Teresa Gamucci Direttore Oncologia Medica ASL Frosinone Adiuvante


































- Slides: 55

Teresa Gamucci Direttore Oncologia Medica ASL Frosinone

Adiuvante e neoadiuvante: quali dubbi? • Dubbi? SI TANTI • Adiuvante o neoadiuvante : non è tanto questo il problema • Sottotipi molecolari: partiamo da qui

Malattia HER 2 positiva Quali dubbi? • Donna di 70 anni, giovanile • Lieve ipertensione arteriosa, lieve ipercolesterolemia • Nodulo mammario di 2 cm, linfonodi ascellari ecograficamente negativi E. I. carcinoma duttale infiltrante G 2 ER=neg Pg. R=neg ki 67=25% HER 2= 3+ • Seno piccolo DECISIONE

Scenari • Chemioterapia neoadiuvante con 4 EC 12 Paclitaxel + Trastuzumab (+ Pertuzumab) probabile RC intervento “minimo” + BLS Trastuzumab • Intervento chirurgico “più ampio” +BLS Chemioterapia adiuvante EC x SBAGLIATO! 4 12 Paclitaxel + Trastuzumab x 1 anno • Intervento chirurgico “più ampio”+ BLS Chemioterapia adiuvante 12 Paclitaxel + Trastuzumab x 1 anno


205 pts 10 Centri Oncologici Italiani 2003 -2011


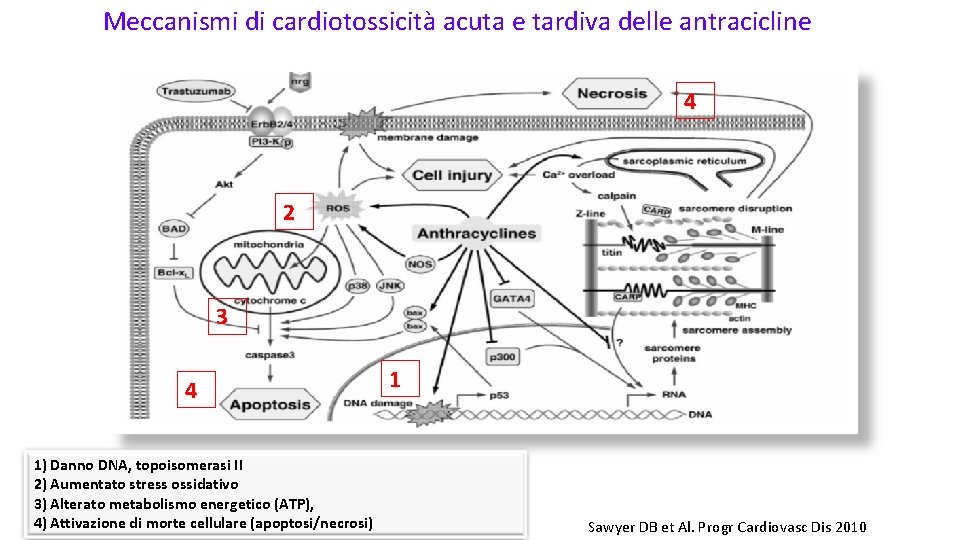
Meccanismi di cardiotossicità acuta e tardiva delle antracicline 4 2 3 4 1) Danno DNA, topoisomerasi II 2) Aumentato stress ossidativo 3) Alterato metabolismo energetico (ATP), 4) Attivazione di morte cellulare (apoptosi/necrosi) 1 Sawyer DB et Al. Progr Cardiovasc Dis 2010

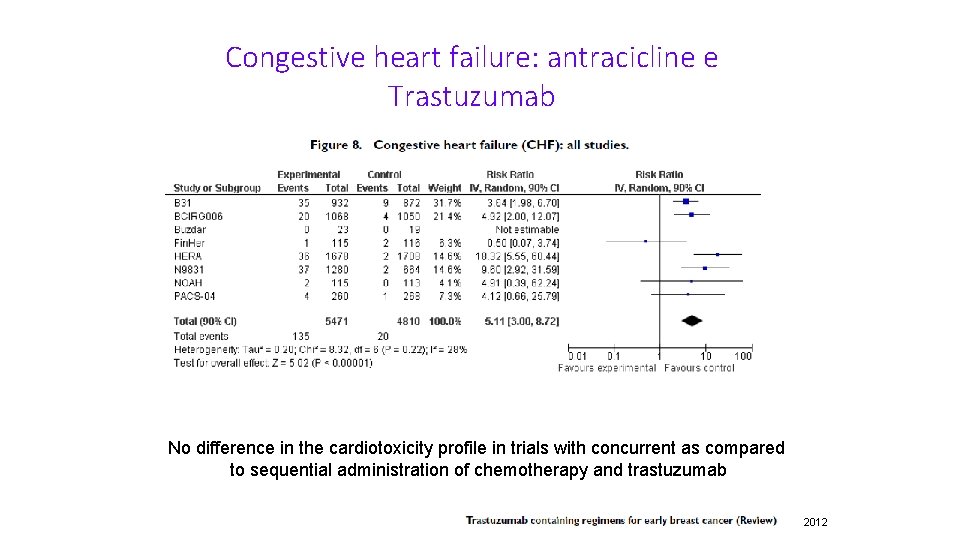
Congestive heart failure: antracicline e Trastuzumab No difference in the cardiotoxicity profile in trials with concurrent as compared to sequential administration of chemotherapy and trastuzumab 2012


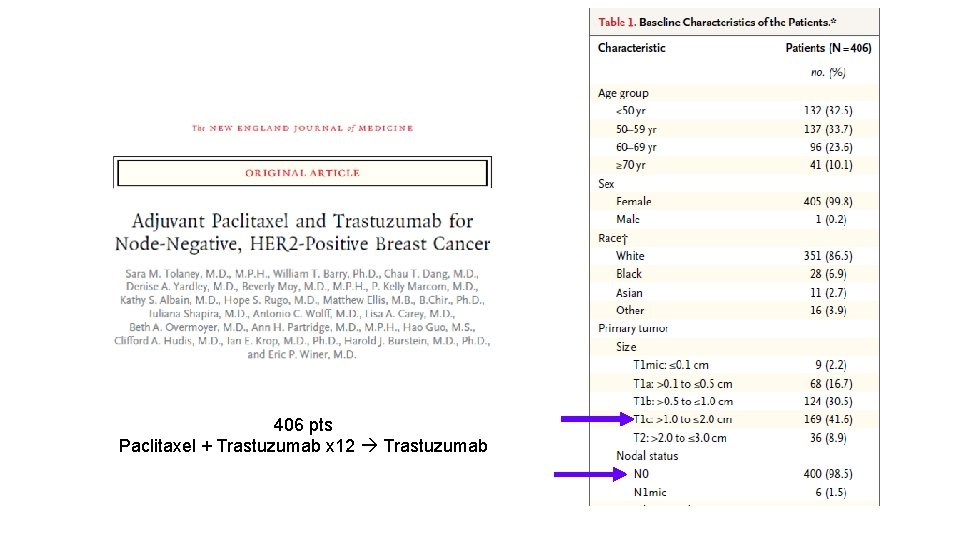
406 pts Paclitaxel + Trastuzumab x 12 Trastuzumab

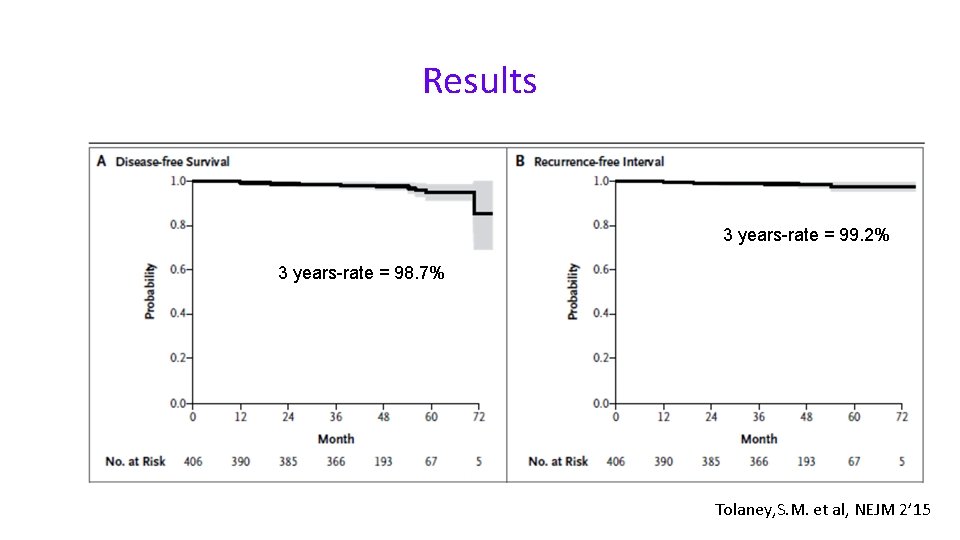
Results 3 years-rate = 99. 2% 3 years-rate = 98. 7% Tolaney, S. M. et al, NEJM 2’ 15

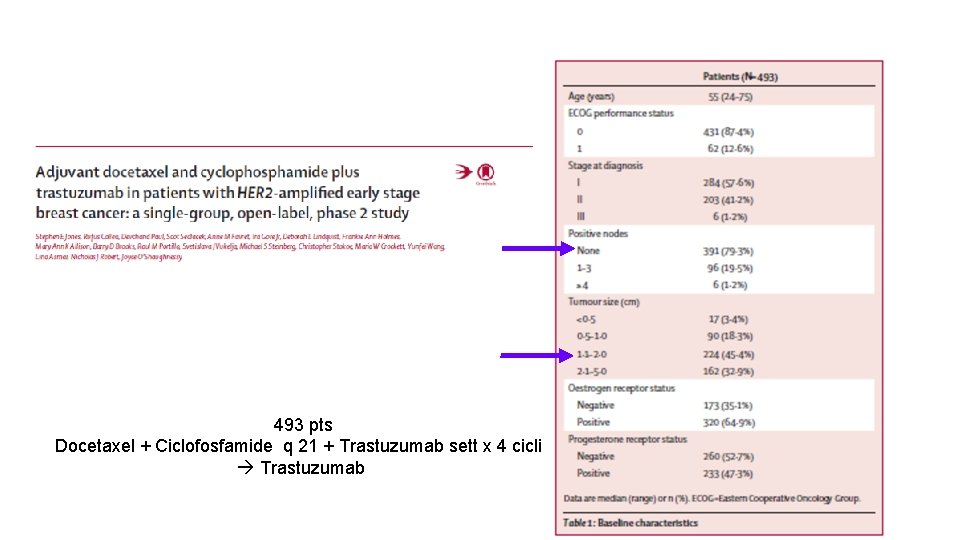
493 pts Docetaxel + Ciclofosfamide q 21 + Trastuzumab sett x 4 cicli Trastuzumab

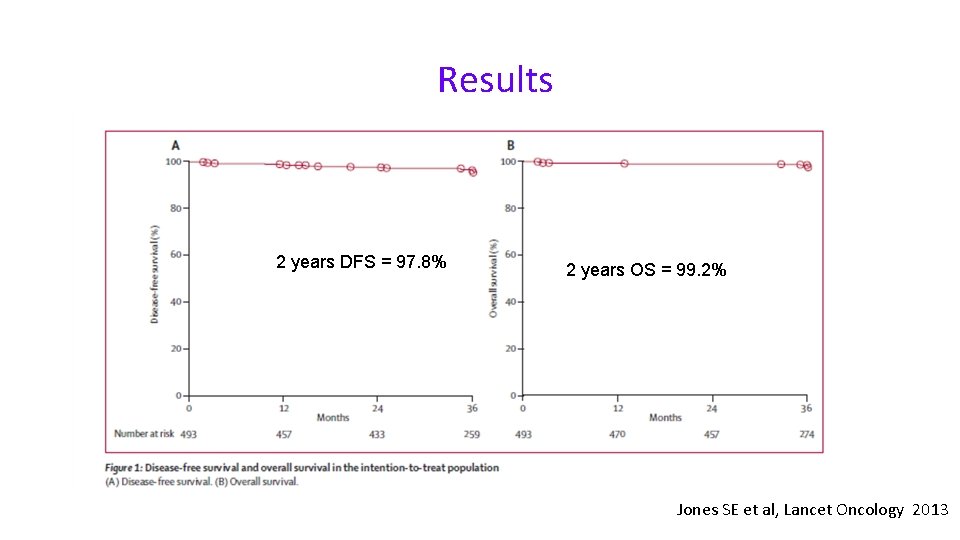
Results 2 years DFS = 97. 8% 2 years OS = 99. 2% Jones SE et al, Lancet Oncology 2013



Malattia triplo-negativa Quali dubbi? • Donna di 39 anni • Zia materna operata a 50 anni per ca ovaio • Nodulo mammario di 3 cm, linfonodi ascellari ecograficamente positivi E. I. carcinoma duttale infiltrante G 3 ER=neg Pg. R=neg ki 67=35% HER 2= neg

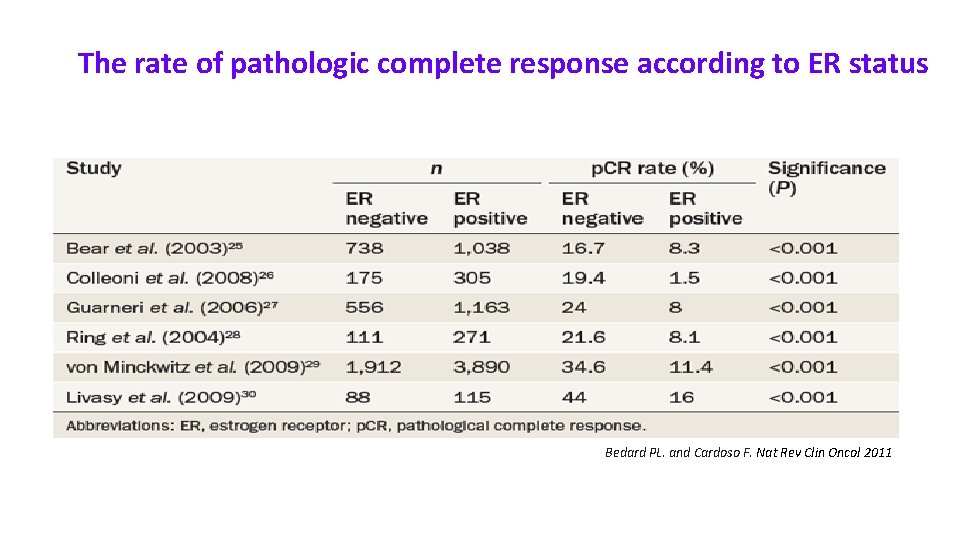
The rate of pathologic complete response according to ER status Bedard PL. and Cardoso F. Nat Rev Clin Oncol 2011


196 pts ESMO 2015

Malattia triplo-negativa Quali dubbi? DECISIONE CHEMIOTERAPIA NEOADIUVANTE QUALE? Platino si/Platino no

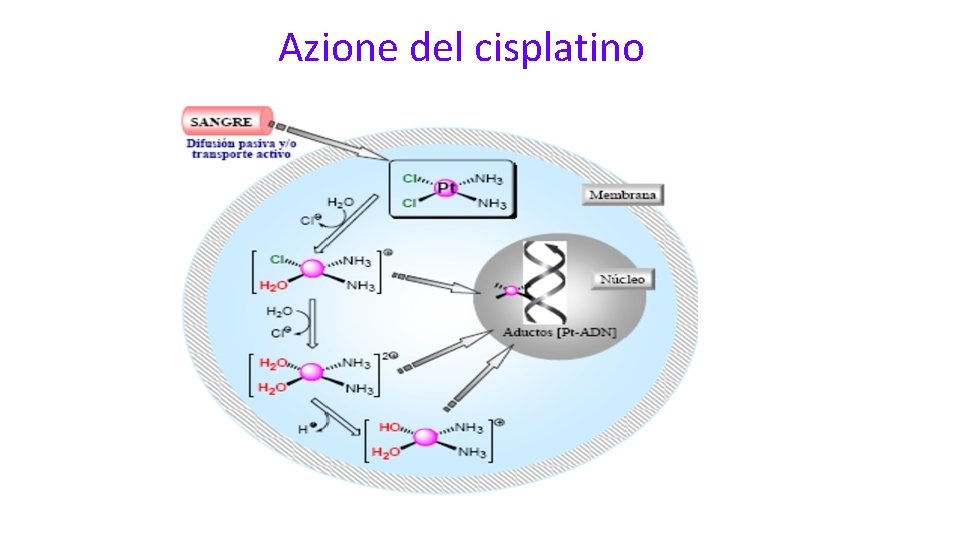
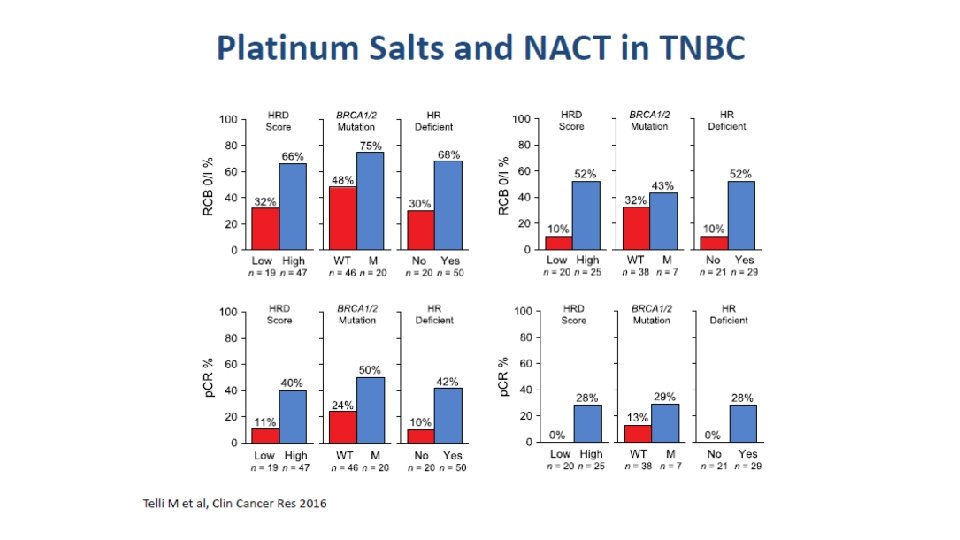
Critiche all’utilizzo dei Sali di platino nelle pazienti TN • I Sali di platino funzionano nelle BRCA mutate • La maggior parte delle paz BRCA mutate sono TN • Negli studi con sali di platino, la risposta ottenuta è dovuta al grippo delle BRCA mutate IN REALTA’ FORSE NO…

Azione del cisplatino

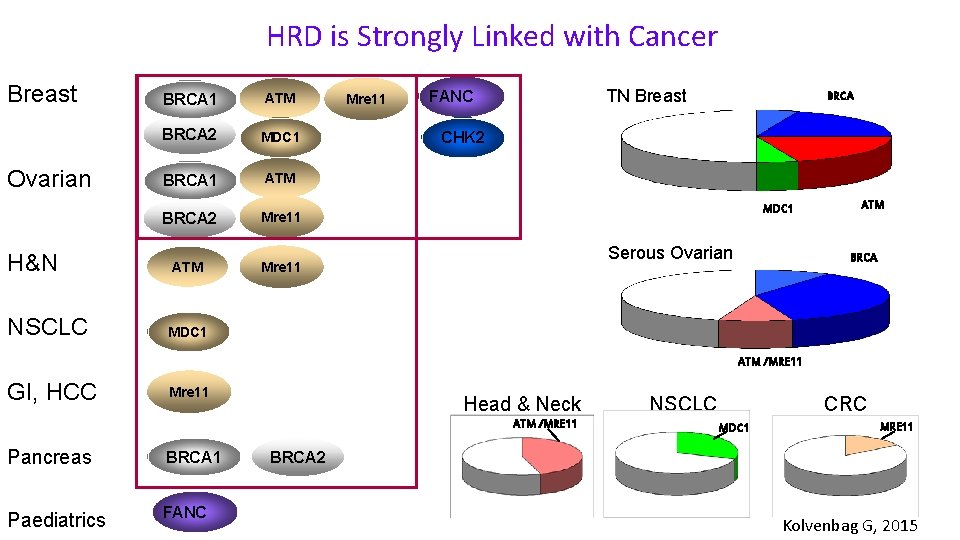
Sali di platino • In situations where the DNA repair is compromised synthetic lethality of the cell depends on • DNA repair factors deficient in functioning: • BRCA gene deficient in genotype or phenotype • Other Homologues Recombination Repair factors deficient in functioning (HRD) , eg ATM, MDC 1, MRE 11 • In presence of DNA damaging agents • Chemotherapy (Especially Platinum salts) • Radiotherapy

HRD is Strongly Linked with Cancer Breast BRCA 1 ATM BRCA 2 MDC 1 BRCA 1 ATM BRCA 2 Mre 11 H&N ATM Mre 11 NSCLC MDC 1 Ovarian Mre 11 TN Breast FANC BRCA CHK 2 MDC 1 Serous Ovarian ATM BRCA ATM /MRE 11 GI, HCC Mre 11 Head & Neck ATM /MRE 11 Pancreas BRCA 1 Paediatrics FANC NSCLC CRC MDC 1 MRE 11 BRCA 2 Kolvenbag G, 2015









Malattia Luminale Quali dubbi? Tumori Luminali: le decisioni difficili Luminal B = ER e/o Pg. R bassi e/o ki 67 < 20% HER 2 – Quando abbiamo davvero bisogno di aiuto da un test genomico?




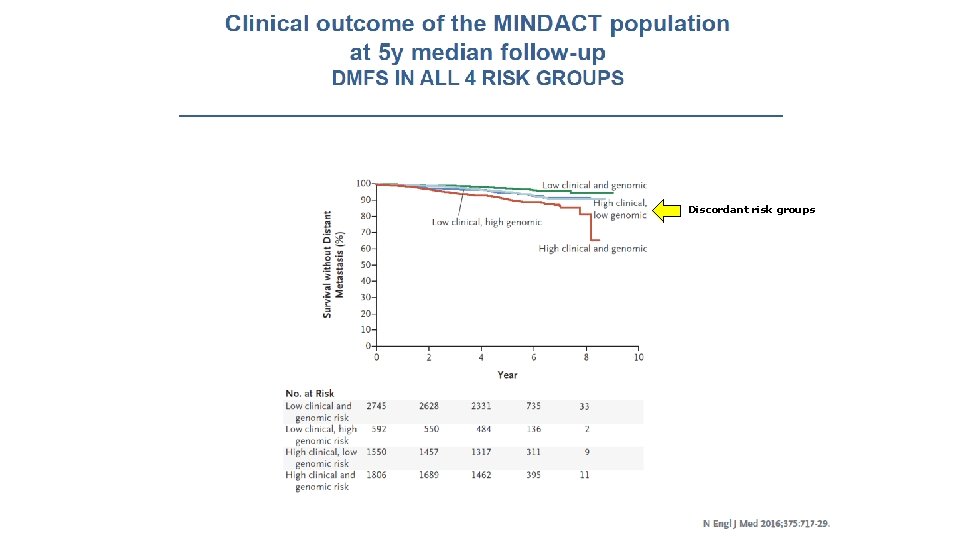
Discordant risk groups

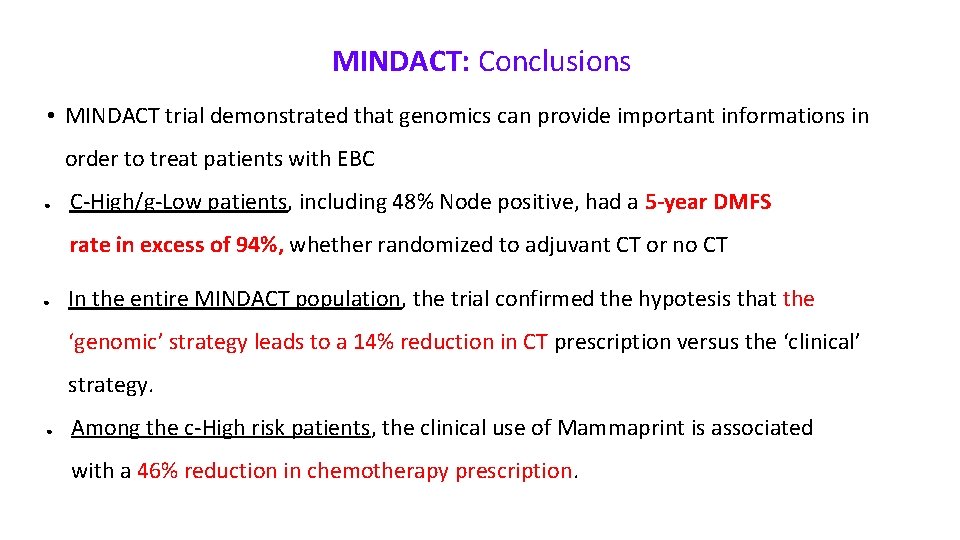
MINDACT: Conclusions • MINDACT trial demonstrated that genomics can provide important informations in order to treat patients with EBC ● C-High/g-Low patients, including 48% Node positive, had a 5 -year DMFS rate in excess of 94%, whether randomized to adjuvant CT or no CT ● In the entire MINDACT population, the trial confirmed the hypotesis that the ‘genomic’ strategy leads to a 14% reduction in CT prescription versus the ‘clinical’ strategy. ● Among the c-High risk patients, the clinical use of Mammaprint is associated with a 46% reduction in chemotherapy prescription.

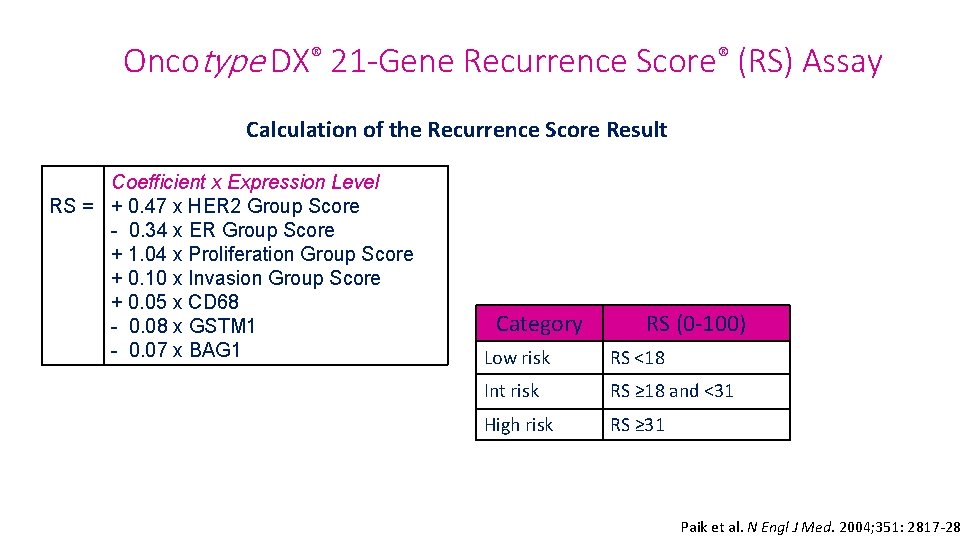
Oncotype DX® 21 -Gene Recurrence Score® (RS) Assay Calculation of the Recurrence Score Result Coefficient x Expression Level RS = + 0. 47 x HER 2 Group Score - 0. 34 x ER Group Score + 1. 04 x Proliferation Group Score + 0. 10 x Invasion Group Score + 0. 05 x CD 68 - 0. 08 x GSTM 1 - 0. 07 x BAG 1 Category RS (0 -100) Low risk RS <18 Int risk RS ≥ 18 and <31 High risk RS ≥ 31 Paik et al. N Engl J Med. 2004; 351: 2817 -28

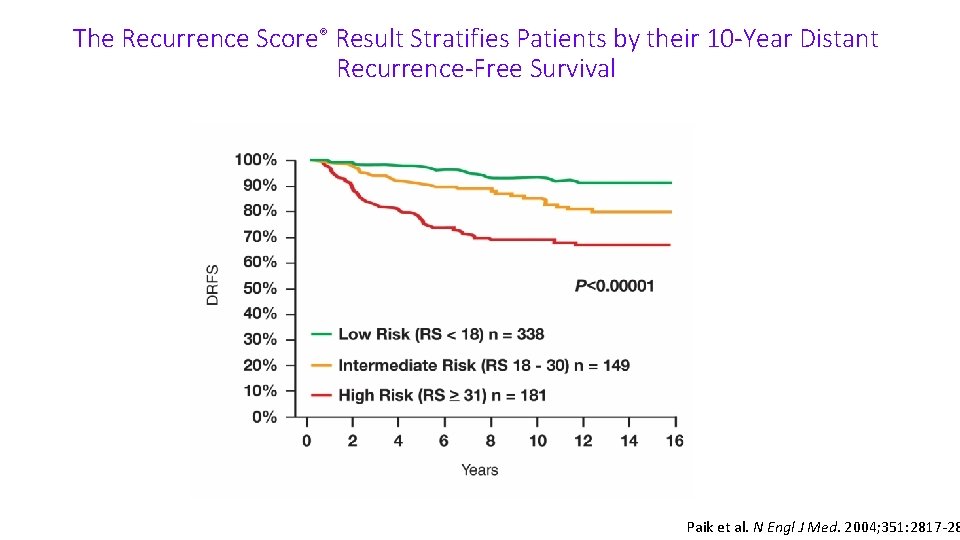
The Recurrence Score® Result Stratifies Patients by their 10 -Year Distant Recurrence-Free Survival Paik et al. N Engl J Med. 2004; 351: 2817 -2826 Paik et al. N Engl J Med. 2004; 351: 2817 -28

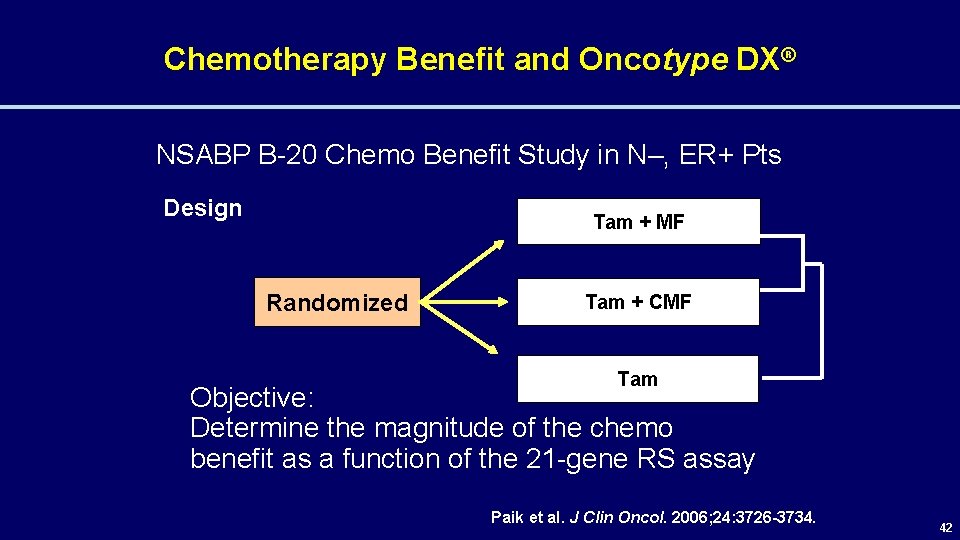
Chemotherapy Benefit and Oncotype DX® NSABP B-20 Chemo Benefit Study in N–, ER+ Pts Design Tam + MF Randomized Tam + CMF Tam Objective: Determine the magnitude of the chemo benefit as a function of the 21 -gene RS assay Paik et al. J Clin Oncol. 2006; 24: 3726 -3734. 42

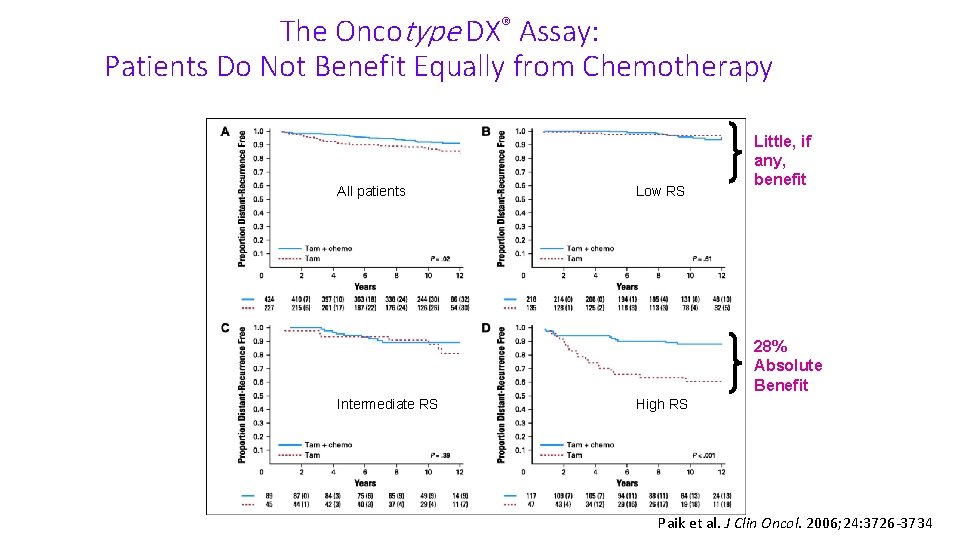
The Oncotype DX® Assay: Patients Do Not Benefit Equally from Chemotherapy All patients Low RS Little, if any, benefit 28% Absolute Benefit Intermediate RS High RS Paik et al. J Clin Oncol. 2006; 24: 3726 -3734

Prognostic and Predictive Value of the 21 -Gene Recurrence Score Assay in Postmenopausal Women with Node-Positive, Estrogen- Receptor-Positive Breast Cancer on Chemotherapy: A Retrospective Analysis of a Randomised Trial Albain KS et al. Lancet Oncol 2010; 11(1): 55 -65. Albain KS et al. San Antonio Breast Cancer Symposium 2009; Abstract 112.

21 -Gene Recurrence Score Assay in Postmenopausal Women with Node-Positive, Estrogen- Receptor-Positive Breast Cancer • • • A low 21 -gene recurrence score (RS) in postmenopausal patients with ER-positive, node-negative breast cancer predicts a lack of benefit from the addition of chemotherapy to tamoxifen (T) treatment (JCO 2006; 24: 3726). The value of the 21 -gene recurrence score assay in patients with ER-positive, nodepositive breast cancer that are treated with T alone is unknown. Study objectives: • Assess prognostic value of the 21 -gene recurrence score in patients with node -positive breast cancer treated only with T. • Assess whether 21 -gene recurrence assay allows for the prediction of a nodepositive subset of patients who do not benefit from anthracycline-based chemotherapy. Albain KS et al. Lancet Oncol 2010; 11(1): 55 -65.

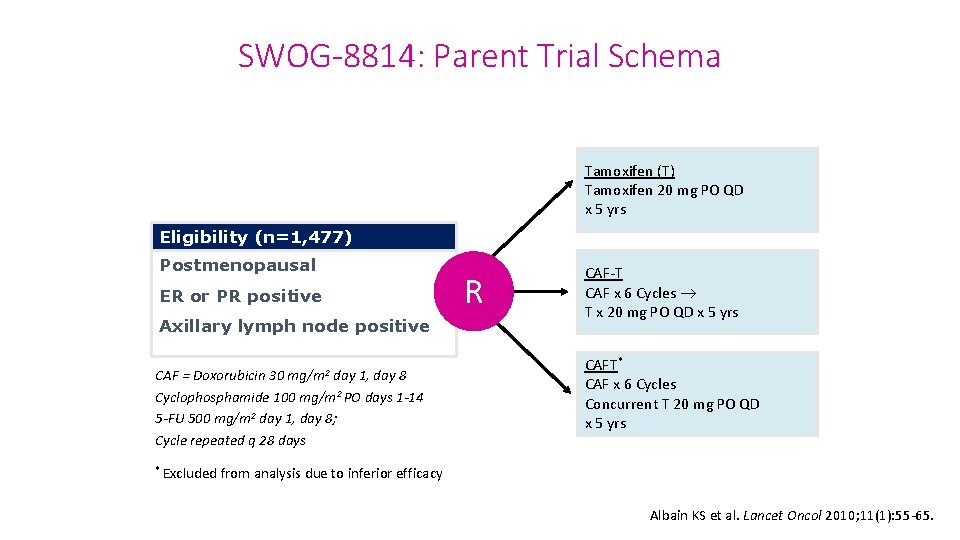
SWOG-8814: Parent Trial Schema Tamoxifen (T) Tamoxifen 20 mg PO QD x 5 yrs Eligibility (n=1, 477) Postmenopausal ER or PR positive Axillary lymph node positive mg/m 2 CAF = Doxorubicin 30 day 1, day 8 Cyclophosphamide 100 mg/m 2 PO days 1 -14 5 -FU 500 mg/m 2 day 1, day 8; Cycle repeated q 28 days * Excluded R CAF-T CAF x 6 Cycles T x 20 mg PO QD x 5 yrs CAFT* CAF x 6 Cycles Concurrent T 20 mg PO QD x 5 yrs from analysis due to inferior efficacy Albain KS et al. Lancet Oncol 2010; 11(1): 55 -65.

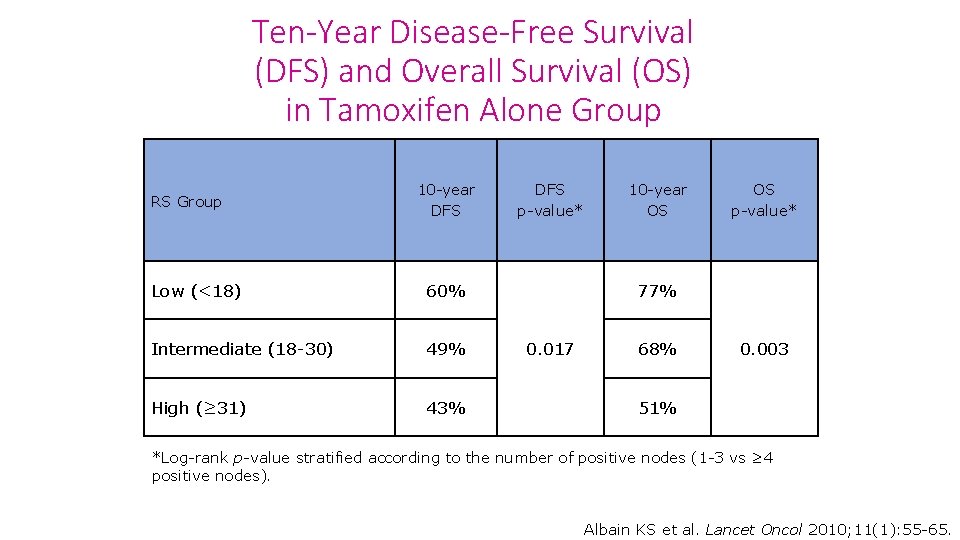
Ten-Year Disease-Free Survival (DFS) and Overall Survival (OS) in Tamoxifen Alone Group RS Group 10 -year DFS Low (<18) 60% Intermediate (18 -30) 49% High (≥ 31) 43% DFS p-value* 10 -year OS OS p-value* 77% 0. 017 68% 0. 003 51% *Log-rank p-value stratified according to the number of positive nodes (1 -3 vs ≥ 4 positive nodes). Albain KS et al. Lancet Oncol 2010; 11(1): 55 -65.

Hazard Ratio: Ten-Year DFS, T versus CAF-T Groups RS Group HR (95% CI) p-value* Low (<18) 1. 02 (0. 54 -1. 93) 0. 97 Intermediate (18 -30) 0. 72 (0. 39 -1. 31) 0. 48 High (≥ 31) 0. 59 (0. 35 -1. 01) 0. 033 Entire RS sample — 0. 054 *Log-rank p-value stratified according to the number of positive nodes (1 -3 vs ≥ 4 positive nodes); HR = hazard ratio. Albain KS et al. Lancet Oncol 2010; 11(1): 55 -65

Conclusions • The RS is prognostic for patients with node-positive breast cancer treated with tamoxifen alone. • A high RS score predicts an improved DFS in patients with node-positive breast cancer treated with anthracyline-based chemotherapy followed by tamoxifen compared to tamoxifen alone. • A low RS score identifies women with node-positive breast cancer who may not benefit from the addition of anthracycline-based chemotherapy to tamoxifen treatment. Albain KS et al. Lancet Oncol 2010; 11(1): 55 -65.

Caso clinico (1) • Donna 42 anni • Premenopausa • Marzo 2016 Intervento di quadrantectomia SE mammella dx + biopsia linfonodo sentinella - E. I. Carcinoma duttale infiltrante della mammella G 3 - p. T 1 c(18 mm) p. N 0(sn) ER=100% Pg. R=100% ki 67=30% HER 2=score 0 ?



Caso clinico (2) • Donna 65 anni • Postmenopausa • Febbraio Intervento di quadrantectomia SI mammella dx + linfectomia ascellare - E. I. Carcinoma duttale infiltrante della mammella G 2 - p. T 1 c (1. 4 cm) p. N 1 a(1/14) ER=98% Pg. R=80% ki 67=32% HER 2=score 0 ?


Grazie per l’attenzione Sora Frosinone Cassino
 Sudorazione notturna cause
Sudorazione notturna cause Frosinone quarto circolo
Frosinone quarto circolo Marina maccari
Marina maccari Master infermieristica oncologica
Master infermieristica oncologica Oncologia sondrio
Oncologia sondrio Oncologia alghero
Oncologia alghero Ritardo diagnostico in oncologia risarcimento
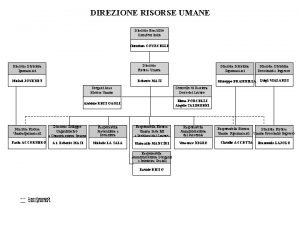
Ritardo diagnostico in oncologia risarcimento Organigramma carrefour italia
Organigramma carrefour italia Distanza punto retta
Distanza punto retta Cicognani alessandro bologna
Cicognani alessandro bologna Nomina direttore tecnico soa
Nomina direttore tecnico soa Direttore generale anpal
Direttore generale anpal Avaliação médica dano corporal
Avaliação médica dano corporal Documentos para apelar licencia medica en compin
Documentos para apelar licencia medica en compin Unife scuole di specializzazione
Unife scuole di specializzazione Domus medica
Domus medica Kalmia materia medica
Kalmia materia medica Polgonos
Polgonos Son los dos tipos de receta médica sicad
Son los dos tipos de receta médica sicad Asterias rubens materia medica
Asterias rubens materia medica Academia nacional de educacion medica
Academia nacional de educacion medica Que es rp en receta médica
Que es rp en receta médica Lex artis
Lex artis Materia medica reloaded
Materia medica reloaded Art. 8 legge gelli
Art. 8 legge gelli Modelo de prescrição médica
Modelo de prescrição médica Mi vocacion medica
Mi vocacion medica Atlas medica cartella clinica
Atlas medica cartella clinica 1. ingeniera médica programadora periodista hijastra
1. ingeniera médica programadora periodista hijastra Academia nacional de educación médica
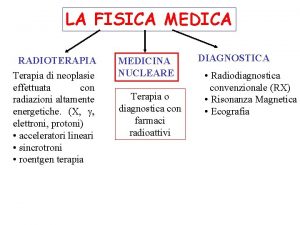
Academia nacional de educación médica Master fisica medica uv
Master fisica medica uv Diceologia
Diceologia Juan carlos arango barrientos
Juan carlos arango barrientos Receta cuantificada veterinaria requisitos
Receta cuantificada veterinaria requisitos Clinica carmelitas soyapango
Clinica carmelitas soyapango Iatrogenia medica
Iatrogenia medica Talasemia
Talasemia Ant tart materia medica
Ant tart materia medica Historia de la informatica medica
Historia de la informatica medica Losavio neurologo
Losavio neurologo Gestin medica
Gestin medica Orari dottoressa manna
Orari dottoressa manna Ricetta bianca esempio
Ricetta bianca esempio Specialista in fisica medica
Specialista in fisica medica Auditoria
Auditoria Graphites homeopathic materia medica
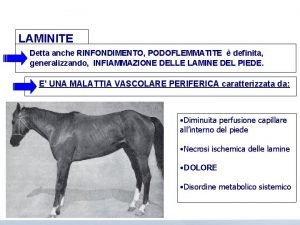
Graphites homeopathic materia medica Erba medica laminite
Erba medica laminite Moto medica
Moto medica Mousie in the snow
Mousie in the snow Teresa valderrama
Teresa valderrama No me tienes que dar porque te quiera
No me tienes que dar porque te quiera Santa teresa andariega
Santa teresa andariega Maria teresa miceli kerbauy
Maria teresa miceli kerbauy Isabel teresa de lorena
Isabel teresa de lorena Ensinar a ler, ensinar a compreender teresa colomer resumo
Ensinar a ler, ensinar a compreender teresa colomer resumo Teresa grimaldi capitello
Teresa grimaldi capitello