APAP Li Fe Toxicity Heather Patterson PGY 4
























- Slides: 88

APAP Li Fe Toxicity Heather Patterson PGY 4 Dr. Ingrid Vicas October 8, 2008

Learning Outcomes • By the end of the session learners will be able to: – Identify clinical presentations, appropriate diagnostic and therapeutic management for acetaminophen, lithium, and iron toxicity

Acetaminophen • Accounts for more deaths than any other pharmaceutical agent • Most common ingestion in all age groups • One of the most common causes of acute hepatic failure • Mainstays of Rx – GI decontamination/A. C. – NAC

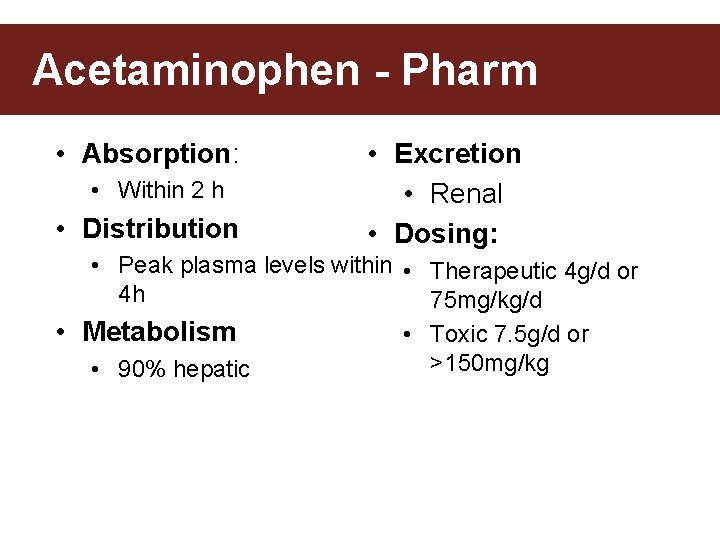
Acetaminophen - Pharm • Absorption: • Within 2 h • Distribution • Excretion • Renal • Dosing: • Peak plasma levels within • Therapeutic 4 g/d or 4 h 75 mg/kg/d • Metabolism • Toxic 7. 5 g/d or >150 mg/kg • 90% hepatic

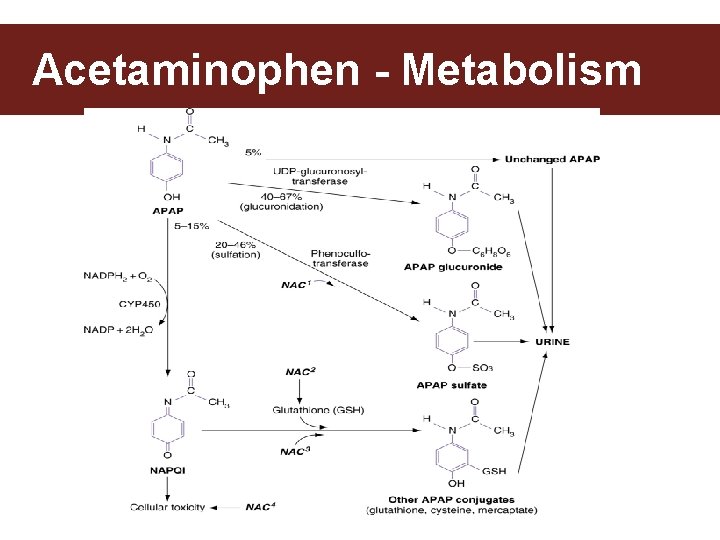
Acetaminophen - Metabolism

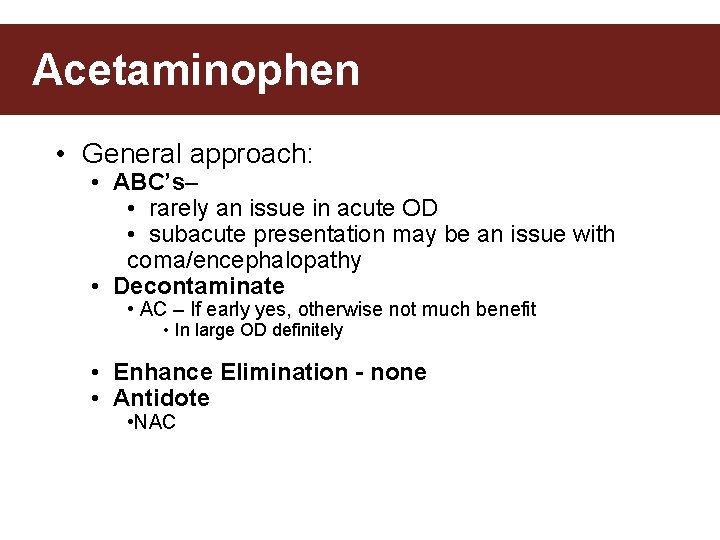
Acetaminophen • General approach: • ABC’s– • rarely an issue in acute OD • subacute presentation may be an issue with coma/encephalopathy • Decontaminate • AC – If early yes, otherwise not much benefit • In large OD definitely • Enhance Elimination - none • Antidote • NAC

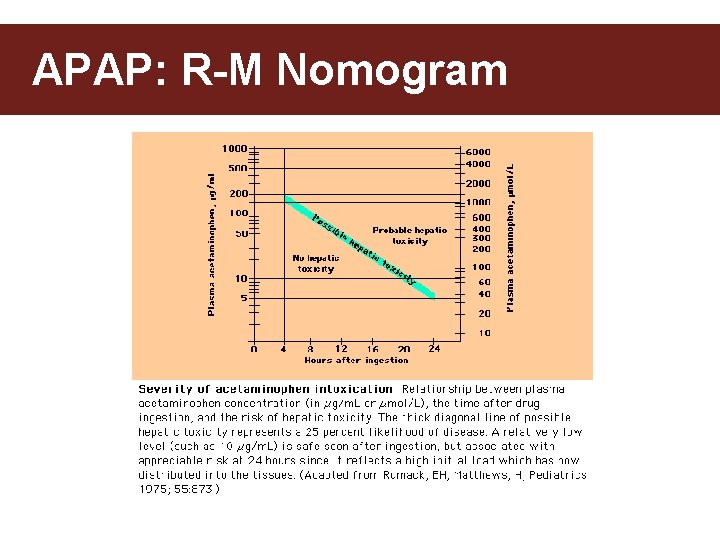
Acetaminophen - Levels • When should we get the first APAP level? • No added benefit of getting a level <4 h • Peak levels at 4 h • 1 st point on the RM nomogram

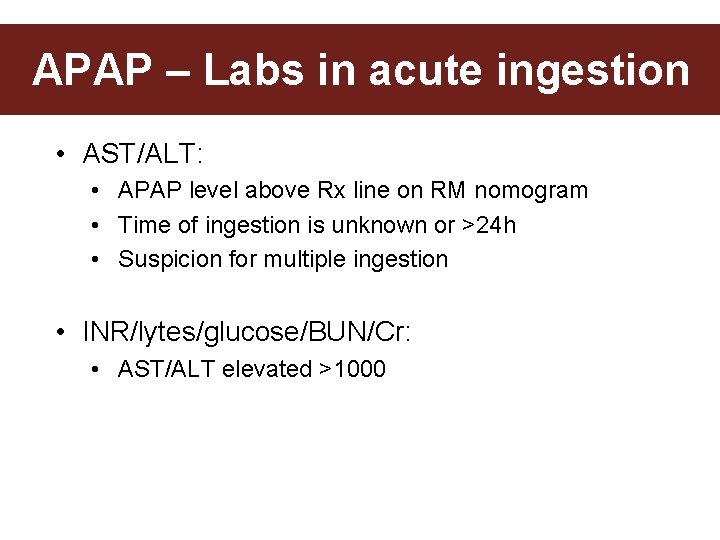
APAP – Labs in acute ingestion • AST/ALT: • APAP level above Rx line on RM nomogram • Time of ingestion is unknown or >24 h • Suspicion for multiple ingestion • INR/lytes/glucose/BUN/Cr: • AST/ALT elevated >1000

APAP: R-M Nomogram

APAP: R-M Nomogram • When can we NOT use the Nomogram to guide our therapy? • • Chronic OD 24 h since time of ingestion >Unknown time of ingestion ? ? ? Extended release preps? ? ?

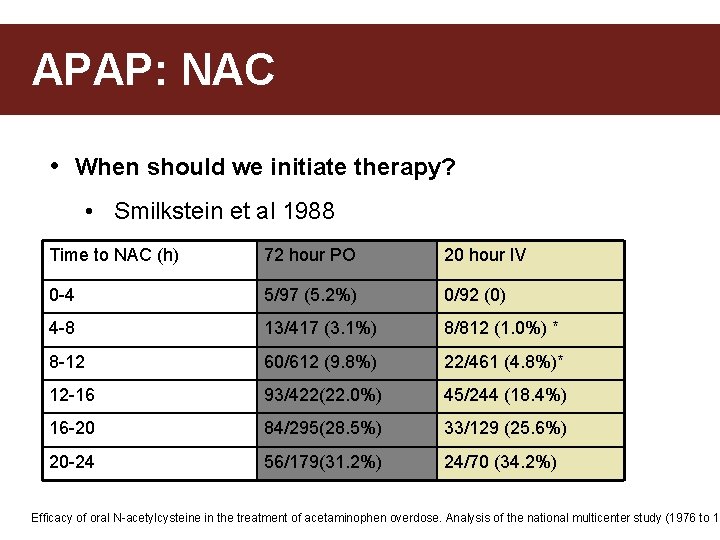
APAP: NAC • When should we initiate therapy? • Smilkstein et al 1988 Time to NAC (h) 72 hour PO 20 hour IV 0 -4 5/97 (5. 2%) 0/92 (0) 4 -8 13/417 (3. 1%) 8/812 (1. 0%) * 8 -12 60/612 (9. 8%) 22/461 (4. 8%)* 12 -16 93/422(22. 0%) 45/244 (18. 4%) 16 -20 84/295(28. 5%) 33/129 (25. 6%) 20 -24 56/179(31. 2%) 24/70 (34. 2%) Efficacy of oral N-acetylcysteine in the treatment of acetaminophen overdose. Analysis of the national multicenter study (1976 to 19

APAP – NAC • What is NAC and what is the MOA? 1. 2. 3. 4. 5. Glutathione precursor Glutathione substitute Enhances sulfate conjugation of APAP Anti-inflammatory and anti-oxidant effects Inotropic and vasodilatory properties that improve microcirculation

APAP – NAC • What are 5 indications for NAC? 1. Level above “possible hepatic toxicity” line on nomogram 2. Hx of ingestions & level not available before 8 h 3. Hx APAP ingestion + evidence of hepatic toxicity 4. Multiple APAP doses, RFs for hepatotoxicity + >66 mcg/m. L 5. Unknown time of ingestion and level >66 mcg/m. L? ?

APAP – NAC • How is NAC administered? • Initial: 150 mg/kg in 250 -500 cc D 5 W over 3060 min • 2 nd infusion: 50 mg/kg in 500 -1000 cc D 5 W over 4 h • 3 rd infusion: 100 mg/kg in 1000 cc D 5 W over 16 h • Better way of doing this is CPS: There is a chart that looks at dosing and mixing of NAC in a user friendly format.

Extended release preps • Tabs have ½ immediate release acetaminophen and ½ as extended release • Study by Cetaruk et al showed that ordering only a 4 h level would have missed 3/14 pts when treatment was indicated by a second level • Suggests that ongoing absorption occurs beyond the 2 -4 h absorption seen with non-ER tabs

Extended release preps • How should we manage these overdoses? • Cetaruk et al – repeat APAP level 4 -8 h post first level (if first level within 8 h) • Manufacturer – • Undetectable @4 h – no Rx, no repeat level • Above Rx line – treat • Detectable but below line@4 h – repeat level 4 -6 h later • Rosen’s – single level at 4 h and treat based on that level

Case 2: • 75 M. Hx of RA & Et. OH abuse. • Presents to ED with FOOSH injury and distal radius #. • d/c’d with a script of Percocets – which he took faithfully, but did not stop his regular Rx • Returns to ED two days later c/o of abdominal pain – especially RUQ • MEDS: • tylenol daily ( 2 -3 gm/day), dilantin.

Stages of Acetaminophen Toxicity • Stage 1: <24 h • Historical features • N/V/anorexia, malaise, diaphoresis • Stage 2: 24 -72 h • P/E features • Stage 1 plus RUQ/epigastric pain

Stages of Acetaminophen Toxicity • Stage 3: 2 -7 d • Lab features: AST/ALT >1000 • Fulminant hepatic failure, hypoglycemia, coagulopathy, encephalopathy/coma • Stage 4: >7 d • Recovery • Enzymes return to N within 5 -7 d

Acute vs. Chronic Ingestions • Acute: • Entire ingestion within 4 h • Chronic • All other repeated supratherapeutic ingestions • Factors that complicate acute ingestions: • Unknown time of ingestion • >24 h after ingestion • ? peds

Risk Factors for Chronic Toxicity • Chronic Et. OH abusers in subacute/chronic OD • Medications that induce P 450 system • Malnourished • Depleted glutathione stores • Less NAPQI binding with glutathione • More hepatotoxicity • Febrile illness in peds <5 y

Indications for Labs in Chronic 1. S+S of hepatotoxicity 2. Peds: • >75 mg/kg in 24 h with risk factors • >150 mg/kg in 24 h with risk factors 3. Adults: • >4 g/24 h with risk factors • >7. 5 g/24 h without risk factors

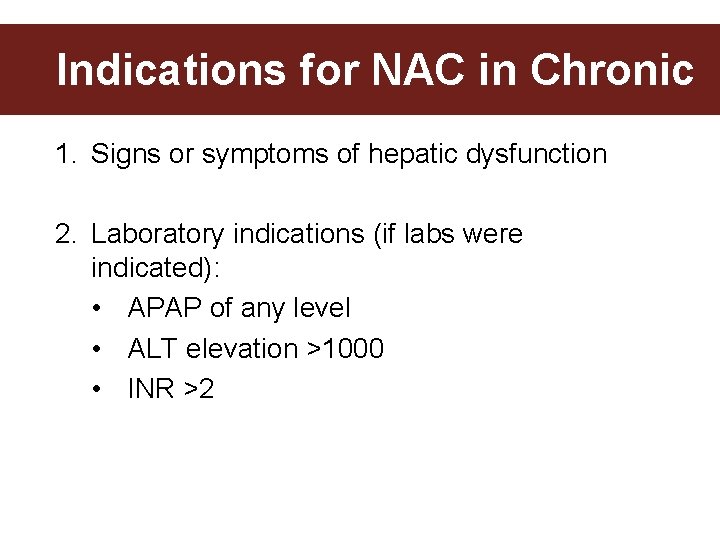
Indications for NAC in Chronic 1. Signs or symptoms of hepatic dysfunction 2. Laboratory indications (if labs were indicated): • APAP of any level • ALT elevation >1000 • INR >2

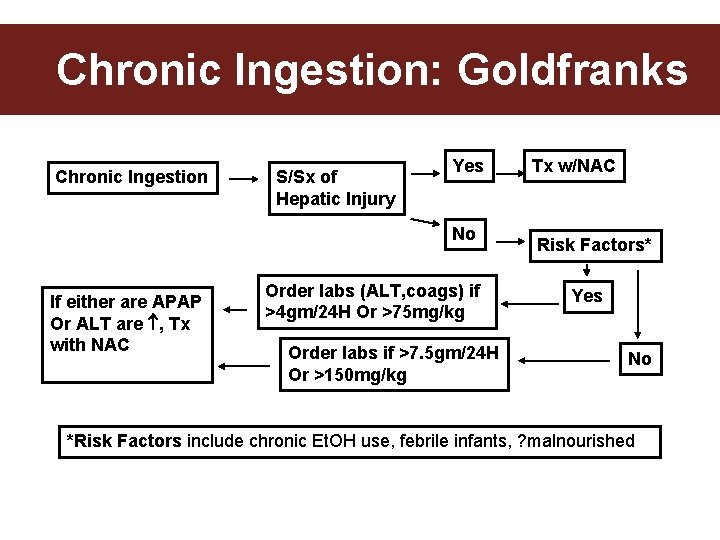
Chronic Ingestion: Goldfranks Chronic Ingestion S/Sx of Hepatic Injury Yes No If either are APAP Or ALT are , Tx with NAC Order labs (ALT, coags) if >4 gm/24 H Or >75 mg/kg Order labs if >7. 5 gm/24 H Or >150 mg/kg Tx w/NAC Risk Factors* Yes No *Risk Factors include chronic Et. OH use, febrile infants, ? malnourished

Case 3 • 18 F suicide attempt a few days ago. Told friend but swore her to secrecy. • Nausea and vomiting x 1 day. Improved for a day. Now returns with N/V and RUQ pain. Feeling ++ unwell.

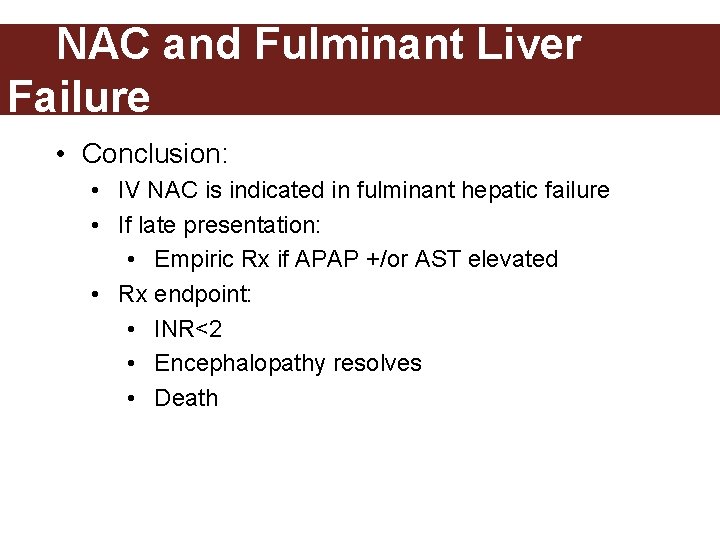
NAC and Fulminant Liver Failure • Keays et al (late 80 s) • Population: 50 pts with liver failure secondary to APAP toxicity without NAC treatment • Intervention: IV NAC or standard therapy

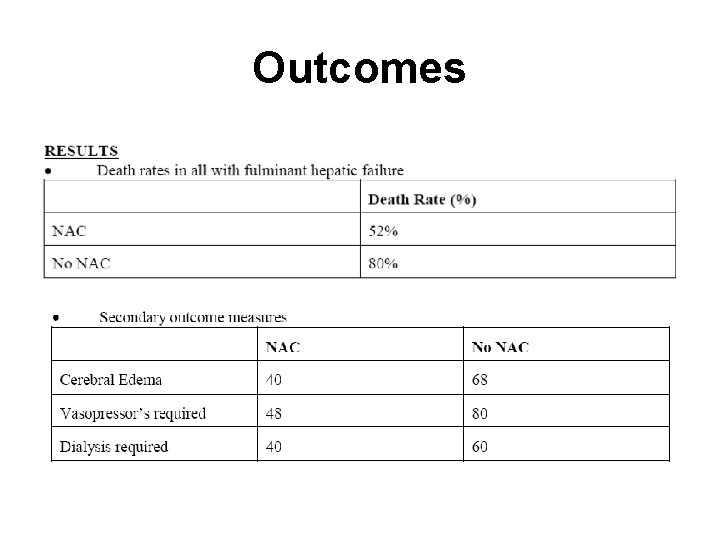
Outcomes

NAC and Fulminant Liver Failure • Conclusion: • IV NAC is indicated in fulminant hepatic failure • If late presentation: • Empiric Rx if APAP +/or AST elevated • Rx endpoint: • INR<2 • Encephalopathy resolves • Death

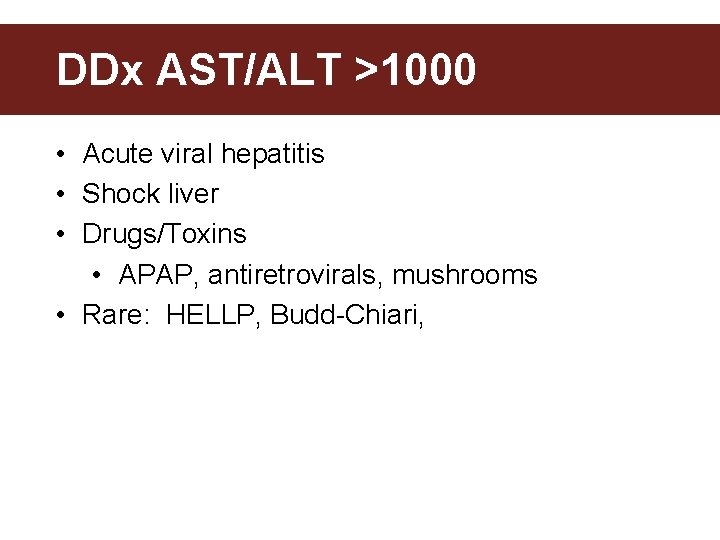
DDx AST/ALT >1000 • Acute viral hepatitis • Shock liver • Drugs/Toxins • APAP, antiretrovirals, mushrooms • Rare: HELLP, Budd-Chiari,

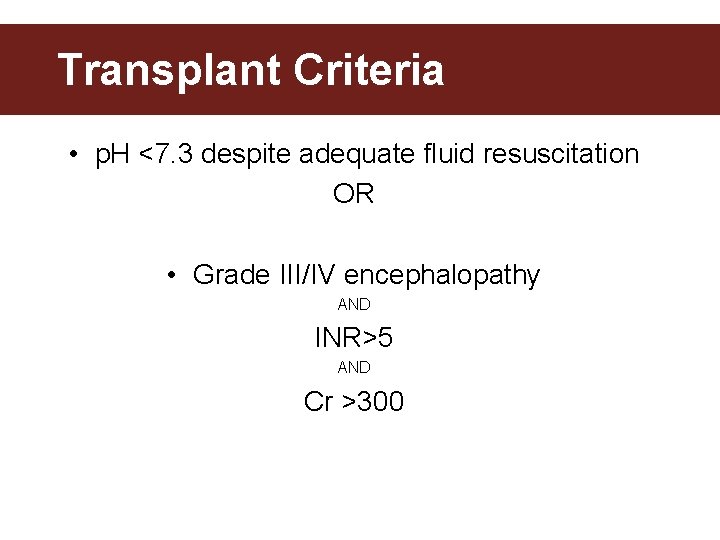
Transplant Criteria • p. H <7. 3 despite adequate fluid resuscitation OR • Grade III/IV encephalopathy AND INR>5 AND Cr >300

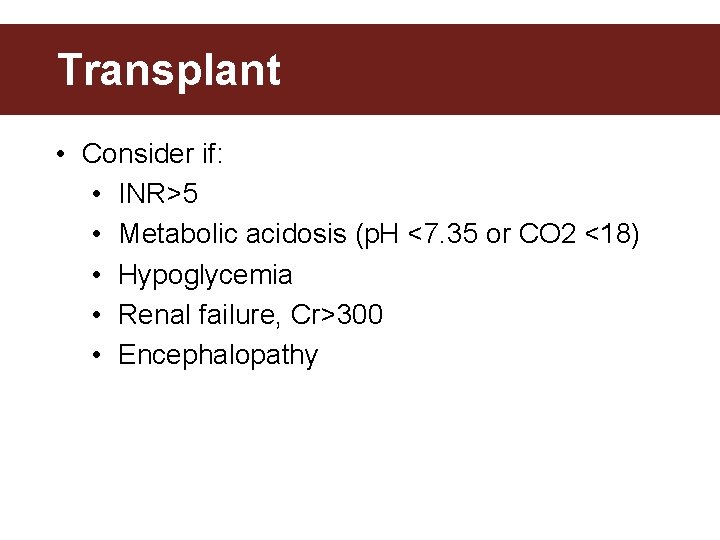
Transplant • Consider if: • INR>5 • Metabolic acidosis (p. H <7. 35 or CO 2 <18) • Hypoglycemia • Renal failure, Cr>300 • Encephalopathy

Case 4 • Unknown time of ingestion

Indications for NAC • Unknown time of ingestion • APAP detectable regardless of level? ? ? • Repeat levels/half life • AST elevated regardless of APAP level • NO Rx if APAP –ve and AST is normal

Anaphylactoid reactions to NAC • Can look like anaphylaxis • <20% of IV NAC infusions • Management: • • Prevent if possible use ideal body wt Temporarily stop infusion, wait 10 -15 min Give benadryl IV Restart at a 1/2 rate and over next hour increase to original rate


Iron • MC of death due to poisoning in children – Traditionally iron OD’s where in children < 6 yo taking excess multi-vits • Less of an issue now because of the packaging of iron and the different formulations of children multi-vits

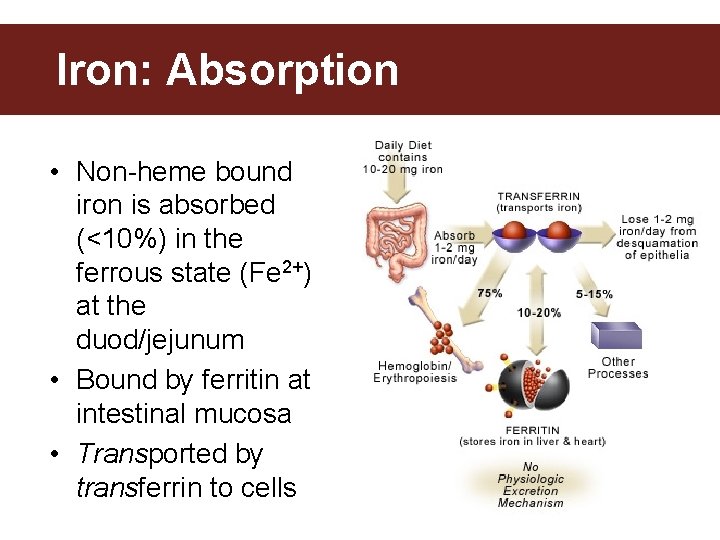
Iron: Absorption • Non-heme bound iron is absorbed (<10%) in the ferrous state (Fe 2+) at the duod/jejunum • Bound by ferritin at intestinal mucosa • Transported by transferrin to cells

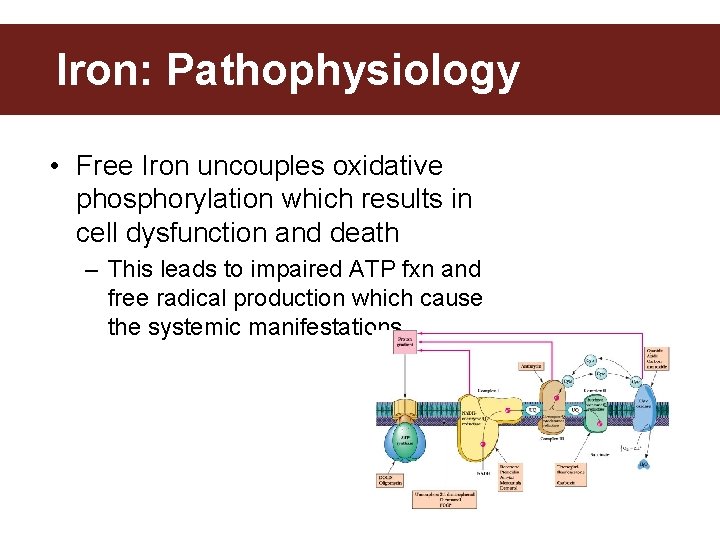
Iron: Pathophysiology • Free Iron uncouples oxidative phosphorylation which results in cell dysfunction and death – This leads to impaired ATP fxn and free radical production which cause the systemic manifestations

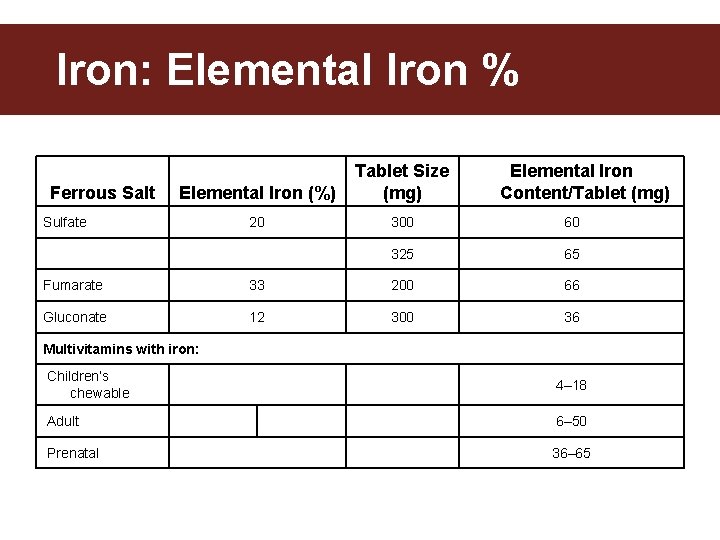
Iron Toxicity • Toxicity of Fe compounds depends on amount of elemental iron • (% elemental iron x mg tabs) x number of tabs = amt of elemental iron * ie. 100 tabs of Ferrous sulphate (325 mg each) =20% x 325 x 100 =6500 mg of elemental iron

Iron: Elemental Iron % Ferrous Salt Elemental Iron (%) Tablet Size (mg) 20 300 60 325 65 Sulfate Elemental Iron Content/Tablet (mg) Fumarate 33 200 66 Gluconate 12 300 36 Multivitamins with iron: Children’s chewable 4– 18 Adult 6– 50 Prenatal 36– 65

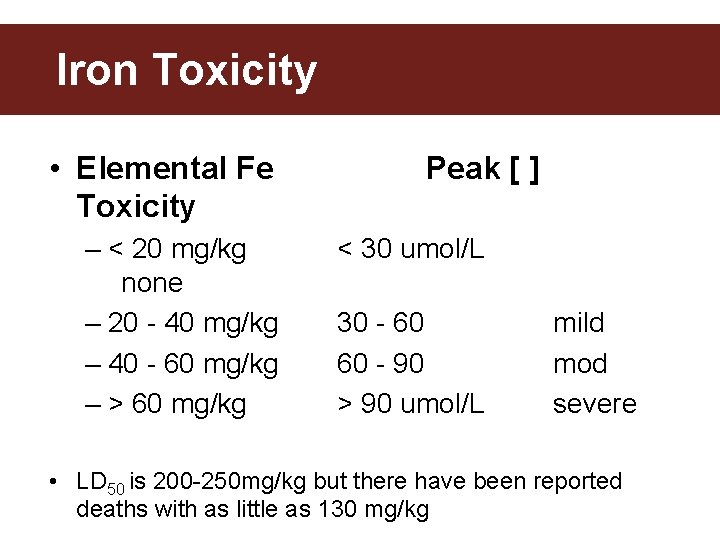
Iron Toxicity • Elemental Fe Toxicity – < 20 mg/kg none – 20 - 40 mg/kg – 40 - 60 mg/kg – > 60 mg/kg Peak [ ] < 30 umol/L 30 - 60 60 - 90 > 90 umol/L mild mod severe • LD 50 is 200 -250 mg/kg but there have been reported deaths with as little as 130 mg/kg

Iron: Local toxicity • Dose: 10 -20 mg/kg lower doses than systemic toxicity • Mech: Direct GI corrosive/irritant – N, V, abdominal pain, – diarrhea, – hematemasis, melena, hematochezia • Must consider on ddx of gastroenteritis/ GI bleed in peds

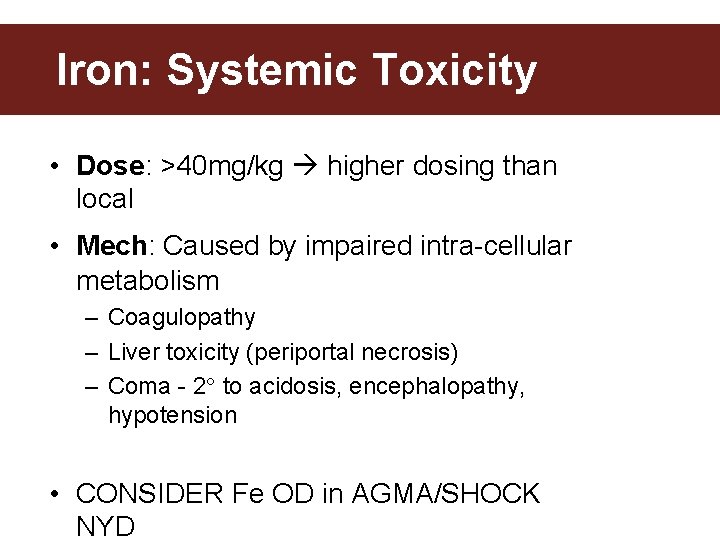
Iron: Systemic Toxicity • Dose: >40 mg/kg higher dosing than local • Mech: Caused by impaired intra-cellular metabolism – Coagulopathy – Liver toxicity (periportal necrosis) – Coma - 2 to acidosis, encephalopathy, hypotension • CONSIDER Fe OD in AGMA/SHOCK NYD

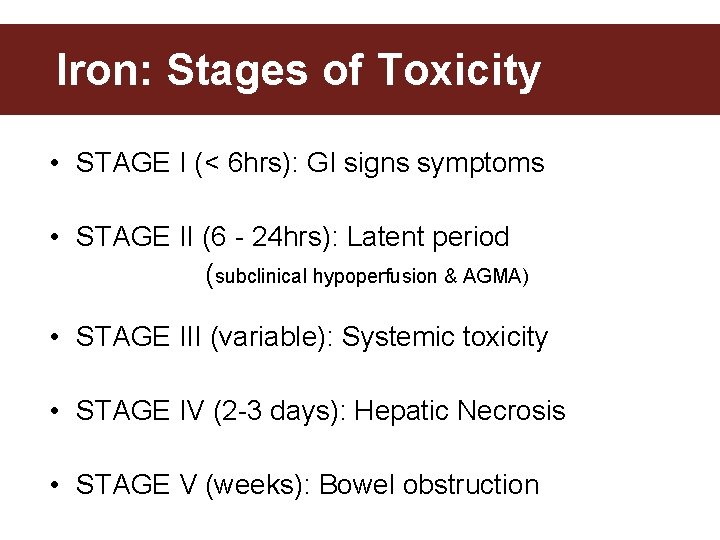
Iron: Stages of Toxicity • STAGE I (< 6 hrs): GI signs symptoms • STAGE II (6 - 24 hrs): Latent period (subclinical hypoperfusion & AGMA) • STAGE III (variable): Systemic toxicity • STAGE IV (2 -3 days): Hepatic Necrosis • STAGE V (weeks): Bowel obstruction

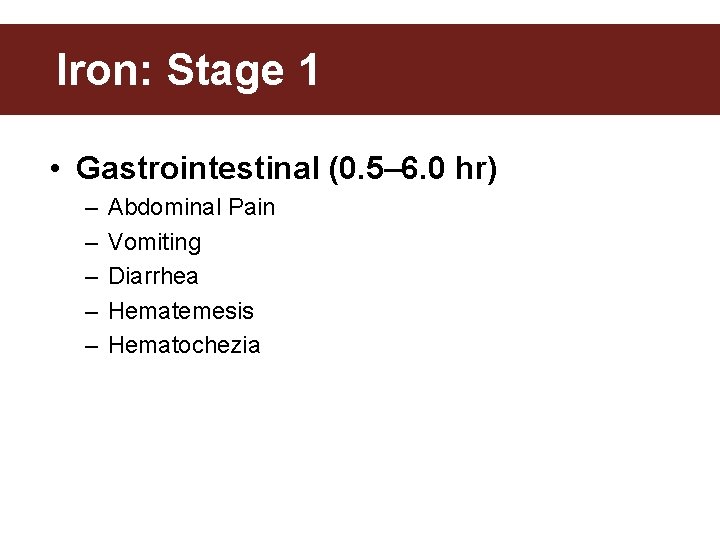
Iron: Stage 1 • Gastrointestinal (0. 5– 6. 0 hr) – – – Abdominal Pain Vomiting Diarrhea Hematemesis Hematochezia

Iron: Stage 2 • Latent Stage/Relative Stability (4 -12 H) – Aka “CALM BEFORE THE STORM” – May not have obvious signs or symptoms but they are getting sick – GI Sx usually resolve during this stage – Evidence of subclinical hypoperfusion and AGMA

Iron: Stage 3 • Systemic toxicity- Shock and Acidosis (672 H) – – – Hypoperfusion Metabolic acidosis Coma Coagulopathy MODS

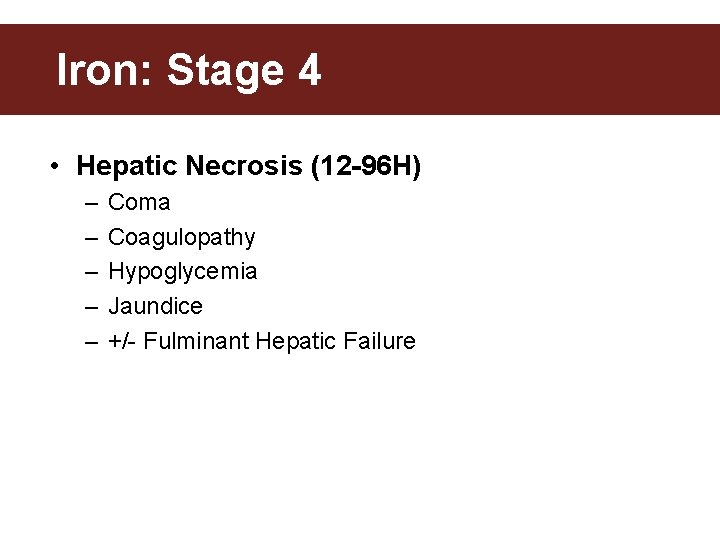
Iron: Stage 4 • Hepatic Necrosis (12 -96 H) – – – Coma Coagulopathy Hypoglycemia Jaundice +/- Fulminant Hepatic Failure

Iron: Stage 5 • Bowel Obstruction (2 -4 wks) – Abdominal pain – Vomiting – Dehydration

Iron: Investigations • Name 3 ways to directly assess for Iron? – Serum Iron levels (4 -6 h post ingestion) – Deferoxamine Challenge • What is this? – AXR

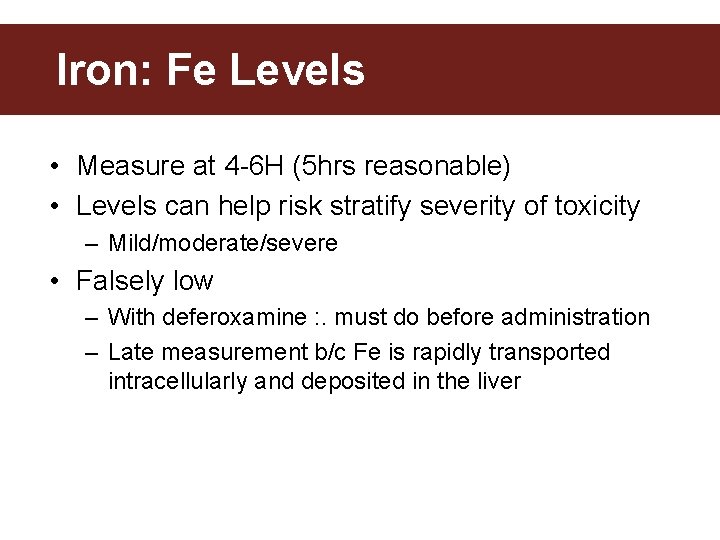
Iron: Fe Levels • Measure at 4 -6 H (5 hrs reasonable) • Levels can help risk stratify severity of toxicity – Mild/moderate/severe • Falsely low – With deferoxamine : . must do before administration – Late measurement b/c Fe is rapidly transported intracellularly and deposited in the liver

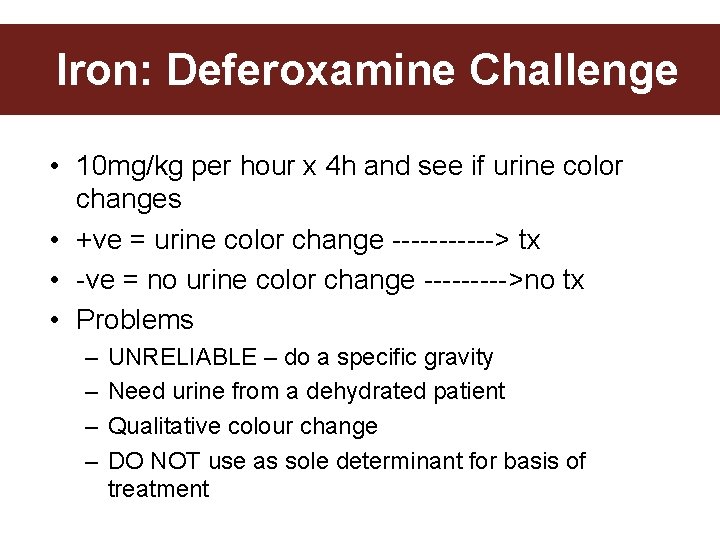
Iron: Deferoxamine Challenge • 10 mg/kg per hour x 4 h and see if urine color changes • +ve = urine color change ------> tx • -ve = no urine color change ----->no tx • Problems – – UNRELIABLE – do a specific gravity Need urine from a dehydrated patient Qualitative colour change DO NOT use as sole determinant for basis of treatment

Iron: AXR • Which toxins are visible on AXR? – – – Chloral hydrate, crack vials, Ca carbonate Heavy metals Iron, iodides Psychotropics, phosphates Enteric –coated preps Slow-release preparations

Iron: AXR • Absence on AXR does NOT r/o ingestion • How can one have a –ve AXR? – Pt did not take Iron – Iron solutions (liquid) are not radiopaque – Chewables formulations are not radiopaque – Iron tabs have already dissolved

Iron: Investigations • Which labs are you going to order? – CH 6, serum iron, LFTs, coags – ABG – +/-APAP/ASA • 3 Abnormalities on CH 6 – Increased WBC – AG – Hypoglycemia

Iron: Decontamination • Charcoal does not bind Fe • Gastric Lavage – tabs are large therefore may not work – Use tap water or saline • Whole Bowel Irrigation – Indicated if iron tabs visible past stomach on AXR – PEG- 1. 5 -2 L/H (20 -40 m. L/kg/H) until clear PR effluent & no iron tabs seen on AXR • OR 10 L then stop

Decontamination • What substances will NOT bind with AC? • C - caustics • H – heavy metals, hydrocarbons • I - Iron • L - Lithium • E – ethanol, methanol, EG

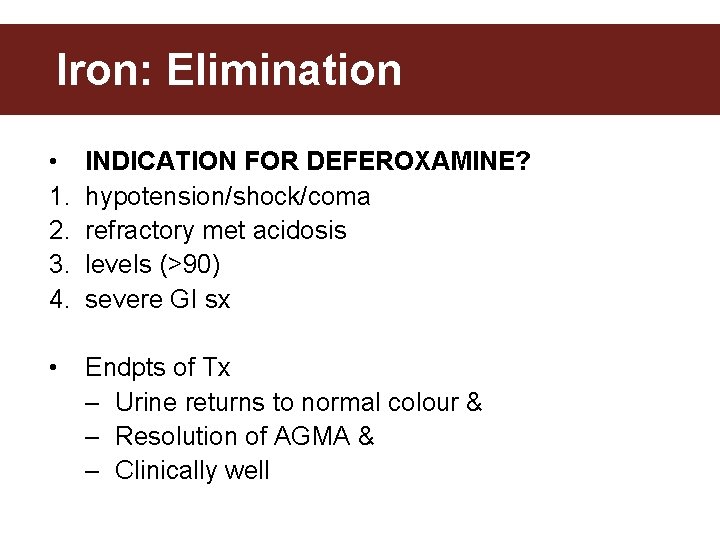
Iron: Elimination • 1. 2. 3. 4. INDICATION FOR DEFEROXAMINE? hypotension/shock/coma refractory met acidosis levels (>90) severe GI sx • Endpts of Tx – Urine returns to normal colour & – Resolution of AGMA & – Clinically well

Iron: Elimination • Administration of Deferoxamine – IV dosing – Goal is 15 mg/kg/hr – Replete intra-vascular volume prior to deferoxamine

Iron: Elimination • Adverse Effects of Deferoxamine – Hypotension with rapid administration – ARDS – usually if deferoxamine therapy for >24 • Stop infusion at 12 h – Increased risk of yersinia infections

Iron: Shock • What are the causes of shock in Fe OD? – Hypovolemia from vomiting and hemorrhage (onset within 1 st 24 H) – Distributive shock from vasodilation (onset 24 -48 H) – Cardiogenic Shock 2 to –ve inotropy (onset >2 days)

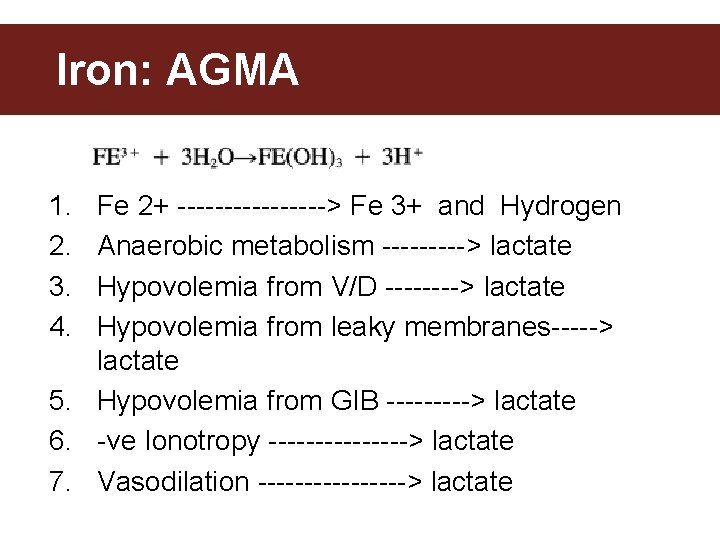
Iron: AGMA 1. 2. 3. 4. Fe 2+ --------> Fe 3+ and Hydrogen Anaerobic metabolism -----> lactate Hypovolemia from V/D ----> lactate Hypovolemia from leaky membranes-----> lactate 5. Hypovolemia from GIB -----> lactate 6. -ve Ionotropy --------> lactate 7. Vasodilation --------> lactate

Iron: Approach to Management

Iron: Mild Toxicity • Ingestion < 20 mg/kg and asymptomatic • Management – Observe 6 -8 hrs – D/C if asymptomatic – Consider iron levels but not necessary if story is reliable

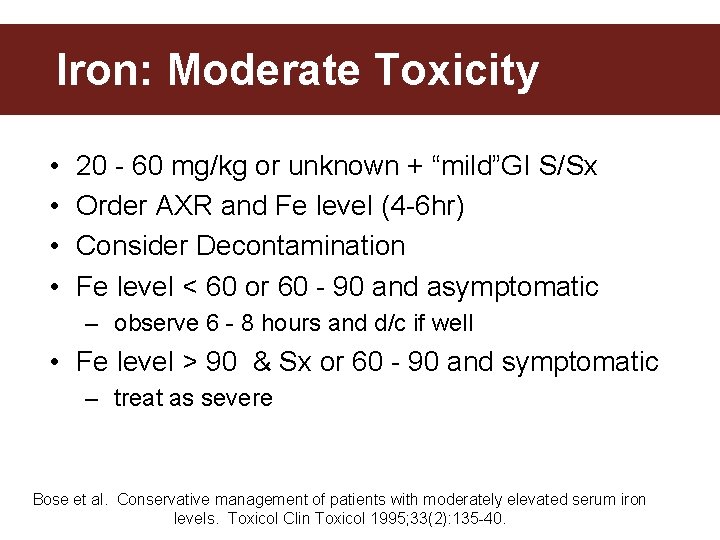
Iron: Moderate Toxicity • • 20 - 60 mg/kg or unknown + “mild”GI S/Sx Order AXR and Fe level (4 -6 hr) Consider Decontamination Fe level < 60 or 60 - 90 and asymptomatic – observe 6 - 8 hours and d/c if well • Fe level > 90 & Sx or 60 - 90 and symptomatic – treat as severe Bose et al. Conservative management of patients with moderately elevated serum iron levels. Toxicol Clin Toxicol 1995; 33(2): 135 -40.

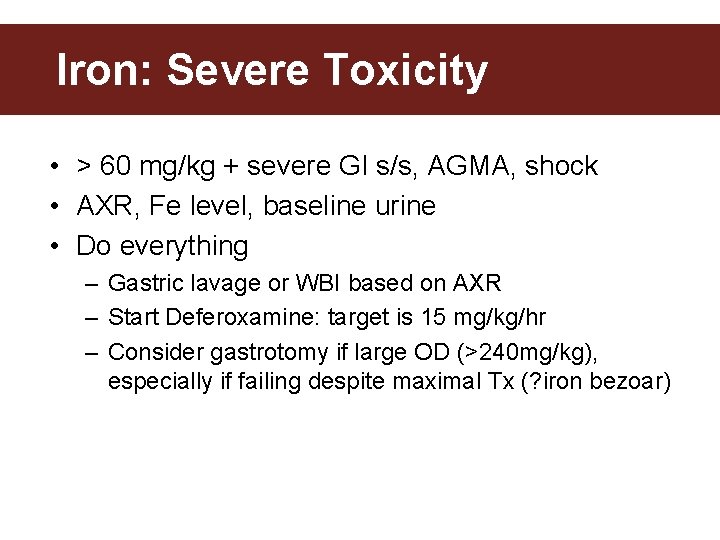
Iron: Severe Toxicity • > 60 mg/kg + severe GI s/s, AGMA, shock • AXR, Fe level, baseline urine • Do everything – Gastric lavage or WBI based on AXR – Start Deferoxamine: target is 15 mg/kg/hr – Consider gastrotomy if large OD (>240 mg/kg), especially if failing despite maximal Tx (? iron bezoar)

Iron: Disposition • Asymptomatic after 6 - 8 hrs rules out significant ingestion and d/c home • Management of moderate to severe ingestions depends on ……. – Clinical assessment: hx, physical, labs – Amount ingested: > 60 mg/kg is bad – Iron level: > 90 umol/L is bad

Iron: Pitfalls in Management • Early levels • Inaccurate calculation of dose of elemental iron ingested • Excessive reliance on the serum iron concentration (SIC) in management decisions rather than looking at the whole clinical picture • Reliance on the abdominal radiograph, or associated laboratory tests (including the deferoxamine challenge test) to predict toxicity • Failure to recognize patients in the latent phase Mills KC; Curry SC SOEmerg Med Clin North Am 1994 May; 12(2): 397 -413

Iron: Pitfalls in Management • Inadequate hydration • Withholding deferoxamine from pregnant patients • Inadequate deferoxamine dose • Intramuscular administration of deferoxamine • Discontinuation of deferoxamine on the basis of urine color alone Mills KC; Curry SC SOEmerg Med Clin North Am 1994 May; 12(2): 397 -413

Iron: Summary • • LOCAL and SYSTEMIC toxicity Risk stratify patients and treat accordingly Know the indications for deferoxamine Don’t wait for iron level if good story and pt has s/sx of severe intoxication • Treat pregnant patients the same


Lithium • MC used for Tx bipolar disorders • Treatment and prophylaxis • Narrow Toxic: Therapeutic Ratio • Therapeutic level (comparable to that of Digoxin) • = 0. 6 -1. 5 m. Eq/L • Toxic level • ≥ 2. 5 -4. 0 (chronic or acute) • One of the few Rx that can ppt it’s own toxicity – Polyuria (from nephrogenic DI) can lead to volume depletion and increased renal re-absorption of Li

Lithium • Mortality • acute OD 25% • chronic OD 9% • Morbidity: • 10% of the survivors from chronic OD have permanent neurologic sequelae

Li – Pharmacokinetics • MOA • Not really understood • Metabolism: • Not metabolized • Absorption • Elimination: • Peak 2 -3 h • SR 2 -6 h • Renal 95% • Fecal 5% • Distribution • Slow • Low Vd • Not protein bound • 12 -22 h • Delayed in elderly and chronic OD

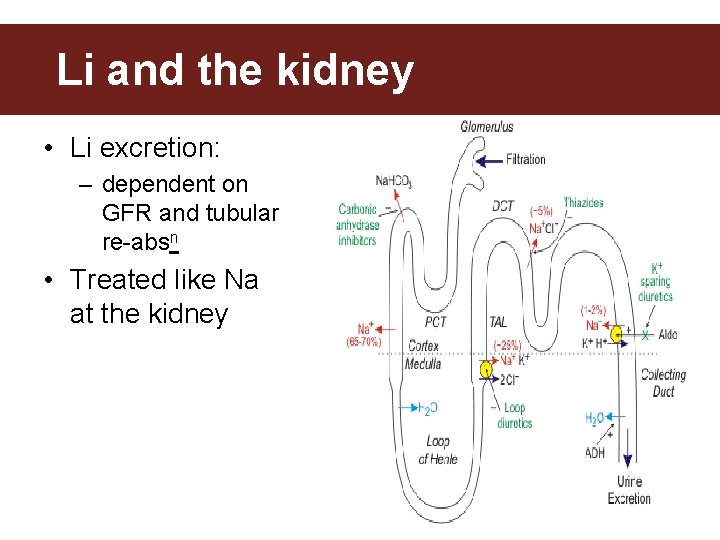
Lithium & the Kidney Li and the kidney • Li excretion: – dependent on GFR and tubular re-absn • Treated like Na at the kidney

Li: Clinical features SNAP MUD • Seizures • N, V, D • Ataxia • Parkinsonian mvmts • Myoclonus • UMN signs • Delirium/Decr LOC

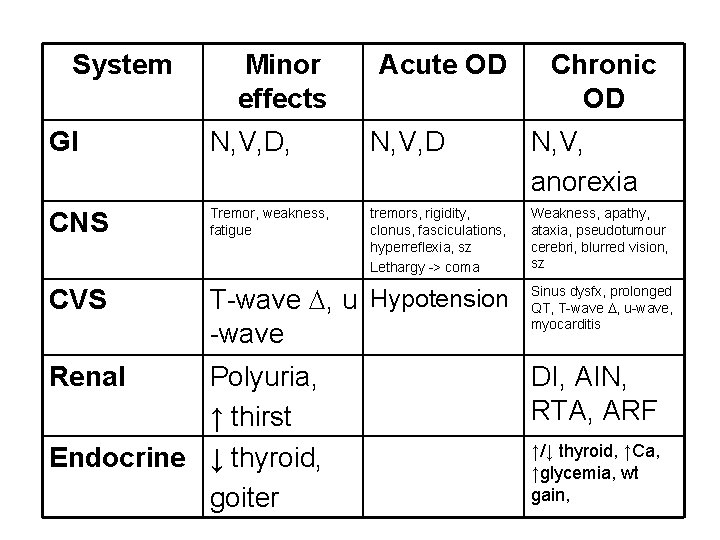
System Minor effects Acute OD Chronic OD GI N, V, D, N, V, D N, V, anorexia CNS Tremor, weakness, fatigue tremors, rigidity, clonus, fasciculations, hyperreflexia, sz Lethargy -> coma Weakness, apathy, ataxia, pseudotumour cerebri, blurred vision, sz CVS T-wave , u Hypotension -wave Renal Polyuria, ↑ thirst Endocrine ↓ thyroid, goiter Sinus dysfx, prolonged QT, T-wave , u-wave, myocarditis DI, AIN, RTA, ARF ↑/↓ thyroid, ↑Ca, ↑glycemia, wt gain,

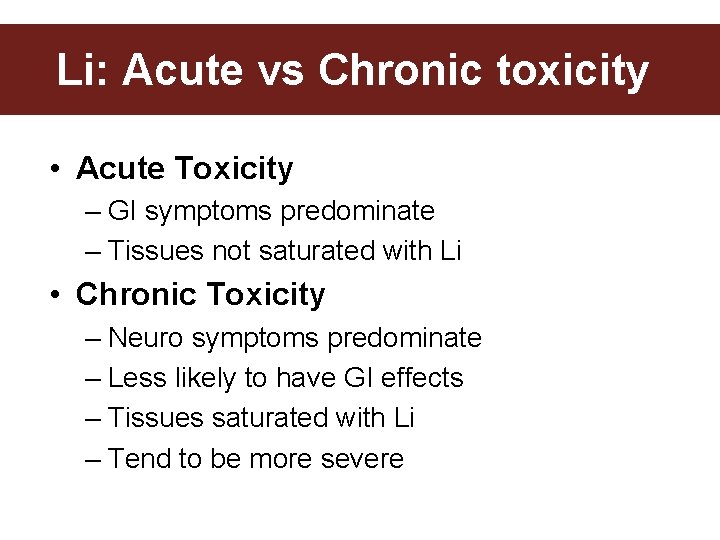
Li: Acute vs Chronic toxicity • Acute Toxicity – GI symptoms predominate – Tissues not saturated with Li • Chronic Toxicity – Neuro symptoms predominate – Less likely to have GI effects – Tissues saturated with Li – Tend to be more severe

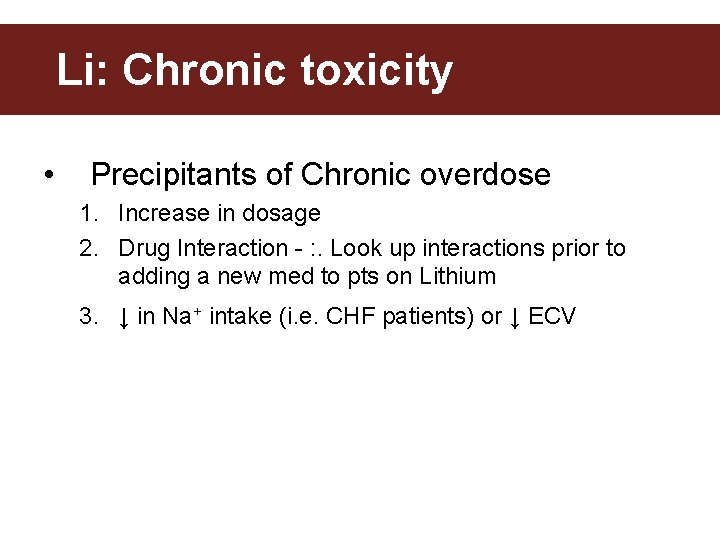
Li: Chronic toxicity • Precipitants of Chronic overdose 1. Increase in dosage 2. Drug Interaction - : . Look up interactions prior to adding a new med to pts on Lithium 3. ↓ in Na+ intake (i. e. CHF patients) or ↓ ECV

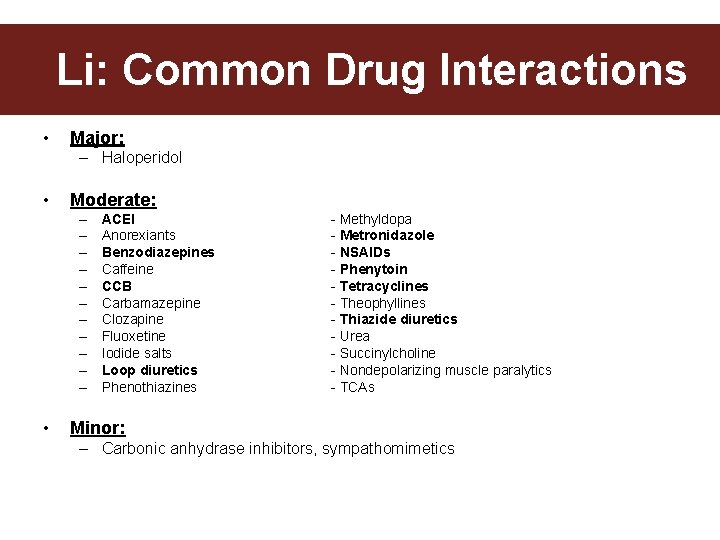
Common Drug Interactions Li: Common Drug Interactions • Major: – Haloperidol • Moderate: – – – • ACEI Anorexiants Benzodiazepines Caffeine CCB Carbamazepine Clozapine Fluoxetine Iodide salts Loop diuretics Phenothiazines - Methyldopa - Metronidazole - NSAIDs - Phenytoin - Tetracyclines - Theophyllines - Thiazide diuretics - Urea - Succinylcholine - Nondepolarizing muscle paralytics - TCAs Minor: – Carbonic anhydrase inhibitors, sympathomimetics

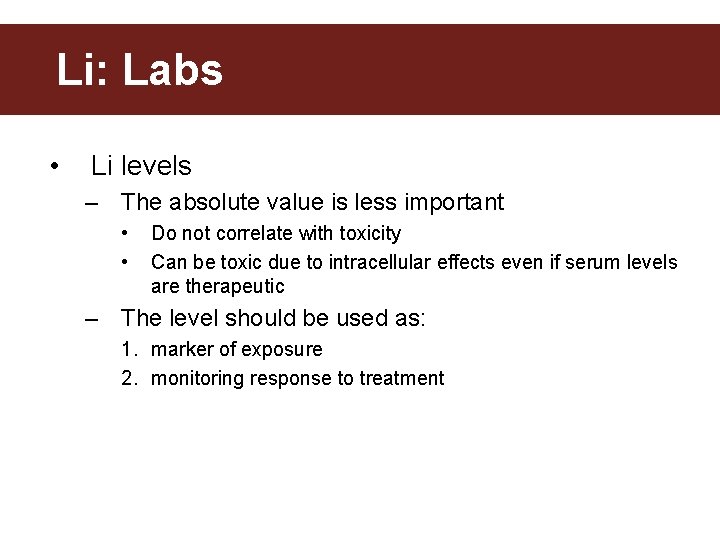
Li: Labs • Li levels – The absolute value is less important • • Do not correlate with toxicity Can be toxic due to intracellular effects even if serum levels are therapeutic – The level should be used as: 1. marker of exposure 2. monitoring response to treatment

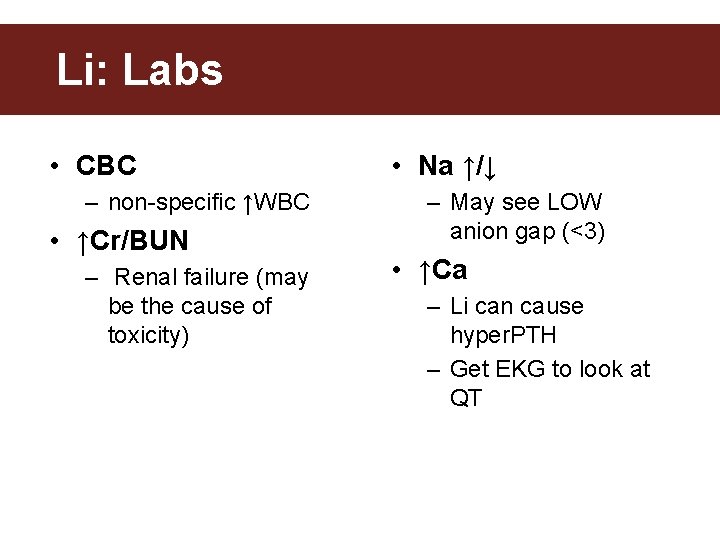
Li: Labs • CBC – non-specific ↑WBC • ↑Cr/BUN – Renal failure (may be the cause of toxicity) • Na ↑/↓ – May see LOW anion gap (<3) • ↑Ca – Li can cause hyper. PTH – Get EKG to look at QT

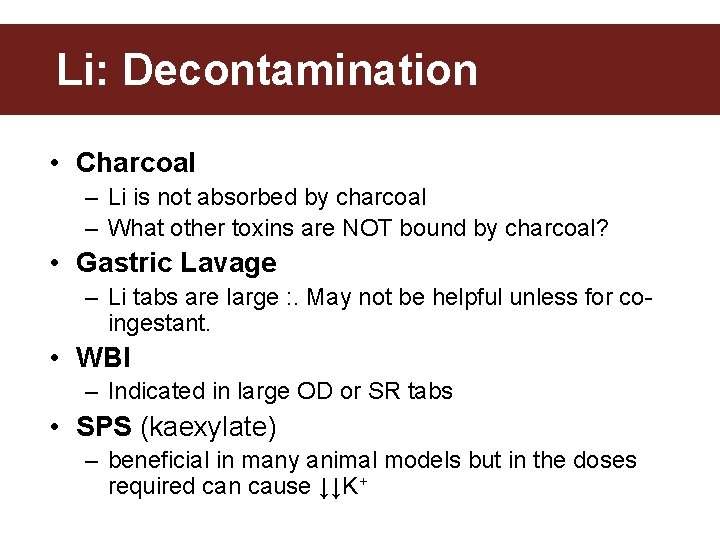
Li: Decontamination • Charcoal – Li is not absorbed by charcoal – What other toxins are NOT bound by charcoal? • Gastric Lavage – Li tabs are large : . May not be helpful unless for coingestant. • WBI – Indicated in large OD or SR tabs • SPS (kaexylate) – beneficial in many animal models but in the doses required can cause ↓↓K+

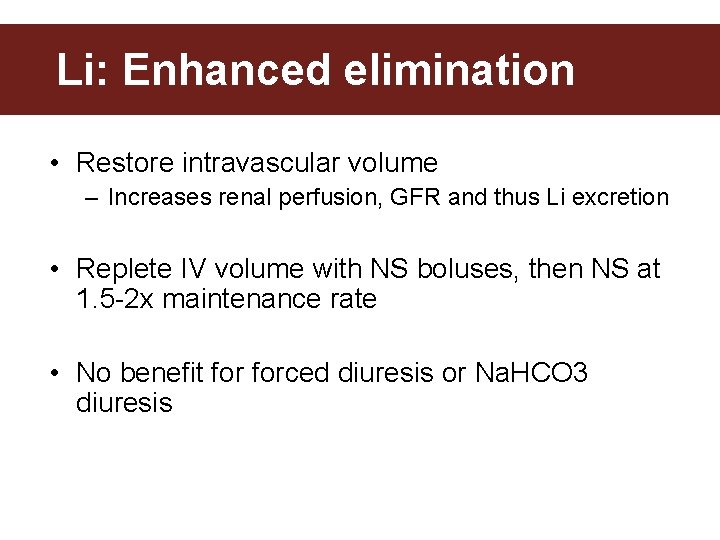
Li: Enhanced elimination • Restore intravascular volume – Increases renal perfusion, GFR and thus Li excretion • Replete IV volume with NS boluses, then NS at 1. 5 -2 x maintenance rate • No benefit forced diuresis or Na. HCO 3 diuresis

Li: Dialysis 1. Clinical indications 1. Severe S/Sx of neurotoxicity 2. Renal Failure and S/Sx of toxicity 3. Pts that unable to tolerate Na repletion (i. e. CHF, cirrhotic? , pancreatitis? ) 2. Laboratory indications 1. Level >2. 5 if chronic or >4. 0 in anyone • What if the patient is hemodynamically unstable?

Li: CRRT • ICU can arrange CRRT which is a safer method to enhance elimination in unstable patients • Lower elimination rate/hr, but same daily clearance rate since CRRT is continuous and IHD is only done in 4 -6 H/session – CRRT also has essentially no occurrence or rebound phenom

Li: Endocrine effects • 4 Common Endocrine disorders – Hypothyroid – Hyperparathyroid – Nephrogenic DI

Li: Summary • Important to recognize Li overdoses early based on Hx, RF & Physical exam • Management revolves around decontamination and adequate volume resuscitation • +/- WBI or hemodialysis in certain situations