MECHANISMS OF TOXICITY ACUTE TOXICITY LD 50 lethal

![ACUTE TOXICITY - LD 50 (lethal dose) [g/kg or mg/kg] - LC 50 (lethal ACUTE TOXICITY - LD 50 (lethal dose) [g/kg or mg/kg] - LC 50 (lethal](https://slidetodoc.com/presentation_image/8c19d45f86c8fdec9659e904b4498587/image-2.jpg)





- Slides: 10

MECHANISMS OF TOXICITY
![ACUTE TOXICITY LD 50 lethal dose gkg or mgkg LC 50 lethal ACUTE TOXICITY - LD 50 (lethal dose) [g/kg or mg/kg] - LC 50 (lethal](https://slidetodoc.com/presentation_image/8c19d45f86c8fdec9659e904b4498587/image-2.jpg)
ACUTE TOXICITY - LD 50 (lethal dose) [g/kg or mg/kg] - LC 50 (lethal concentration) [g/m 3/kg or mg/m 3/ kg]

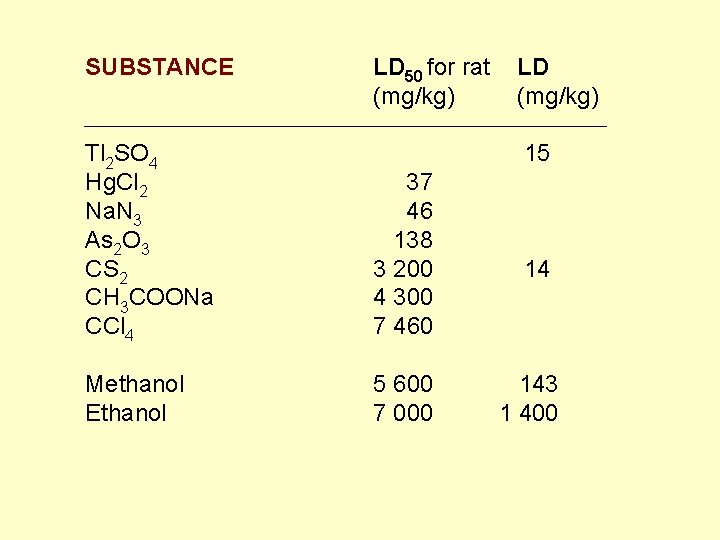
SUBSTANCE Tl 2 SO 4 Hg. Cl 2 Na. N 3 As 2 O 3 CS 2 CH 3 COONa CCl 4 Methanol Ethanol LD 50 for rat (mg/kg) 37 46 138 3 200 4 300 7 460 LD (mg/kg) 15 14 5 600 143 7 000 1 400

SUBSTANCE Botulinum toxin HCN As 2 O 3 KNO 2 Glycerol LD 50 for mouse (mg/kg) 0. 000 03 10 100 30 000

A) ESPECIALLY DANGEROUS POISONS Examples: Hg (CH 3)2 As 2 O 3 HCN CNSe. O 2 Pb (CH 3)4 Gases As. H 3 PH 3 COCl 2 (phosgene)

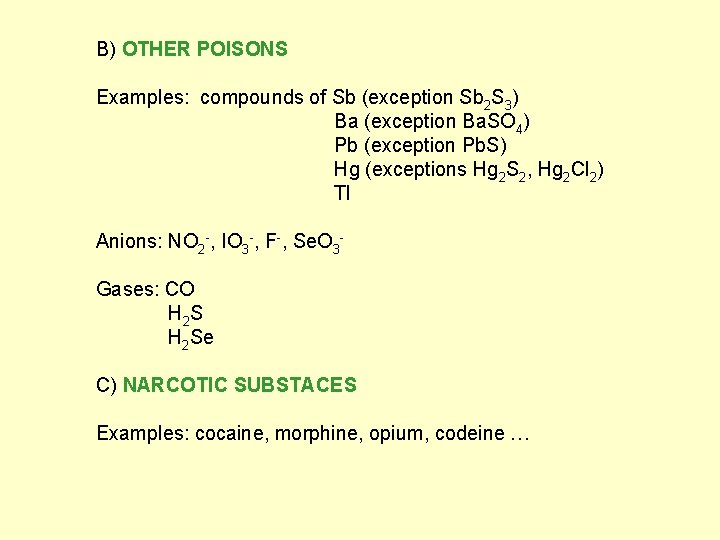
B) OTHER POISONS Examples: compounds of Sb (exception Sb 2 S 3) Ba (exception Ba. SO 4) Pb (exception Pb. S) Hg (exceptions Hg 2 S 2, Hg 2 Cl 2) Tl Anions: NO 2 -, IO 3 -, F-, Se. O 3 Gases: CO H 2 Se C) NARCOTIC SUBSTACES Examples: cocaine, morphine, opium, codeine …

The effect of the poison depends on: 1. Physical and chemical properties of substances State (gaseous, liquid, solid) The toxicity of Hg depends above all on its state. Fumes causes bronchopneumonia. (Neural and psychic failure appears after long-duration contact). Consumption of metal mercury causes diarrhea. Soluble salts of mercury are toxic (Hg. Cl 2 , Hg(NO 3)2). Ba. SO 4, Ba 2+ , Ba. SO 4 is used as a contrast matter at X-ray examination of alimentary tract. Be. O (insoluble), Be 2+ (berylliosis), Si. O 2 (silicosis is progressive disorder of lung). Oxidation number: As 3+ is more toxic than As 5+ Type of bonds: compare CN- and K 3[Fe(CN)6], K 4[Fe(CN)6] (ferri- and ferrocyanide).

2. Exposure (dose, concentration in the environment, duration of contact …) LD 50, LC 50 3. Organism (its individual, hereditary and acquired characteristics, age …)

Action of poisons 1) Irritation of mucous membranes and skin (trauma, injury) Strong acids and bases, organic solvent (HCl, H 2 SO 4, Na. OH, KOH …) 2) Inhibition of enzymes Hg, Pb, As, Cd (binding on – SH group of proteins, especially in the active centre of enzymes) F-, S-, CN- combine with metals, that activate enzymes, or are in enzyme molecule Ca 2+, Mg 2+, Mn 2+, Zn 2+, Fe 2+, Cu 2+… CN- group inactivates more than 40 enzymes. 3) Inhibition of the electrons and oxygen transport CO, HCN, CN-, H 2 S Hb. O – oxyhemoglobin Hb. CO – carbonyl hemoglobin. CO has 200 – 300 x higher affinity to Hb than oxygen. This binding is strong, but reversible. If the affected person is transported on the open air, the oxygen replaces CO. Hb. CN – cyanhemoglogin, CN- group binds firmly to central atom of Fe, Hb cannot transport the oxygen. CN- groups inactivate enzyme cytochromoxidase (respiratory chain) because of their strong bonding of Cu 2+ and Fe 2+ in enzyme molecule. Hb. S - sulfohemoglobin NO 2 -, NO 3 -, Cl. O 3 - (ox. of Fe 2+ of hemoglobin to Fe 3+ methemoglobin)

4) Changes in genetic information: As, Cr, Ni, Cd, Be … 5) Origin of reactive radicals O 3 , reactive radicals of oxygen, lipoperoxidation (phospholipids of biomembranes) 6) Breakdown of cell walls CS 2, organic nonpolar solvents – narcotic effect. Benzene, toluene, carbon tetrachloride. (Carbon disulfide – degeneration of fat of ganglia cells)