The CCHS Now Registry Sarah Yang Ph D






















- Slides: 29

The CCHS Now Registry Sarah Yang, Ph. D, Vandana Dole, Ph. D, Melinda Riccitelli, Ph. D

National Organization of Rare Disorders • The patient advocates who lobbied for the Orphan Drug Act of 1983, incorporated to become NORD after the passing of the act. • The Orphan Drug act gave financial incentives for the development of treatments for rare diseases. • Since then, NORD has served to represent the shared interests and goals of all Americans affected by rare diseases.

Being Rare is Hard • Rare diseases present unique challenges for researchers and companies working towards treatments and cures • • Small Patient populations Difficult/delayed diagnosis Clinical endpoints that are often unclear Enrollment and retention challenges for clinical trials • Because of this, there is a poor understanding of the natural history of rare diseases and their progression

There are nearly 7, 000 rare diseases and disorders, 95 percent of which have no treatment.

CCHS is complicated… • In the United States, a disease is defined as rare if it affects fewer than 200, 000 people • In contrast, a disease is generally considered to be ultra-rare if it affects one patient per 50, 000 people Assuming a world population of 7. 6 billion And a CCHS population of 1, 200 CCHS patients are one in 6. 3 million

• With multiple mutations, delineated between PARMS and NPARMS • Varying degrees of affectedness… • Our family members NEED a voice about what is important to them • Our researchers and clinical doctors need more information about day to day life with CCHS to improve outcomes and hopefully develop treatments

IAMRARE™ Registry Program To address the special needs of those developing treatments for rare diseases, NORD has created a Natural Histories Patient Registry Platform, with extensive input from FDA, NIH, patients, organizations and experts in the field.

IAMRARE™ Registry Program NORD’s platform is • An easy to use system that allows patients and organizations to inform and shape medical research and translational science for rare diseases • A high-quality, customized registry to collect data needed to define the natural progression of a rare disease – ultimately advancing treatment and improving outcomes.

NORD Natural History Grant • Natural history studies are longitudinal studies that aim to fill research gaps to help medical researchers better understand how diseases progress over time. • They can yield vital information that is essential to clinical trial design, such as biomarkers, demographics, important clinical symptoms, genetic and environmental variables, and patient perspectives. • NORD’s Natural History Studies project empowers patients and families to help eliminate some of the ‘I don’t know’ in rare disease research, making way for progress

NORD Natural History Grant • 20 NORD member groups were selected by a competitive application process reviewed by an internal committee. • All diseases represented have diagnostic challenges, limited or no research, and cover a broad range of symptoms and medical specialties, including neurology, cardiovascular, musculoskeletal, immunology and endocrinology.

NORD Natural History Grant • Each group was chosen to develop natural history studies with the assistance of the National Organization for Rare Disorders (NORD) supported in part by a cooperative agreement with the U. S. Food and Drug Administration (FDA), through NORD’s Natural History Study research platform. • “Our goal is for the 1 in 10 Americans with rare diseases, most of whom are children, to have a treatment and cure, and we developed NORD’s Natural History Study platform to eliminate challenges standing in the way of that target, ” said NORD President and CEO Peter L. Saltonstall. “We thank the FDA for its support of NORD and ongoing commitment to rare diseases. ”

20 Patient Groups Selected Hereditary Neuropathy Foundation, Organic Acidemia Association, XLH Network, Inc. , CCHS Network, Pitt Hopkins Research Foundation, The OMSLife Foundation, Platelet Disorder Support Association, Global Foundation for Peroxisomal Disorders, APS Type 1 Foundation, Scleroderma Research Foundation, Galactosemia Foundation, Desmoid Tumor Research Foundation, International Pemphigus & Pemphigoid, The Morgan Leary Vaughan Fund, Adult Polyglucosan Body Disease (APBD) Research, Bridge the Gap-SYNGAP Education and Research Foundation, United Leukodystrophy Foundation, AMENSupport (American Multiple Endocrine Neoplasia Support), Lipoprotein(a) Foundation, and Worldwide Syringomyelia & Chiari Task Force.

Iam. Rare platform • Iam. Rare platform was designed and is maintained by NORD and NIH. • Follows government guidelines to protected patient information • Registry information is encrypted • Their maintenance and security allowed the CCHS Network to focus on questionnaire development and data collection/analysis

Consent • Once a participant registers for the CCHS NOW Registry, they must voluntarily agree to participate. • Research Subjects Bill of Rights • Gives information about storing data • Risks/benefits • Confidentiality • Privacy

Consent 1) To characterize and describe the CCHS population as a whole and to gain a better understanding of the spectrum of clinical variants in individuals with CCHS of all stages. This includes but is not limited to collecting information on: diagnosis, treatment, medical history, socio-economic environment and outcomes. 2) To understand the changes of CCHS over a lifetime as well as to gain information on clinical practice patterns and variations over the course of treatment. 3) To facilitate the development of best practice and management guidelines and recommendations to optimize care, improve quality of life and outcomes and standards of care. 4) To provide information regarding ongoing research studies and clinical trials. Study Participants may consent to be contacted by researchers for recruitment into Institutional Review Board (IRB) approved studies.

NORD specific questions • To identify common XXX across different rare disorders • To look for commonalities between disorders in different aspects of

Hummingbird IRB • Developed and staffed by respected members of the IRB community with specialized expertise in medical, ethical and regulatory matters, Hummingbird IRB provides independent central IRB services for institutional and commercial clients. Full accreditation of the human research protection program, inc

nd 2 round CCHS specific

nd 2 round CCHS specific

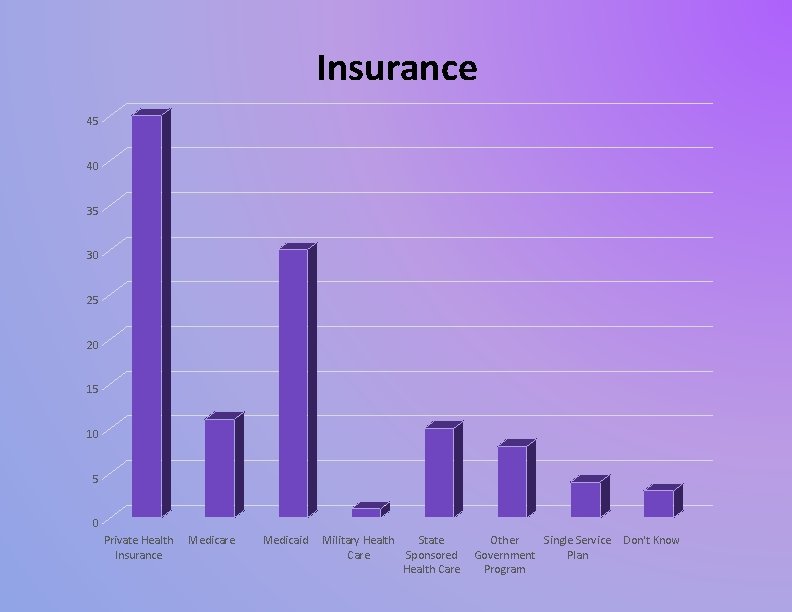
Insurance 45 40 35 30 25 20 15 10 5 0 Private Health Insurance Medicare Medicaid Military Health State Care Sponsored Health Care Other Single Service Don't Know Government Plan Program

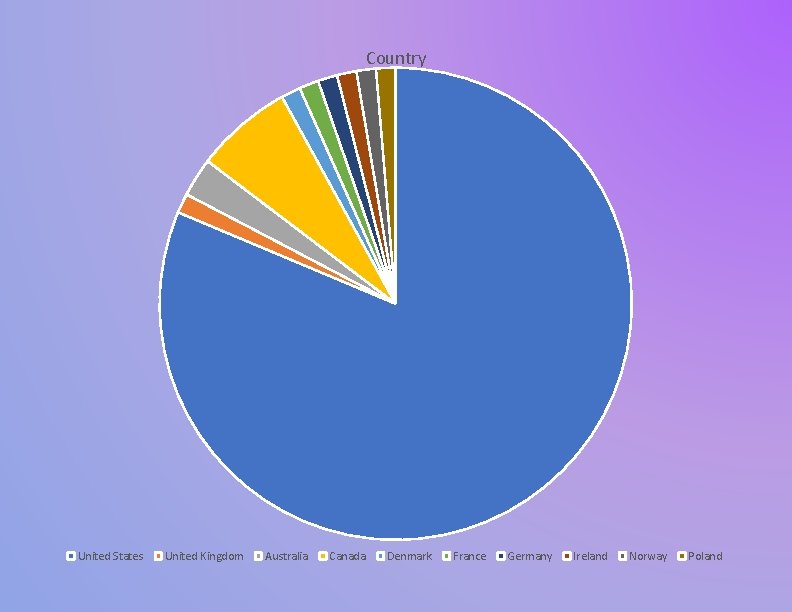
Country United States United Kingdom Australia Canada Denmark France Germany Ireland Norway Poland

Rare Disease Diagnosis 40 30 20 10 0 PARM NPARM HD Clinical Diagnosis Other Rare Disease Diagnosis 20/31 20/30 5% 3% 20/28 3% 20/27 34% 20/32 20/33 5% 3% 20/25 21% 20/26 26%

CCHS cardiac data Cardiac Pacemakers have do not have

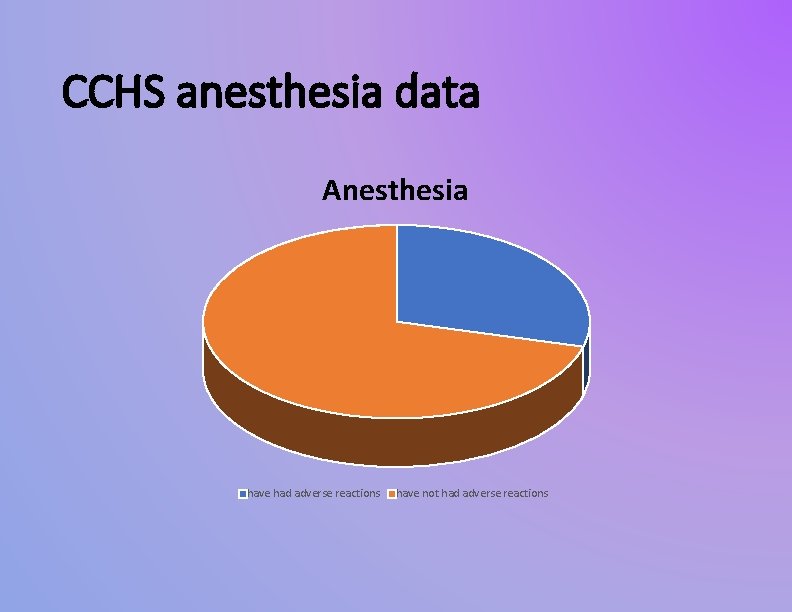
CCHS anesthesia data Anesthesia have had adverse reactions have not had adverse reactions

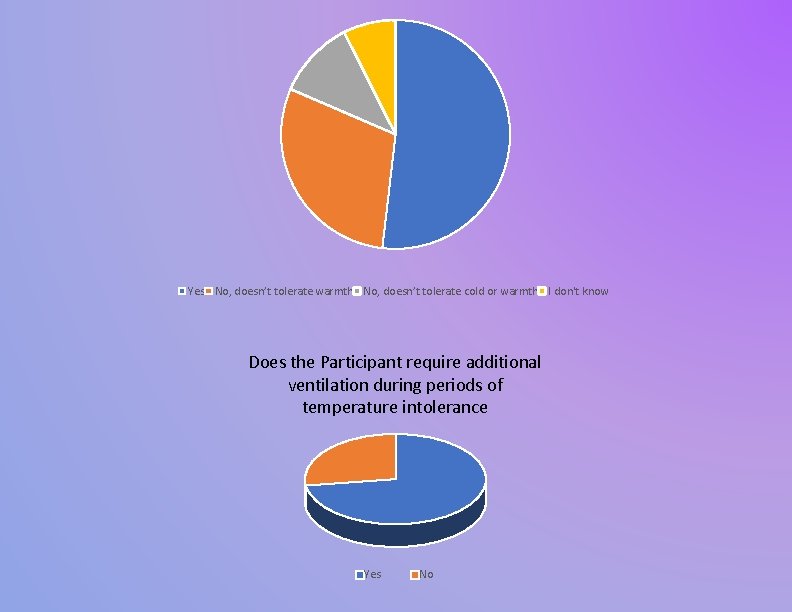
Yes No, doesn’t tolerate warmth No, doesn’t tolerate cold or warmth I don't know Does the Participant require additional ventilation during periods of temperature intolerance Yes No

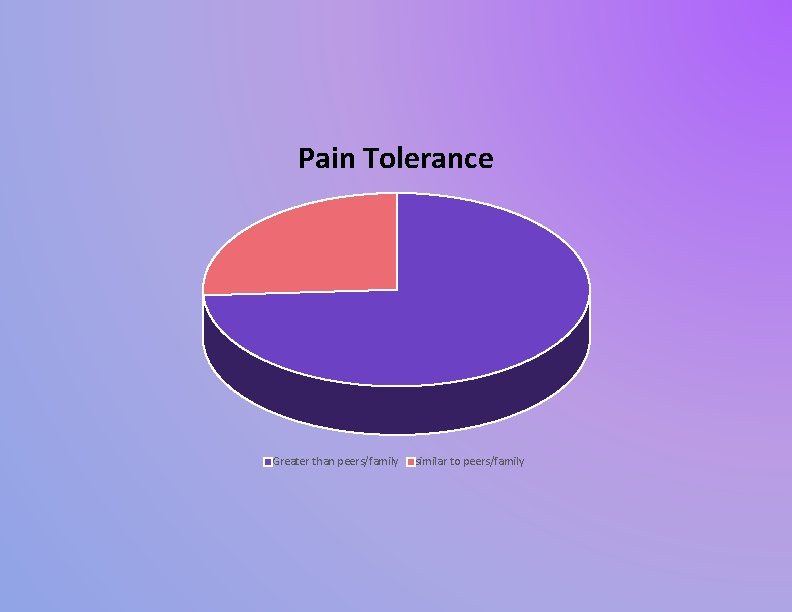
Pain Tolerance Greater than peers/family similar to peers/family

Future data collection • Update current questions with improved wording and response options • Expand upon areas of interest • Add areas that we did not consider in initial database • Look to our CCHS families to see what else they would like to see added

Future goals • Expand to include clinician specific questions • Involve more clinicians to help identify areas of treatment/management that could be improved. • Help identify areas of management that should become mutation specific treatment options for CCHS patients

Thank you • Melinda and Vandana • Registry Board • NORD, NIH, FDA