Mechanisms of toxicity Mechanisms of toxicity l l









- Slides: 25

Mechanisms of toxicity

Mechanisms of toxicity l l l l l Inhibition of oxygen transport Inhibition of electron transport chain Irritating, corrosivity Inhibition of enzymes Penetrating lipid structures, predominantly in the CNS Carcinogenic activity Teratogenic activity Radical damage Block of neurotransmission

The effect depends on: l Physical and chemical properties of the substance: – state, solubility… l Exposure: – dose, concentration, duration … l Organism: – sex, age, condition…

1) Inhibition of oxygen transport l CO: – produced by the incomplete combustion of organic compounds (e. g. gas) – binds to hemoglobin ( carboxyhemoglobin) with higher affinity than oxygen, thus hindering the transport of oxygen – symptoms: at 30 -40% of Hb. CO – headache, dizziness, unconsciousness; at 60 -65% of Hb. CO – coma – intervention: mechanical ventilation (oxygen displaces CO)

l Poisons forming methemoglobin: – nitrites, derivatives of aniline, certain drugs (esters of HNO 3) – Fe 2+ in the molecule of Hb is oxidized to Fe 3+ Hb is converted to methemoglobin which is unable to bind O 2 – symptoms: cyanosis – treatment: toluidine blue: • speeds up the reduction of Met. Hb to Hb . Cl-

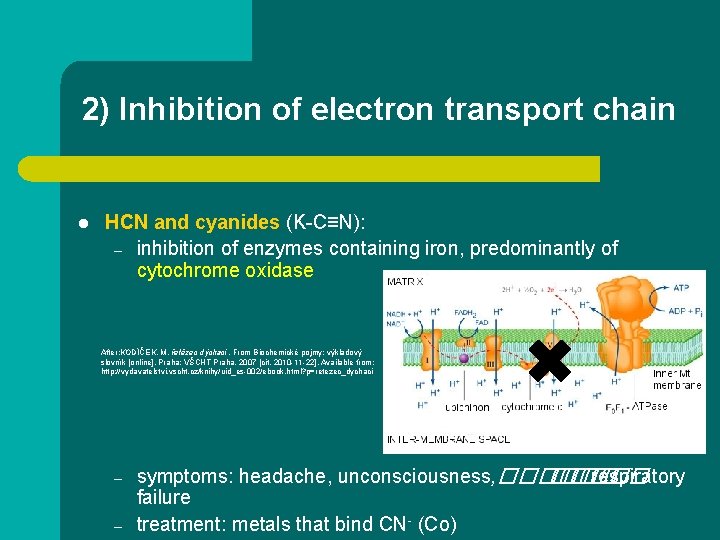
2) Inhibition of electron transport chain l HCN and cyanides (K-C≡N): – inhibition of enzymes containing iron, predominantly of cytochrome oxidase After: KODÍČEK, M. řetězec dýchací. From Biochemické pojmy: výkladový slovník [online]. Praha: VŠCHT Praha, 2007 [cit. 2010 -11 -22]. Available from: http: //vydavatelstvi. vscht. cz/knihy/uid_es-002/ebook. html? p=retezec_dychaci – – symptoms: headache, unconsciousness, ������ respiratory failure treatment: metals that bind CN- (Co)

3) Irritating gases l Cl 2, HCl, HF, halogen derivatives – some of them are used as tear gases – irritate the mucous membranes in the eyes, nose, mouth and lungs: react with –SH groups of proteins – symptoms: conjunctivitis, rhinitis, ﺍﻷﻨﻒ ﺍﻟﺘﻬﺎﺏ bronchitis, sometimes even pulmonary edema (phosgene)

4) Inhibition of enzymes l HCN – see above l H 2 S: – forms insoluble sulfides with transition metals, especially iron inhibits cytochrome oxidase and electron transport chain – symptoms: respiratory difficulties, circulation failure l -amanitin: – poison of „death cap“ – inhibits RNA-polymerase liver damage, heart and kidney failure

l Metals: – react with –SH groups of enzymes – e. g. lead inhibits enzymes participating in the synthesis of porphyrin, and thus hematopoiesis – metals can accumulate in the liver, kidney, and bones – symptoms: glomerular nephritis, neurological symptoms, a grey line along the gum (lead, mercury), anemia (lead)

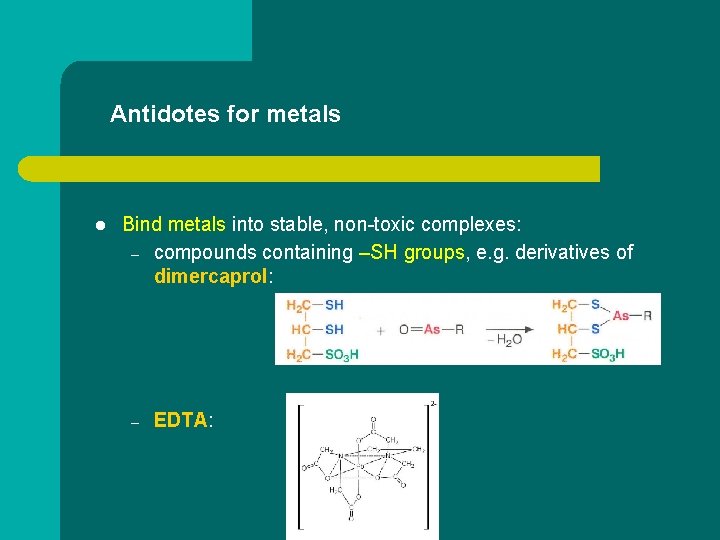
Antidotes for metals l Bind metals into stable, non-toxic complexes: – compounds containing –SH groups, e. g. derivatives of dimercaprol: – EDTA:

5) Corrosivity, acidosis l l Acids: – local effects (hydrolysis of biomolecules, protein coagulation ) – moreover, intake of H+ can cause acidosis: fall of blood p. H • compensation: hyperventilation, ↑ tubular secretion of H+ • treatment: neutralization using Mg. O Bases: tissue damage is more severe than by acids – treatment: large volume of water acidified with a weak acid (acetic)

6) Organic solvents: penetrating the membranes l Organic solvents can easily penetrate lipid structures of the cell l In CNS, they act as anesthetics, sedatives, and hypnotics, ﻭﺍﻟﻤﻨﻮﻣﺎﺕ ﻭﺍﻟﻤﻬﺪﺋﺎﺕ they can cause excitation, inhibition, as well as neurotoxicity l Halogen derivatives – chloroform, vinyl chloride , – they can also damage the liver and kidney

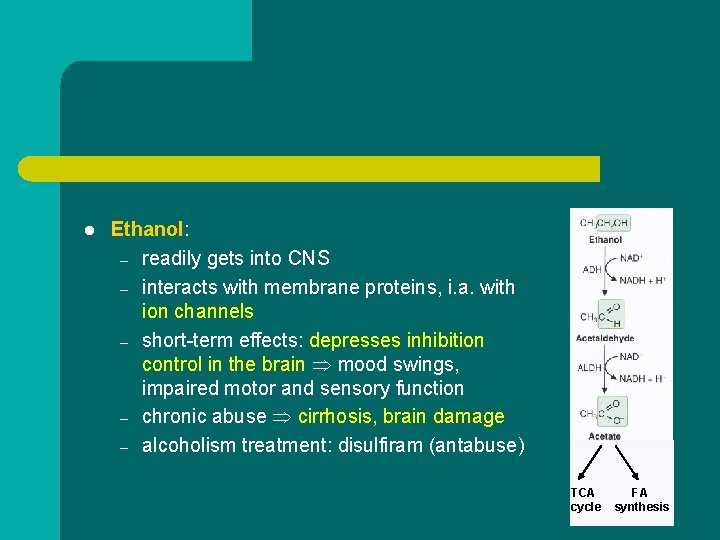
l Ethanol: – readily gets into CNS – interacts with membrane proteins, i. a. with ion channels – short-term effects: depresses inhibition control in the brain mood swings, impaired motor and sensory function – chronic abuse cirrhosis, brain damage – alcoholism treatment: disulfiram (antabuse) TCA cycle FA synthesis

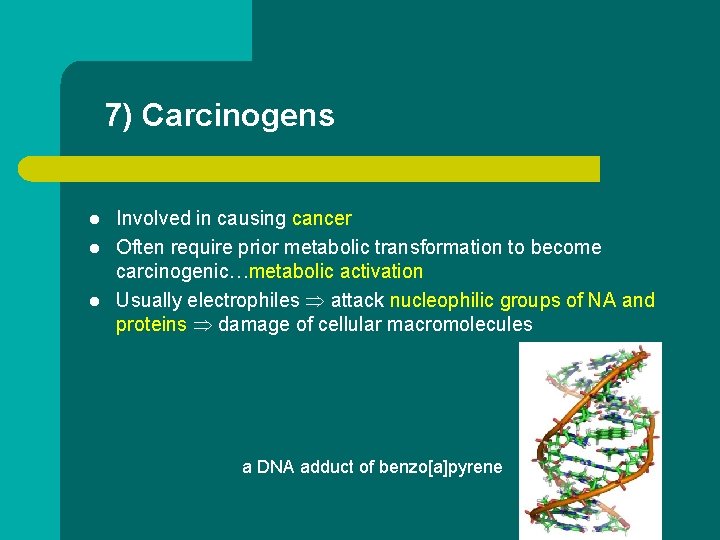
7) Carcinogens l l l Involved in causing cancer Often require prior metabolic transformation to become carcinogenic…metabolic activation Usually electrophiles attack nucleophilic groups of NA and proteins damage of cellular macromolecules a DNA adduct of benzo[a]pyrene

Damage to DNA l Mutations – can be caused by: – alkylating agents – DNA crosslinkers – DNA intercalating agents – usually cationic planar (aromatic) – compounds that form DNA adducts l Some of these agents can also inhibit transcription and replication ethidium bromide

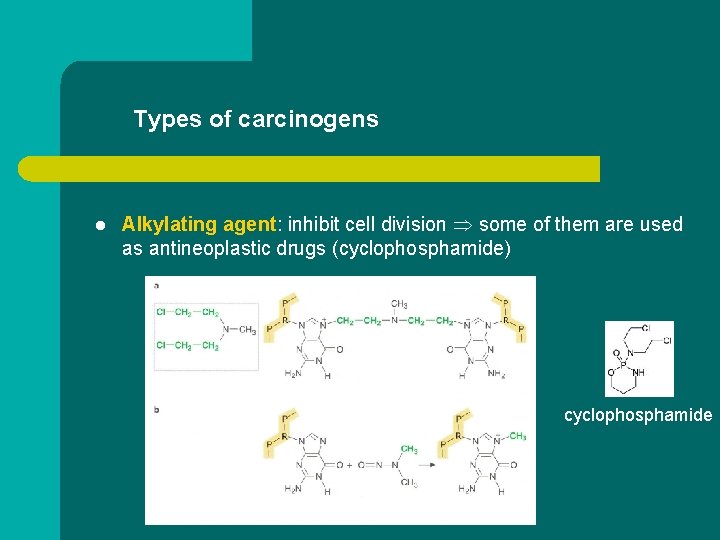
Types of carcinogens l Alkylating agent: inhibit cell division some of them are used as antineoplastic drugs (cyclophosphamide) cyclophosphamide

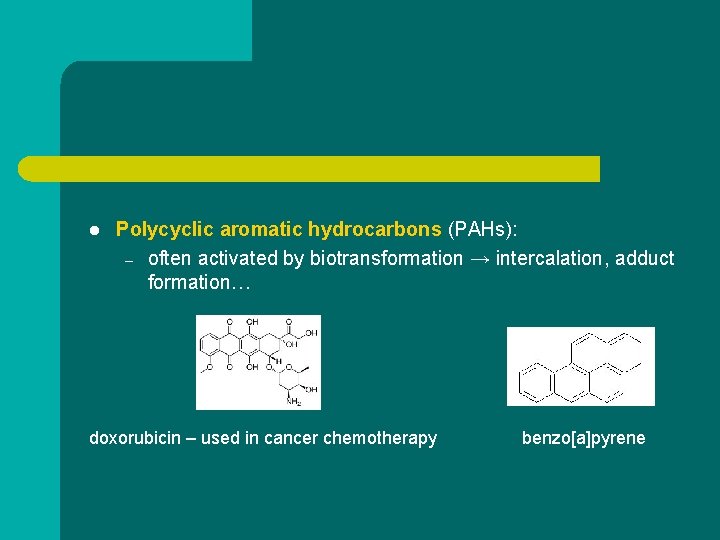
l Polycyclic aromatic hydrocarbons (PAHs): – often activated by biotransformation → intercalation, adduct formation… doxorubicin – used in cancer chemotherapy benzo[a]pyrene

l Inorganic substances: arsenic, chromium salts, asbestos: – l Asbestos = silicate minerals exploited commercially; dust inhalation → phagocytosis, pulmonary fibrosis → carcinoma Naturally occurring compounds: – aflatoxin produced by Aspergillus flavus (a fungus, contaminating peanuts, cereals…)

8) Teratogenic agents l Impair fetal development (depends on developmental stage) l Most of the carcinogens listed above, certain drugs – Thalidomide (Contergan): birth defects l Potential mechanism: – folate antagonism – endocrine disruption – oxidative stress – receptor- or enzyme-mediated teratogenesis

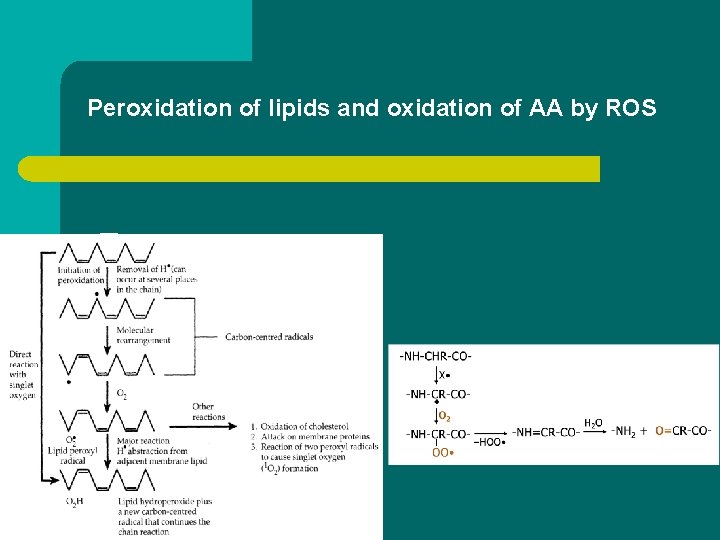
9) Damage by reactive species l Compounds increasing the formation of reactive oxygen species (ROS): H 2 O 2, OH • , O 2 • - – peroxidation of membrane lipids – oxidation of amino acids in proteins – damage to DNA l Paraquat: herbicide, impairs transport of electrons in the electron transport chain and stimulates ROS formation – damage to the liver, kidney, and lung

Peroxidation of lipids and oxidation of AA by ROS

10) Block of neurotransmission l Plant as well as animal toxins – snake venoms: • -bungarotoxin – binds to the acetylcholine receptor at the neuromuscular junction, causing paralysis, respir. failure – tetrodotoxin – concentrated in internal organs of members of the order Tetraodontiformes (fish); blocks Na+ channels paralysis of the diaphragm, respiratory failure – curare: alkaloid; blocks neuromuscular transmission paralysis of the respiratory muscles

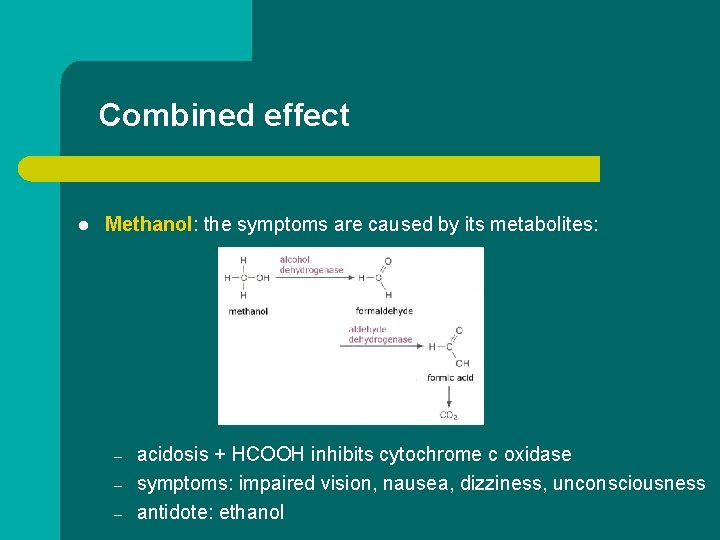
Combined effect l Methanol: the symptoms are caused by its metabolites: – – – acidosis + HCOOH inhibits cytochrome c oxidase symptoms: impaired vision, nausea, dizziness, unconsciousness antidote: ethanol

Treatment of acute poisoning l Decreasing the absorption of the toxic substance: – – – l gastric lavage cathartics (Na 2 SO 4, mannitol) activated charcoal Antidotes

l Enhanced excretion of the toxic substance: – – l forced diuresis (by diuretics) exchange transfusion, hemodialysis (if the toxin is concentrated in the circulation, not bound in tissues) Treatment for symptoms (ensuring adequate cardiopulmonary function, electrolyte and acid-base balance…)