced, the casein precipitates selectively. The first signs of aggregation occur around p. H 5 and once the p. H falls to 4. 6 all the casein becomes insoluble.

Milk Coagulation n Some factors influence coagulation, including: ¡ p. H ¡ Temperature ¡ Heat treatment ¡ Casein concentration ¡ The presence of salt

Milk & Dairy Products Adulteration n n n n Watering of milk Milk of different species Addition of non-dairy protein Altering the casein/whey protein ratio Addition of buttermilk or whey powder to milk powder Addition of vegetable or animal fats to milk fat Addition of reconstituted milk to fluid milk Non-authorized preservatives.

References n Walstra, P. , J. T. M. Wouters & T. J. Geurts. 2006. Dairy Science and Technology 2 nd Edition. Taylor and Francis Group. Boca Raton.

Thank You….
 Ve inneke leala
Ve inneke leala Sensory evaluation of dairy products
Sensory evaluation of dairy products Milk for toddlers with milk allergynon dairy
Milk for toddlers with milk allergynon dairy Human milk vs cow milk
Human milk vs cow milk Contamination of milk and milk products
Contamination of milk and milk products G
G Disaccharide found in milk
Disaccharide found in milk Milk packaging machine
Milk packaging machine His favourite subject is
His favourite subject is Unit 2 food food food
Unit 2 food food food Food chain food chain food chain
Food chain food chain food chain Metric conversion chart king henry died
Metric conversion chart king henry died Milk and food coloring lab report
Milk and food coloring lab report Basic geometric features of symbology
Basic geometric features of symbology Idle time meaning in cost accounting
Idle time meaning in cost accounting Pop culture examples
Pop culture examples Refers to the knowledge language values customs
Refers to the knowledge language values customs All groups create norms to enforce their cultural values.
All groups create norms to enforce their cultural values. Useful and harmful materials in the house
Useful and harmful materials in the house Grade c milk
Grade c milk Scum formation definition milk
Scum formation definition milk Dmse iit delhi
Dmse iit delhi Mit material science
Mit material science Material science lab report
Material science lab report Motif in material science
Motif in material science What is material science
What is material science Materials science oxford
Materials science oxford Natural vs social science
Natural vs social science Branches of natural science
Branches of natural science Natural and physical science
Natural and physical science Applied science vs pure science
Applied science vs pure science Natural science and social science similarities
Natural science and social science similarities Wwwk-6.thinkcentral
Wwwk-6.thinkcentral Tragedy of the commons
Tragedy of the commons Windcube
Windcube Hard science and soft science
Hard science and soft science Ffa food science
Ffa food science Introduction of food web
Introduction of food web Food science careers
Food science careers Unit 4 food
Unit 4 food Chapter 1 food science an old but new subject
Chapter 1 food science an old but new subject Food adulteration investigatory project
Food adulteration investigatory project The science of food the nutrients and substances therein
The science of food the nutrients and substances therein Food science an old but new subject
Food science an old but new subject Principles of carbohydrates
Principles of carbohydrates