Thermodynamics Heat Work Heat is a form of

































- Slides: 54

Thermodynamics Heat Work

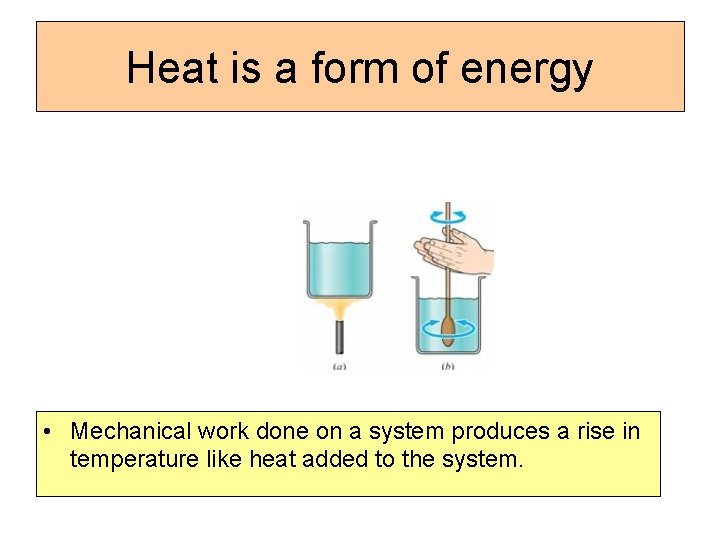
Heat is a form of energy • Mechanical work done on a system produces a rise in temperature like heat added to the system.


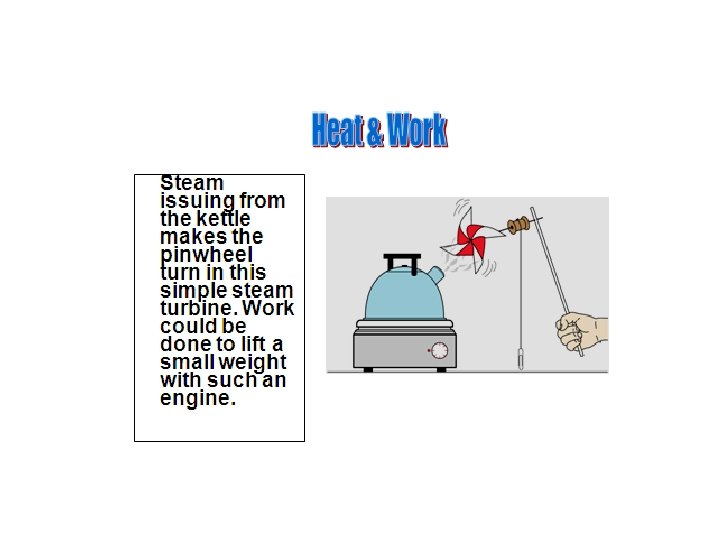
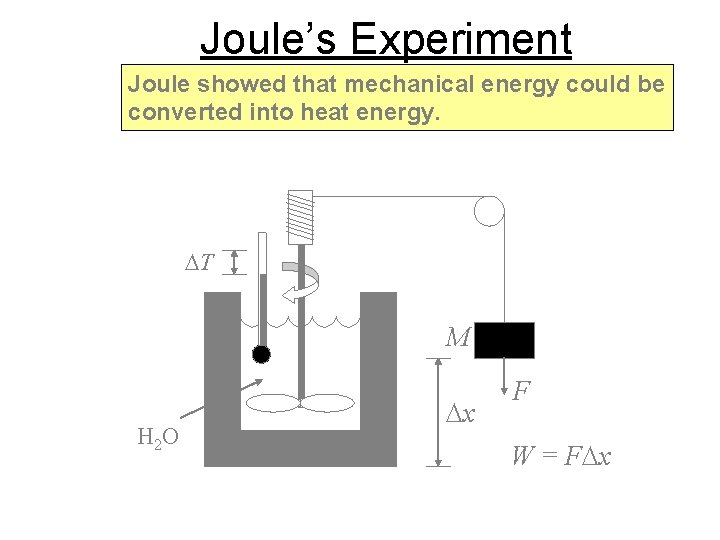
Joule’s Experiment Joule showed that mechanical energy could be converted into heat energy. DT M H 2 O Dx F W = FDx

• Thermodynamics is concerned with interconversions of different forms of energy. • It was developed as a mathematical tool for studying phenomena such as the way in which heat energy can be converted into mechanical energy in heat engines • Thermodynamics provides a means for deciding whether a process will occur spontaneously • Spontaneous” in this context implies nothing about how fast it will take place - e. g. combustion of a diamond • Thermodynamics is linked at a fundamental level to the nature of the universe • The Industrial Revolution depended on heat engines, most of which (like these steam engines) were of very low efficiency. The development of physical theories, and mathematical tools, to analyse these systems led to rapid improvements in technology.

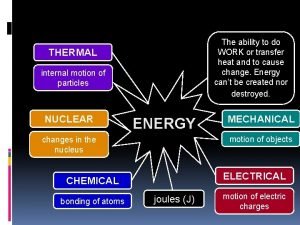
• Energy can be divided into two categories - kinetic energy and potential energy • Kinetic energy includes all forms of energy that result from movement - either linear motion or rotation. Heat, which is molecular motion. Radiant energy - the kinetic energy of photons of light and other electromagnetic radiation • • • Mechanical energy • Electrical energy as currents of moving electrons or charged particles • Potential energy includes all forms of energy that are stored. – Energy stored in chemical bonds – Energy stored in concentration gradients – Energy stored as electrical potential (separation of charges) – Energy stored in the nuclei of atoms • The basic unit of energy is the Joule (J)

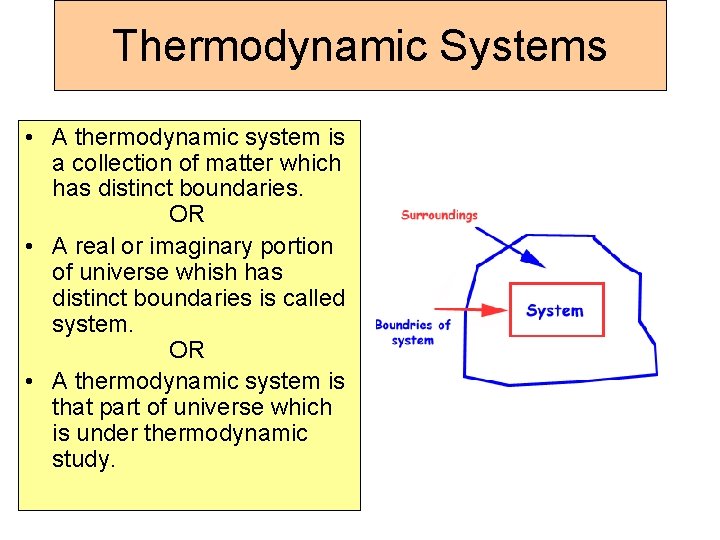
Thermodynamic Systems • A thermodynamic system is a collection of matter which has distinct boundaries. OR • A real or imaginary portion of universe whish has distinct boundaries is called system. OR • A thermodynamic system is that part of universe which is under thermodynamic study.

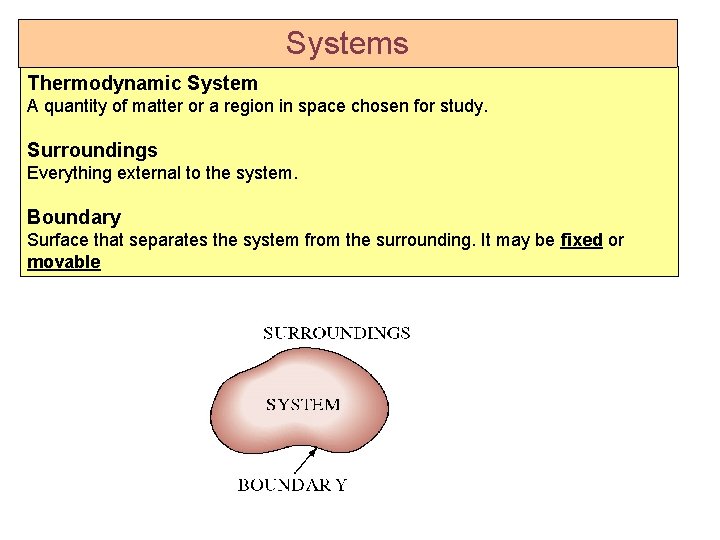
Systems Thermodynamic System A quantity of matter or a region in space chosen for study. Surroundings Everything external to the system. Boundary Surface that separates the system from the surrounding. It may be fixed or movable

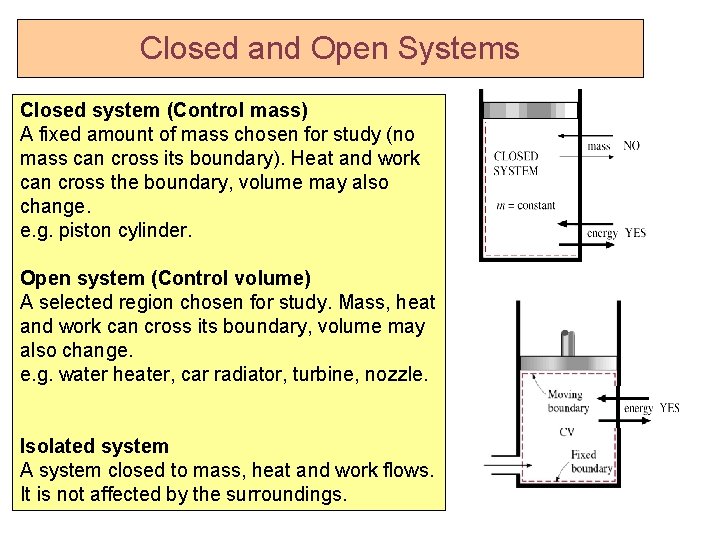
Closed and Open Systems Closed system (Control mass) A fixed amount of mass chosen for study (no mass can cross its boundary). Heat and work can cross the boundary, volume may also change. e. g. piston cylinder. Open system (Control volume) A selected region chosen for study. Mass, heat and work can cross its boundary, volume may also change. e. g. water heater, car radiator, turbine, nozzle. Isolated system A system closed to mass, heat and work flows. It is not affected by the surroundings.

Open Systems

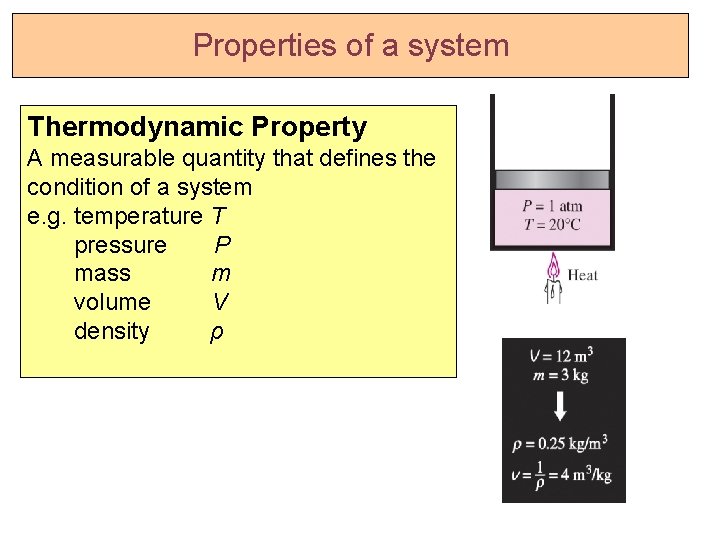
Properties of a system Thermodynamic Property A measurable quantity that defines the condition of a system e. g. temperature T pressure P mass m volume V density ρ

Extensive and Intensive properties • Properties are of 2 types: • Intensive properties Independent of mass. e. g. P, T, v, ρ. • Extensive properties Change with mass. e. g. m, V, Energy


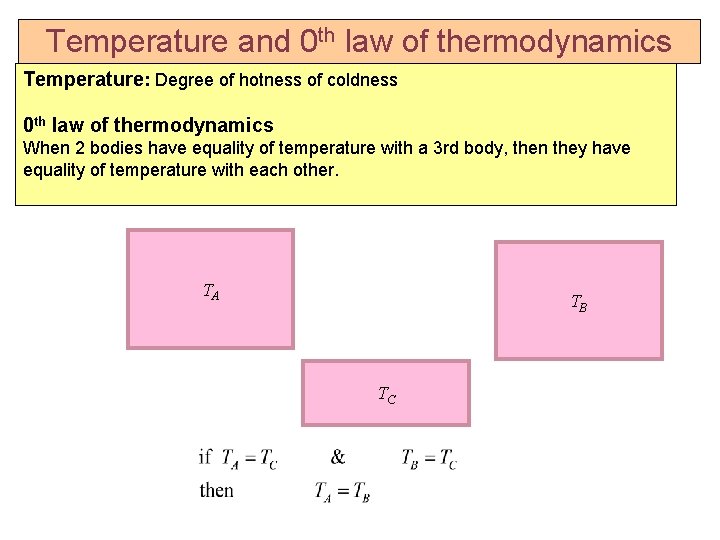
Temperature and 0 th law of thermodynamics Temperature: Degree of hotness of coldness 0 th law of thermodynamics When 2 bodies have equality of temperature with a 3 rd body, then they have equality of temperature with each other. TA TB TC

Absolute scale of temperature:

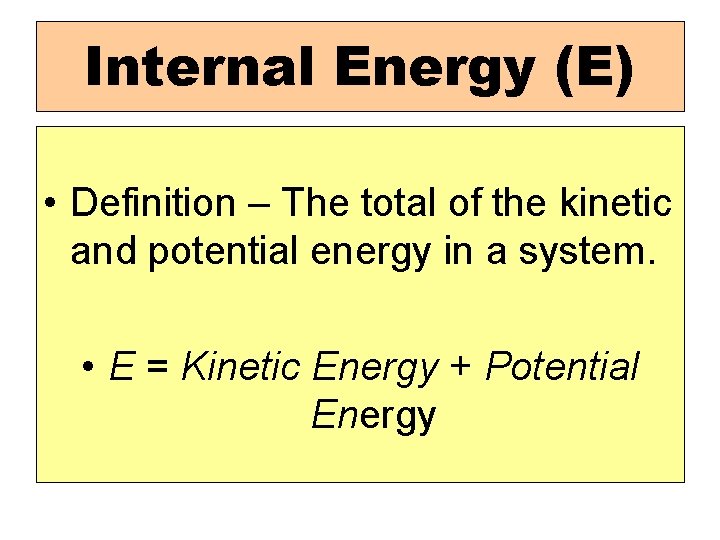
Internal Energy (E) • Definition – The total of the kinetic and potential energy in a system. • E = Kinetic Energy + Potential Energy

The First Law of Thermodynamics • • Internal Energy: total energy of a system. Involves translational, rotational, vibrational motions. Cannot measure absolute internal energy. Change in internal energy,

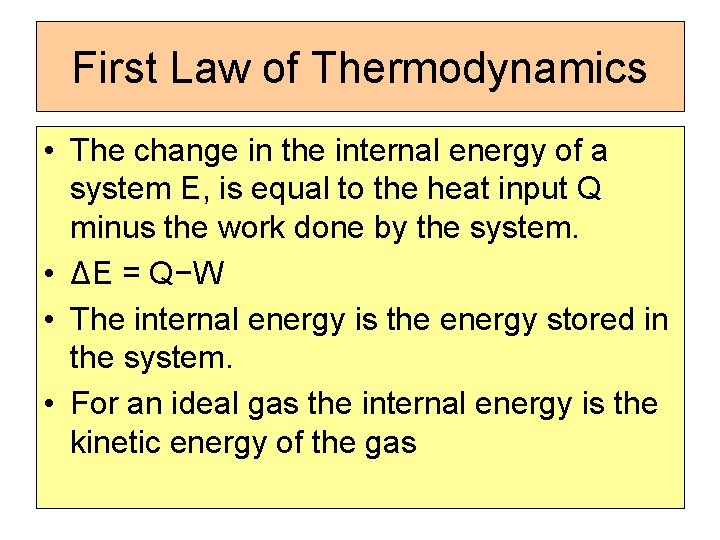
First Law of Thermodynamics • The change in the internal energy of a system E, is equal to the heat input Q minus the work done by the system. • ΔE = Q−W • The internal energy is the energy stored in the system. • For an ideal gas the internal energy is the kinetic energy of the gas

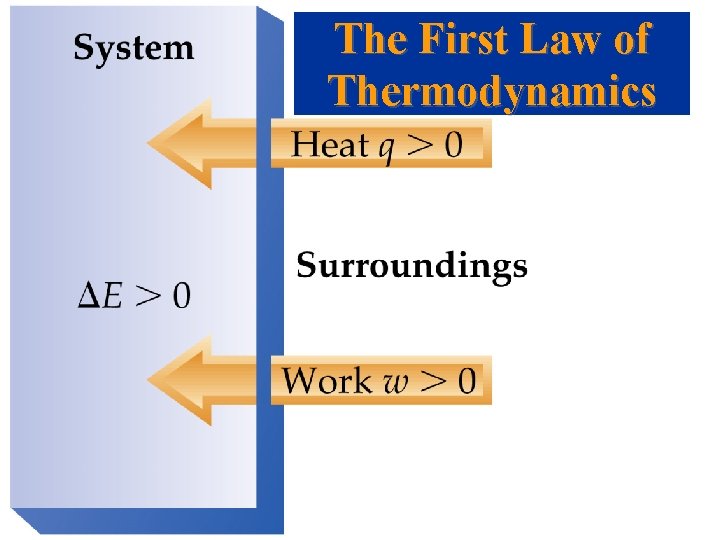
The First Law of Thermodynamics Relating DE to Heat(q) and Work(w) • Energy cannot be created or destroyed. • Energy of (system + surroundings) is constant. • Any energy transferred from a system must be transferred to the surroundings (and vice versa). • From the first law of thermodynamics:

• Work is a form of energy. It’s the energy involved in moving something. If nothing moves, no work is done. • Work in chemical terms is usually done with pressure and volume changes.

The First Law of Thermodynamics

QUIZ…… • Calculate the energy change for a system undergoing a process in which 15. 4 k. J of heat flows and where 6. 3 k. J of work is done on the system.

ANSWER • • ∆E = q + w q = - 15. 40 J w = + 06. 30 J ∆E = - 15. 40 J + 06. 30 J = - 09. 10 J

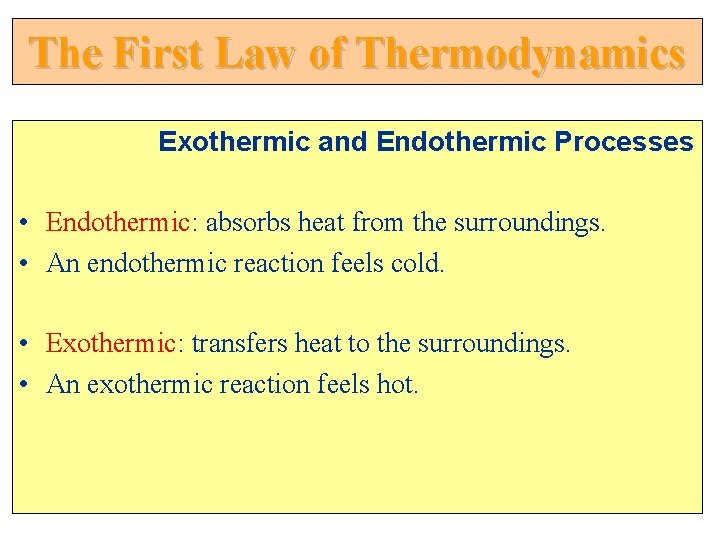
The First Law of Thermodynamics Exothermic and Endothermic Processes • Endothermic: absorbs heat from the surroundings. • An endothermic reaction feels cold. • Exothermic: transfers heat to the surroundings. • An exothermic reaction feels hot.

Pressure-volume work • P = f / A • Work done by the system (gas): • w = – f Δx • w = –P ΔV

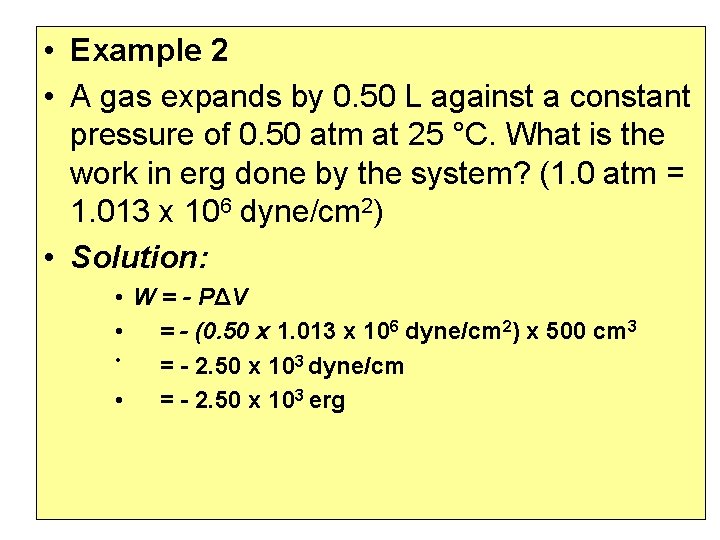
• Example 2 • A gas expands by 0. 50 L against a constant pressure of 0. 50 atm at 25 °C. What is the work in erg done by the system? (1. 0 atm = 1. 013 x 106 dyne/cm 2) • Solution: • W = - PΔV • = - (0. 50 x 1. 013 x 106 dyne/cm 2) x 500 cm 3 • = - 2. 50 x 103 dyne/cm • = - 2. 50 x 103 erg

Reversible work • Isothermal work expansion against variable pressure. • n = number of moles, R = gas constant (=8. 314 JK-1 mol 1, = 1. 987 cal K-1 mol-1, = 0. 0802 L. atm. K-1 mol-1) T = absolute temperature

• Example: • What is the maximum work done in the isothermal reversible expansion of 2 moles of an ideal gas from 1 to 5 litres at 25 °C? • Solution: » = 7976. 43 J

Special Forms of the 1 st law • • • ∆E = q + w Case 1: Isothermal process ∆E = 0, hence, q = - w Case 2: Isochoic process w = 0, ∆E = qv Case 3: Adiabatic process q = 0, ∆E = w Case 4: Isobaric ∆E = q + w



Energy Changes in Chemical Reactions • How are energy changes measured? • One Answer – Calorimetry, q = CcalΔT • What thermodynamic quantities do we get? • Constant Volume: qv = ΔE. • Constant Pressure: q. P = ΔH • In most calorimetry, ΔT is very small, initial and final states are at nearly constant T •


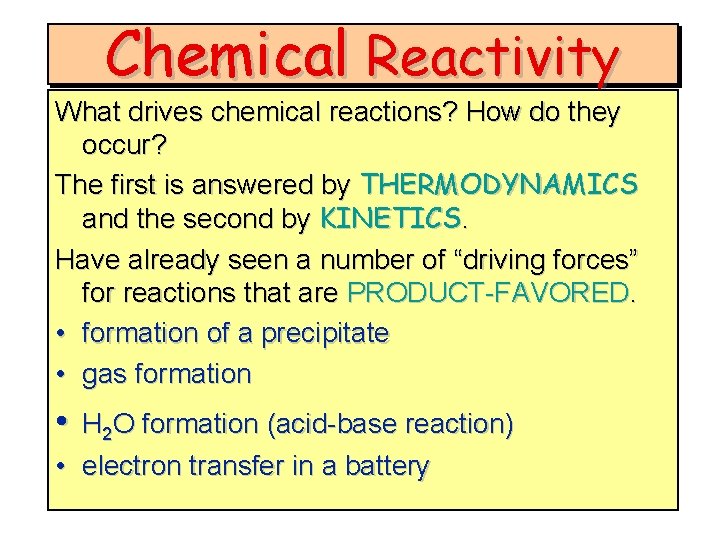
Chemical Reactivity What drives chemical reactions? How do they occur? The first is answered by THERMODYNAMICS and the second by KINETICS. Have already seen a number of “driving forces” for reactions that are PRODUCT-FAVORED. • formation of a precipitate • gas formation • H 2 O formation (acid-base reaction) • electron transfer in a battery

Chemical Reactivity But energy transfer also allows us to predict reactivity. In general, reactions that transfer energy to their surroundings are product-favored. So, let us consider heat transfer in chemical processes.


Enthalpy of Reaction from Heat of Formation • Standard Heat of Formation (ΔHof): The amount of energy gained or lost when 1 mole of the substance is formed from its elements under standard conditions (25°C, 1 atm = 101. 3 k. Pa)

Example 2 Standard enthalpies of formation are: C 2 H 5 OH(l) -228, CO 2 (g) -394, and H 2 O(l) -286 k. J/mol. Calculate the enthalpy of the reaction, • C 2 H 5 OH (l) + 3 O 2 (g) = 2 CO 2 (g) + 3 H 2 O (l) • Solution: • ∆HR = [3 x∆Hf(H 2 O (l)) + 2 x∆Hf(CO 2(g))] – [1 x∆Hf C 2 H 5 OH(l) + 3 x∆Hf. O 2(g)] = [3 mol. X-286 k. J/mol + 2 molx-394 k. J/mol]-[1 molx-228 k. J/mol + 3 molx 0. 0 k. J/mol] = [- 858 + (-788)] – [-228] = - 1546 + 228 = - 1418 k. J

Heat of Reactions from Bond Energies • When bonds are formed, energy is released. • In order to break bonds, energy must be absorbed • Exothermic: Products have stronger bonds than the reactants. • Endothermic: Products have weaker bonds than the reactants.


• • • O 2(g) = 2 O(g) ∆H° = 490. 4 k. J H 2(g) = 2 H(g) ∆H° = 431. 2 k. J H 2 O(g)= 2 H(g) + O(g) ∆H° = 915. 6 k. J 2 H 2(g) + O 2(g) = 2 H 2 O(g) ∆H° = ? ∆H° reaction = bonds broken Energy (absorbed) ─ bonds formed Energy (released) • =[2 H-H + O=O] – [ 4 O-H] » = [2 x 431. 2 + 1 x 490. 4] – [4 x 457. 8] » = [862. 4 + 490. 4] – [1831. 2] » = 1532. 8 – 1831. 2 » = - 478. 4 k. J


Heat of Combustion IS the Heat Evolved when One mole of a Fuel is Burned in Enough Oxygen

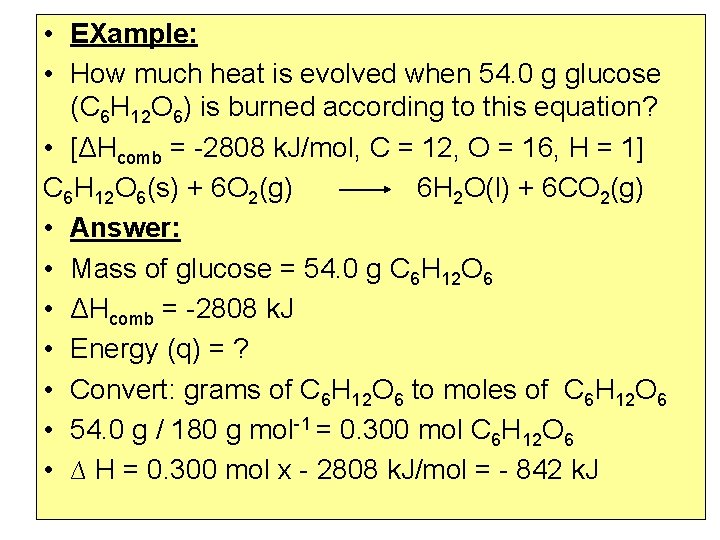
• EXample: • How much heat is evolved when 54. 0 g glucose (C 6 H 12 O 6) is burned according to this equation? • [ΔHcomb = -2808 k. J/mol, C = 12, O = 16, H = 1] C 6 H 12 O 6(s) + 6 O 2(g) 6 H 2 O(l) + 6 CO 2(g) • Answer: • Mass of glucose = 54. 0 g C 6 H 12 O 6 • ΔHcomb = -2808 k. J • Energy (q) = ? • Convert: grams of C 6 H 12 O 6 to moles of C 6 H 12 O 6 • 54. 0 g / 180 g mol-1 = 0. 300 mol C 6 H 12 O 6 • ∆ H = 0. 300 mol x - 2808 k. J/mol = - 842 k. J

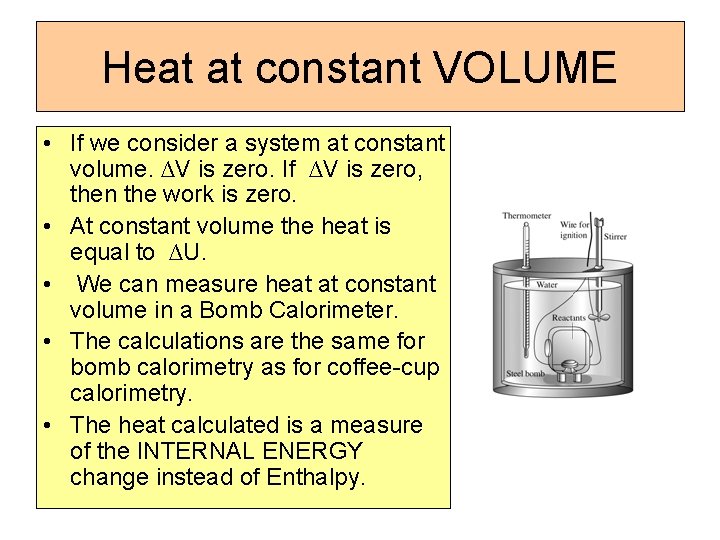
Heat at constant VOLUME • If we consider a system at constant volume. ∆V is zero. If ∆V is zero, then the work is zero. • At constant volume the heat is equal to ∆U. • We can measure heat at constant volume in a Bomb Calorimeter. • The calculations are the same for bomb calorimetry as for coffee-cup calorimetry. • The heat calculated is a measure of the INTERNAL ENERGY change instead of Enthalpy.

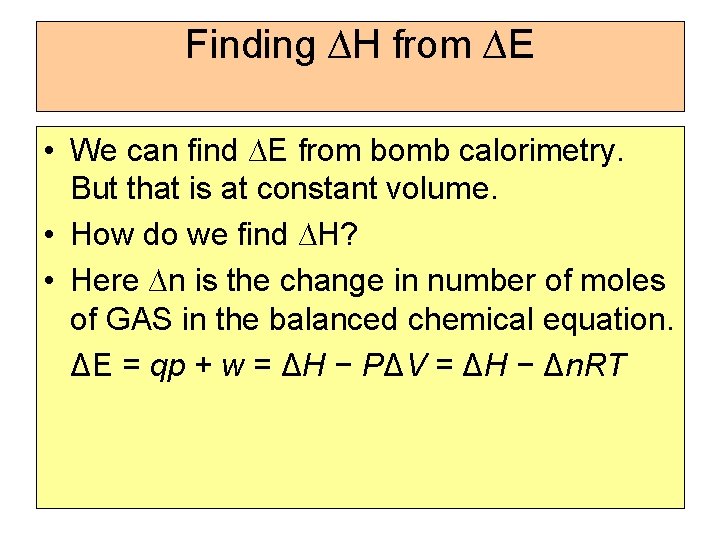
Finding ∆H from ∆E • We can find ∆E from bomb calorimetry. But that is at constant volume. • How do we find ∆H? • Here ∆n is the change in number of moles of GAS in the balanced chemical equation. ΔE = qp + w = ΔH − PΔV = ΔH − Δn. RT

• ∆n. RT • This is an amount of energy that would be represented as work if work could be done. • This can be zero if there is no change in number of moles of gas.

Application • For the reaction Mg (s) + ½ O 2 (g) = Mg. O (s) • the enthalpy change is -601. 2 k. J. What is the internal energy change for this reaction? How many g of magnesium must react to effect an internal energy change of -22. 4 kcal?

Hess’s Law The Enthalpy of a Reaction is the Same if it takes place in One or More than One step.

Hess’s Law & Energy Level Diagrams Forming H 2 O can occur in a single step or in a two steps. ∆Htotal is the same no matter which path is followed.

USING ENTHALPY Making H 2 O from H 2 involves two steps. H 2(g) + 1/2 O 2(g) ---> H 2 O(g) + 242 k. J H 2 O(g) ---> H 2 O(liq) + 44 k. J --------------------------------H 2(g) + 1/2 O 2(g) --> H 2 O(liq) + 286 k. J Example of HESS’S LAW— If a rxn. is the sum of 2 or more others, the net ∆H is the sum of the ∆H’s of the other rxns.


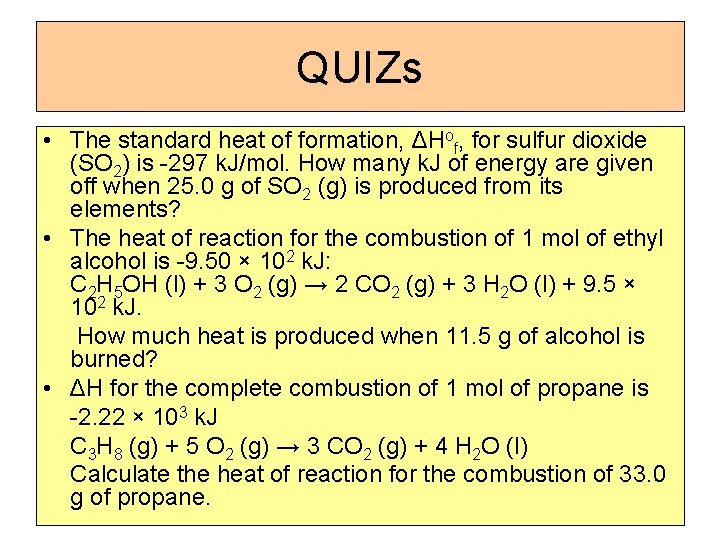
QUIZs • The standard heat of formation, ΔHof, for sulfur dioxide (SO 2) is -297 k. J/mol. How many k. J of energy are given off when 25. 0 g of SO 2 (g) is produced from its elements? • The heat of reaction for the combustion of 1 mol of ethyl alcohol is -9. 50 × 102 k. J: C 2 H 5 OH (l) + 3 O 2 (g) → 2 CO 2 (g) + 3 H 2 O (l) + 9. 5 × 102 k. J. How much heat is produced when 11. 5 g of alcohol is burned? • ΔH for the complete combustion of 1 mol of propane is -2. 22 × 103 k. J C 3 H 8 (g) + 5 O 2 (g) → 3 CO 2 (g) + 4 H 2 O (l) Calculate the heat of reaction for the combustion of 33. 0 g of propane.

 What is flow work in thermodynamics
What is flow work in thermodynamics Present continuous affirmative
Present continuous affirmative Specific heat capacity of lead j/kg c
Specific heat capacity of lead j/kg c Specific latent heat
Specific latent heat Examples of moist heat
Examples of moist heat Relationship between heat and work
Relationship between heat and work Osha heat work/rest chart
Osha heat work/rest chart Osha heat work/rest chart
Osha heat work/rest chart Relationship between heat and work
Relationship between heat and work Relationship between heat and work
Relationship between heat and work Differentiate between heat and work
Differentiate between heat and work Conversion of work into heat and vice versa
Conversion of work into heat and vice versa Three heat switch diagram
Three heat switch diagram The ability to do work or transfer heat
The ability to do work or transfer heat Work immersion grading system
Work immersion grading system Work immersion in barangay
Work immersion in barangay Smart vs hard working
Smart vs hard working Present continuous for work
Present continuous for work Social goals model social work
Social goals model social work Social case work definition
Social case work definition A group vs a team
A group vs a team Physics 03-06 impulse and momentum answer key
Physics 03-06 impulse and momentum answer key Chapter 4 work and energy section 1 work and machines
Chapter 4 work and energy section 1 work and machines I work all day i work all night
I work all day i work all night Smart work vs hard work group discussion
Smart work vs hard work group discussion Compilation of work in work immersion
Compilation of work in work immersion Why study thermodynamics
Why study thermodynamics Thermodynamics webquest
Thermodynamics webquest Rubbing your hands together is an example of which transfer
Rubbing your hands together is an example of which transfer Property tables thermodynamics
Property tables thermodynamics Internal energy formula thermodynamics
Internal energy formula thermodynamics Sssf thermodynamics
Sssf thermodynamics First law of thermodynamics in open system
First law of thermodynamics in open system Thermodynamics deals with
Thermodynamics deals with Tds equation
Tds equation Thermodynamic probability ppt
Thermodynamic probability ppt 0th law of thermodynamics definition
0th law of thermodynamics definition Third law of thermodynamics derivation
Third law of thermodynamics derivation Thermodynamics laws
Thermodynamics laws Engineering thermodynamics
Engineering thermodynamics Isobaric process formula
Isobaric process formula Brayton cycle process
Brayton cycle process Residual properties in thermodynamics
Residual properties in thermodynamics Laws of thermodynamics simple
Laws of thermodynamics simple Statistical thermodynamics in chemistry
Statistical thermodynamics in chemistry Maxwell relations thermodynamics
Maxwell relations thermodynamics What is the change of entropy in an irreversible process
What is the change of entropy in an irreversible process Kelvin statement
Kelvin statement Formula sheet thermodynamics
Formula sheet thermodynamics Van't hoff equation
Van't hoff equation Cop of refrigerator
Cop of refrigerator Entropy thermodynamics
Entropy thermodynamics Properties of pure substances thermodynamics
Properties of pure substances thermodynamics Thermodynamics study guide
Thermodynamics study guide Maxwell thermodynamic relations
Maxwell thermodynamic relations