Mario Scartozzi Clinica di Oncologia Medica Ancona HIGHLIGHTS







- Slides: 61

Mario Scartozzi Clinica di Oncologia Medica Ancona HIGHLIGHTS IN COLORECTAL CANCER MANAGEMENT TREATMENT OF METASTATIC DISEASE

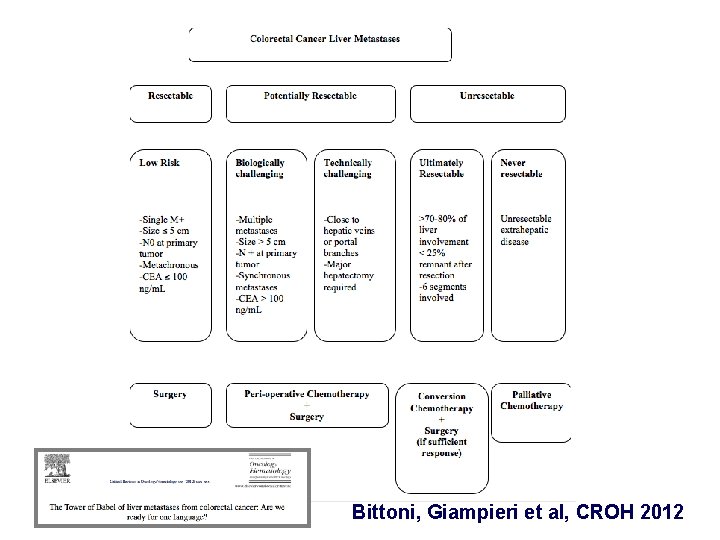
Bittoni, Giampieri et al, CROH 2012

Colon Cancer: what we already know – Chemotherapy has determined a relevant improvement in survival in the last 15 years: from 6 to 18 months – Probably FOLFOX = FOLFIRI and XELOX=FOLFOX (XELIRI has PHYLOSOPHICAL problems with toxicity) – Concept of all three drugs – Some patients with stage IV disease can be cured by an interdisciplinary approach

Colon Cancer: what we already know – Chemotherapy has determined a relevant improvement in survival in the last 15 years: from 6 to 18 months – Probably FOLFOX = FOLFIRI and XELOX=FOLFOX (XELIRI has PHYLOSOPHICAL problems with toxicity) – Concept of all three drugs – Some patients with stage IV disease can be cured by an interdisciplinary approach

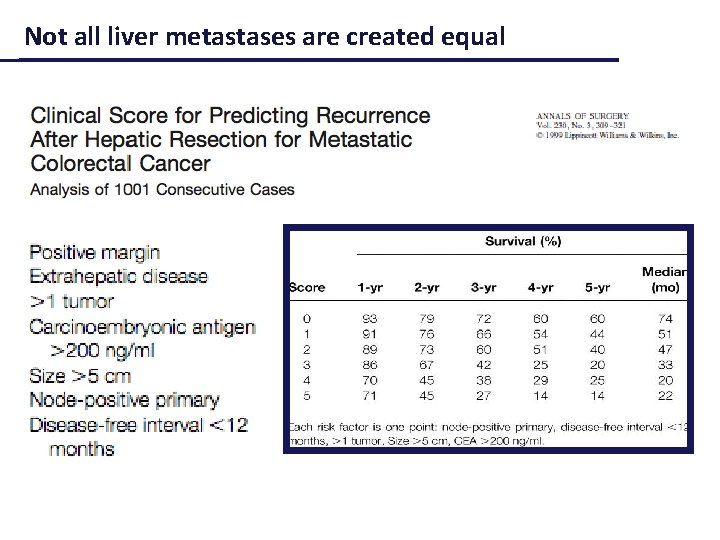
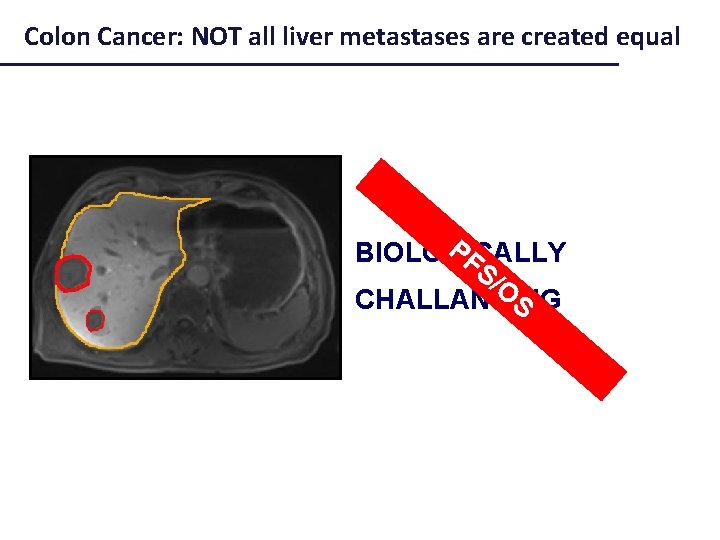
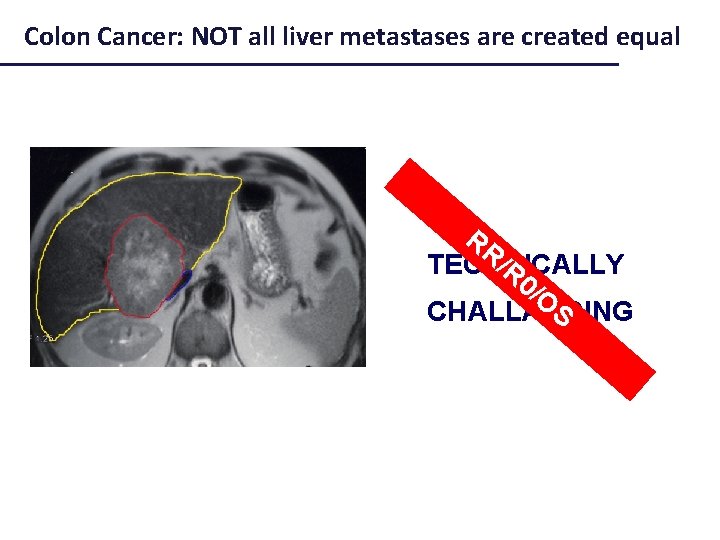
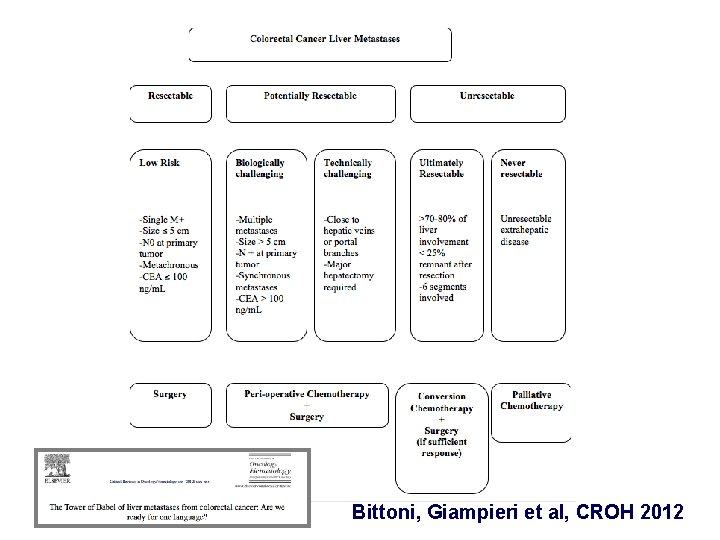
Not all liver metastases are created equal


Bittoni, Giampieri et al, CROH 2012

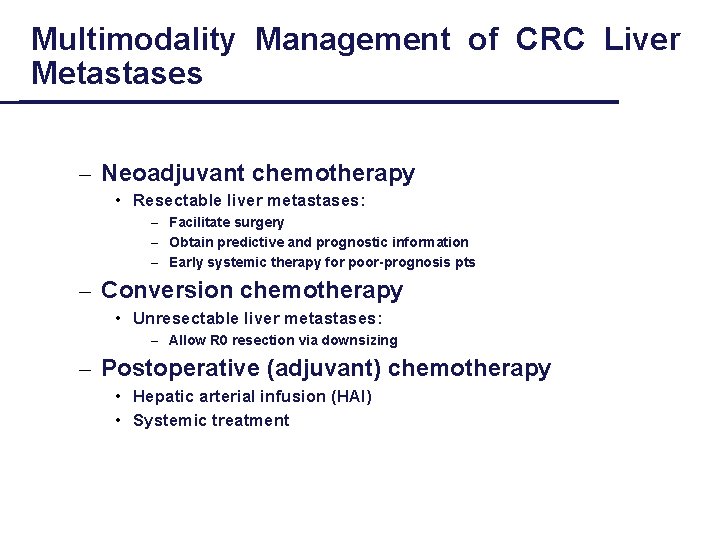
Multimodality Management of CRC Liver Metastases – Neoadjuvant chemotherapy • Resectable liver metastases: – Facilitate surgery – Obtain predictive and prognostic information – Early systemic therapy for poor-prognosis pts – Conversion chemotherapy • Unresectable liver metastases: – Allow R 0 resection via downsizing – Postoperative (adjuvant) chemotherapy • Hepatic arterial infusion (HAI) • Systemic treatment

Colon Cancer: NOT all liver metastases are created equal PF BIOLOGICALLY S/ O S CHALLANGING

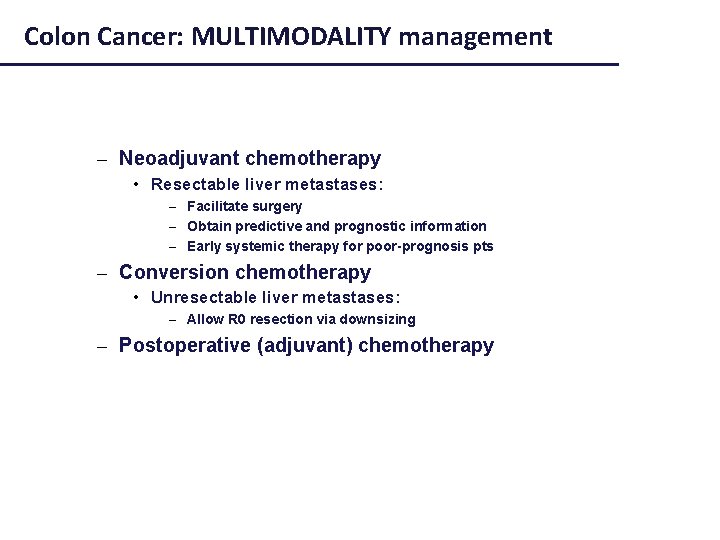
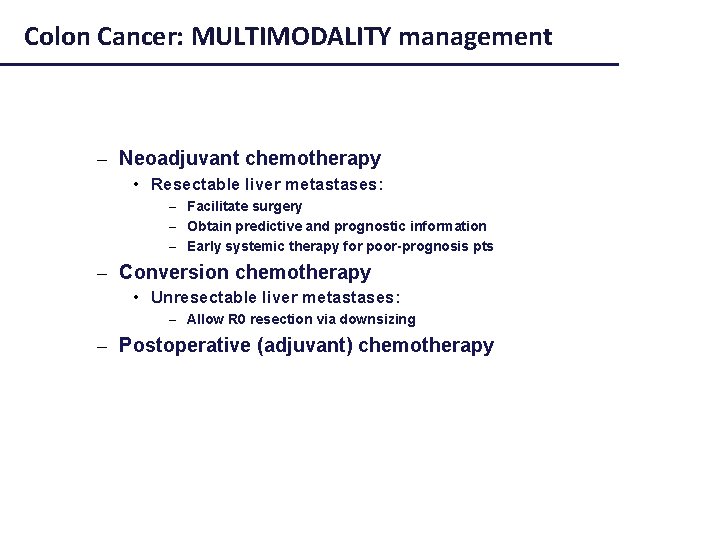
Colon Cancer: MULTIMODALITY management – Neoadjuvant chemotherapy • Resectable liver metastases: – Facilitate surgery – Obtain predictive and prognostic information – Early systemic therapy for poor-prognosis pts – Conversion chemotherapy • Unresectable liver metastases: – Allow R 0 resection via downsizing – Postoperative (adjuvant) chemotherapy

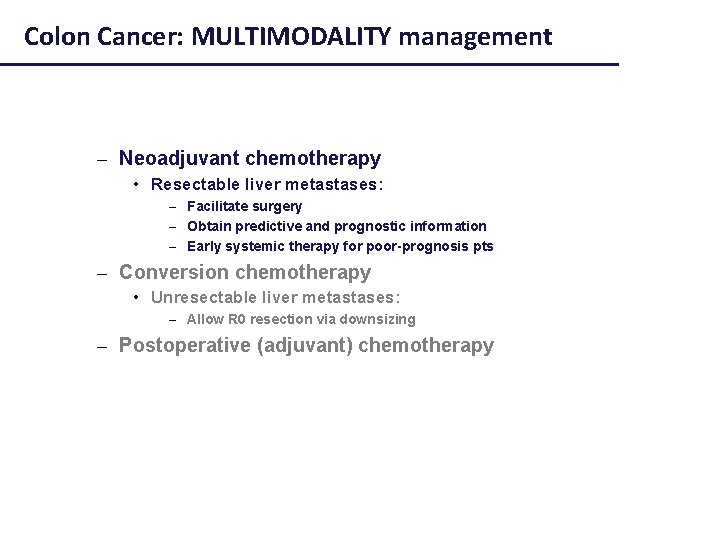
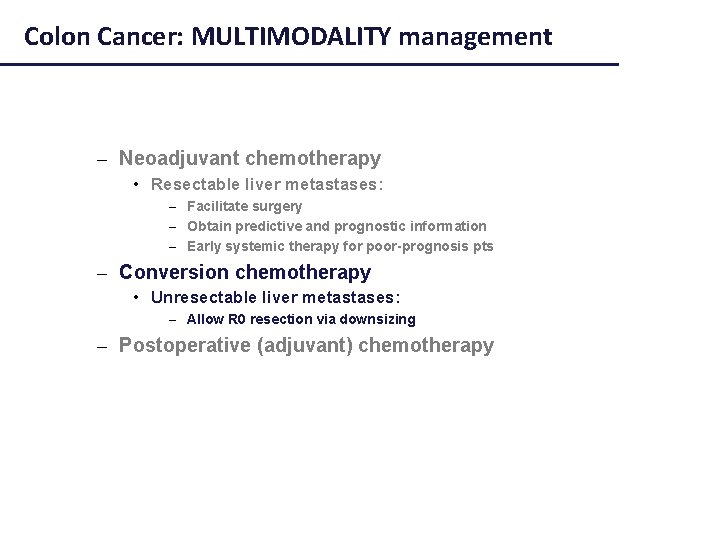
Colon Cancer: MULTIMODALITY management – Neoadjuvant chemotherapy • Resectable liver metastases: – Facilitate surgery – Obtain predictive and prognostic information – Early systemic therapy for poor-prognosis pts – Conversion chemotherapy • Unresectable liver metastases: – Allow R 0 resection via downsizing – Postoperative (adjuvant) chemotherapy

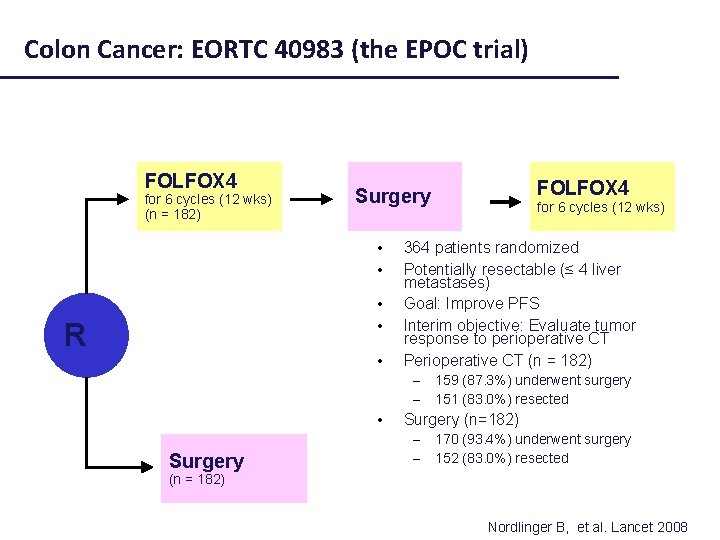
Colon Cancer: EORTC 40983 (the EPOC trial) FOLFOX 4 for 6 cycles (12 wks) (n = 182) • • R • • for 6 cycles (12 wks) 364 patients randomized Potentially resectable (≤ 4 liver metastases) Goal: Improve PFS Interim objective: Evaluate tumor response to perioperative CT Perioperative CT (n = 182) – – Surgery FOLFOX 4 Surgery 159 (87. 3%) underwent surgery 151 (83. 0%) resected Surgery (n=182) – – 170 (93. 4%) underwent surgery 152 (83. 0%) resected (n = 182) Nordlinger B, et al. Lancet 2008

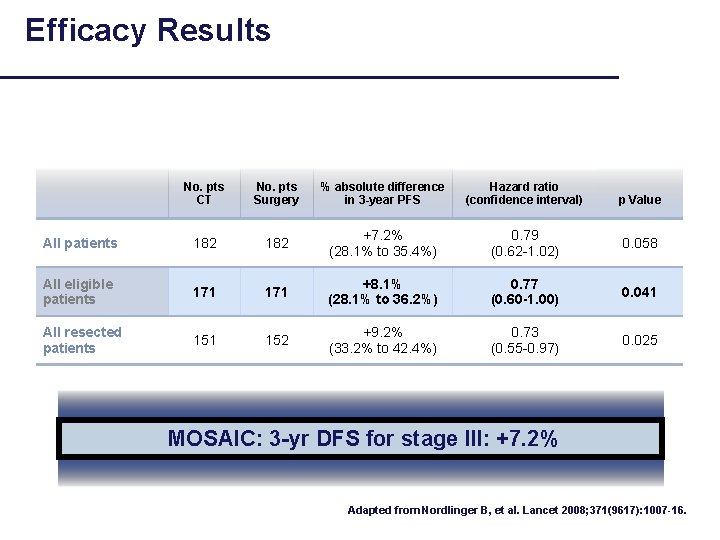
Efficacy Results No. pts CT No. pts Surgery % absolute difference in 3 -year PFS Hazard ratio (confidence interval) All patients 182 +7. 2% (28. 1% to 35. 4%) 0. 79 (0. 62 -1. 02) 0. 058 All eligible patients 171 +8. 1% (28. 1% to 36. 2%) 0. 77 (0. 60 -1. 00) 0. 041 All resected patients 151 152 +9. 2% (33. 2% to 42. 4%) 0. 73 (0. 55 -0. 97) 0. 025 p Value MOSAIC: 3 -yr DFS for stage III: +7. 2% Adapted from Nordlinger B, et al. Lancet 2008; 371(9617): 1007 -16.

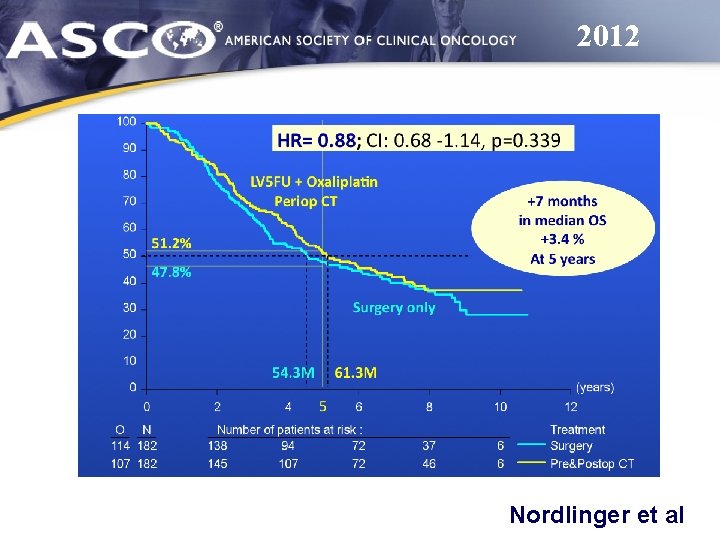
2012 Nordlinger et al

als gic olo Bi Surgery Chemotherapy Biologicals: How Do They Fit Into This Strategy?

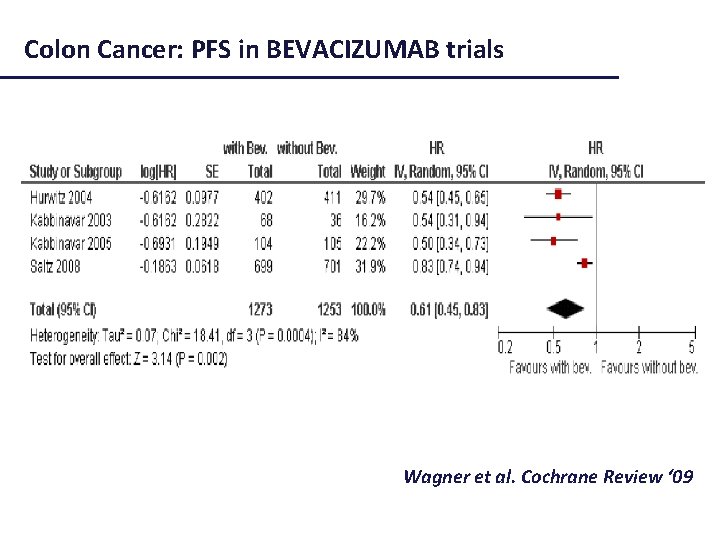
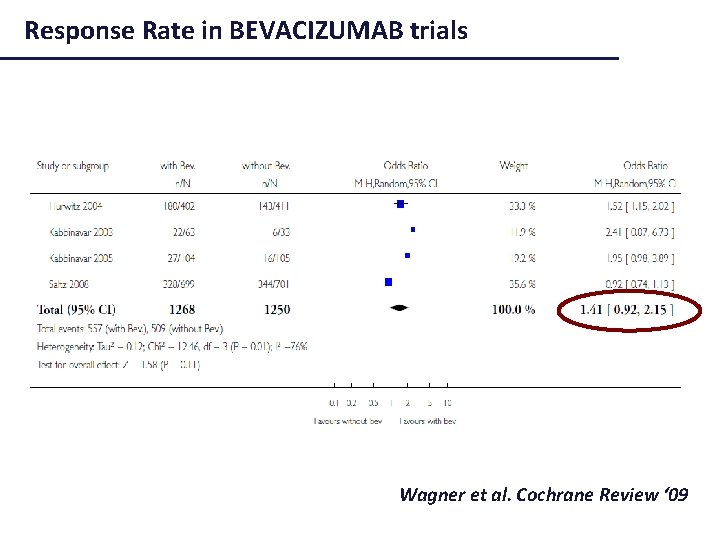
Colon Cancer: PFS in BEVACIZUMAB trials Wagner et al. Cochrane Review ‘ 09

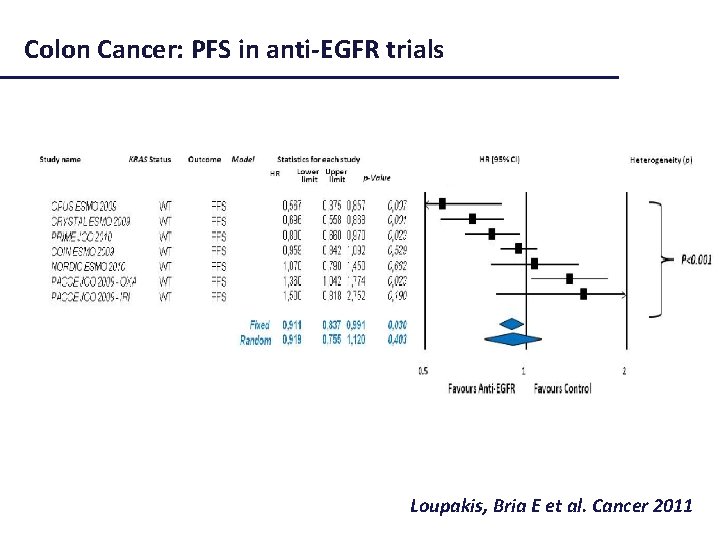
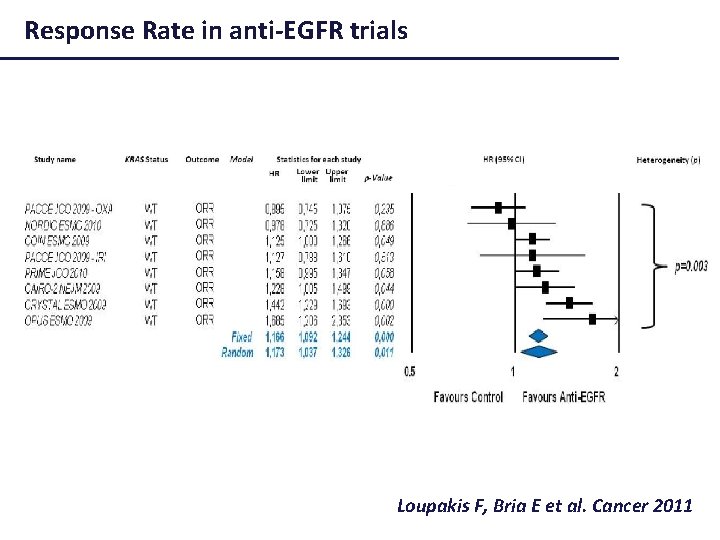
Colon Cancer: PFS in anti-EGFR trials Loupakis, Bria E et al. Cancer 2011

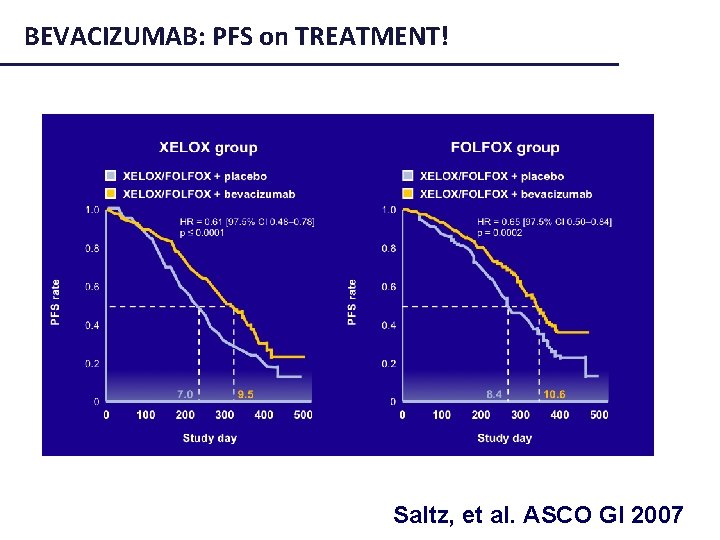
BEVACIZUMAB: PFS on TREATMENT! Saltz, et al. ASCO GI 2007

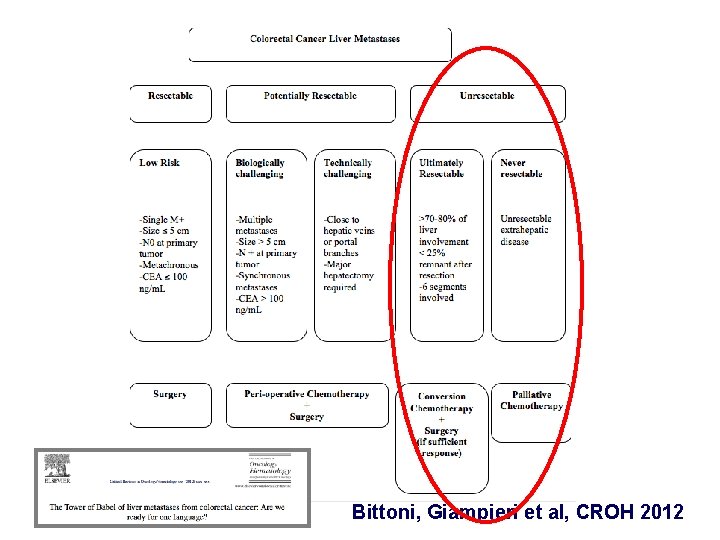
Colon Cancer: NOT all liver metastases are created equal RR /R TECHNICALLY 0/ O S CHALLANGING

Colon Cancer: MULTIMODALITY management – Neoadjuvant chemotherapy • Resectable liver metastases: – Facilitate surgery – Obtain predictive and prognostic information – Early systemic therapy for poor-prognosis pts – Conversion chemotherapy • Unresectable liver metastases: – Allow R 0 resection via downsizing – Postoperative (adjuvant) chemotherapy

Colon Cancer: MULTIMODALITY management – Neoadjuvant chemotherapy • Resectable liver metastases: – Facilitate surgery – Obtain predictive and prognostic information – Early systemic therapy for poor-prognosis pts – Conversion chemotherapy • Unresectable liver metastases: – Allow R 0 resection via downsizing – Postoperative (adjuvant) chemotherapy

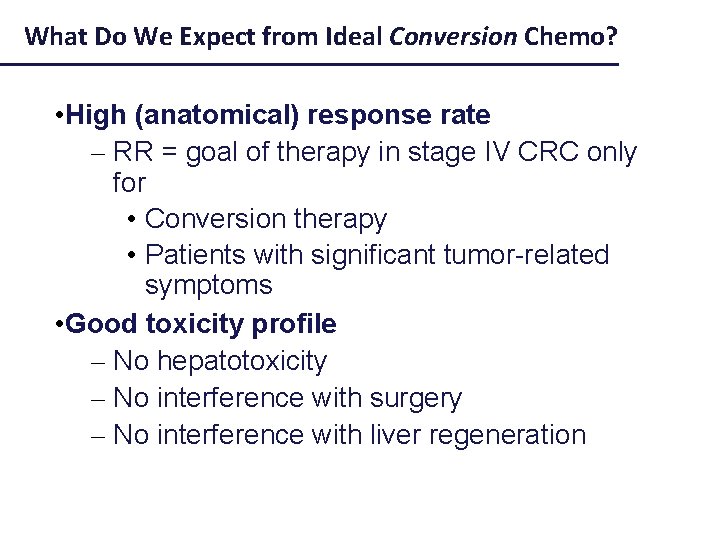
What Do We Expect from Ideal Conversion Chemo? • High (anatomical) response rate – RR = goal of therapy in stage IV CRC only for • Conversion therapy • Patients with significant tumor-related symptoms • Good toxicity profile – No hepatotoxicity – No interference with surgery – No interference with liver regeneration

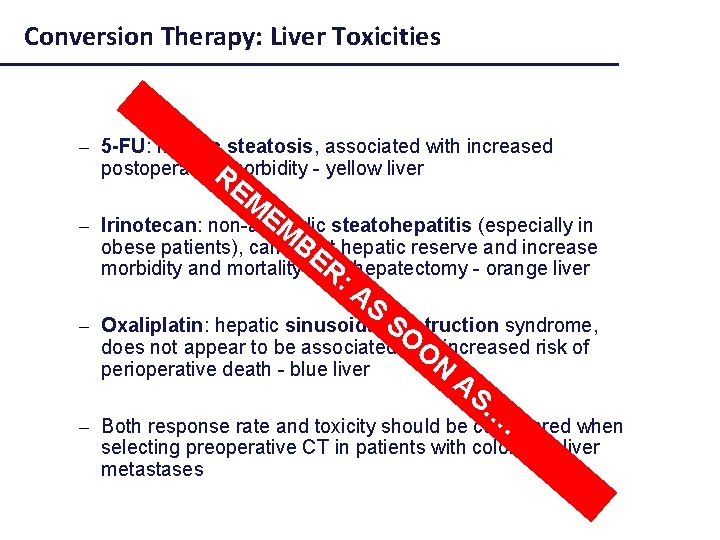
Conversion Therapy: Liver Toxicities – 5 -FU: hepatic steatosis, associated with increased postoperative. Rmorbidity - yellow liver EM E – Irinotecan: non-alcoholic M steatohepatitis (especially in BE hepatic reserve and increase obese patients), can affect R hepatectomy - orange liver morbidity and mortality after : A S – Oxaliplatin: hepatic sinusoidal Sobstruction syndrome, O does not appear to be associated with O increased risk of N perioperative death - blue liver AS … . – Both response rate and toxicity should be considered when selecting preoperative CT in patients with colorectal liver metastases Adapted from Zorzi D, et al. Br J Surg 2007; 94: 274 -86.

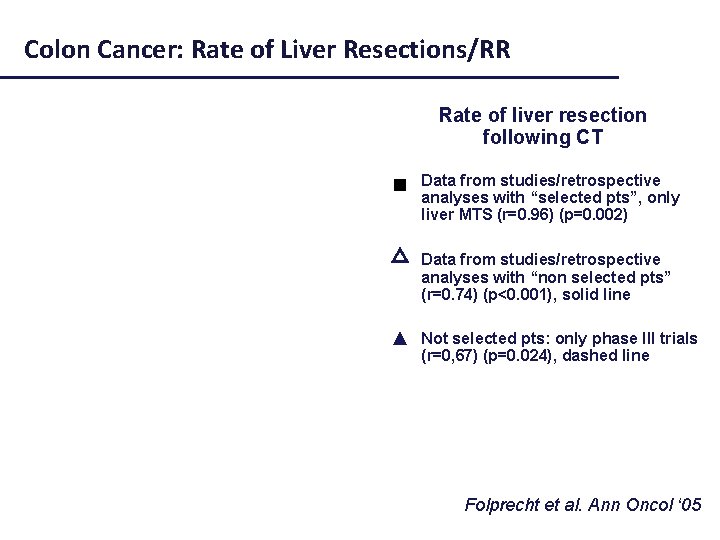
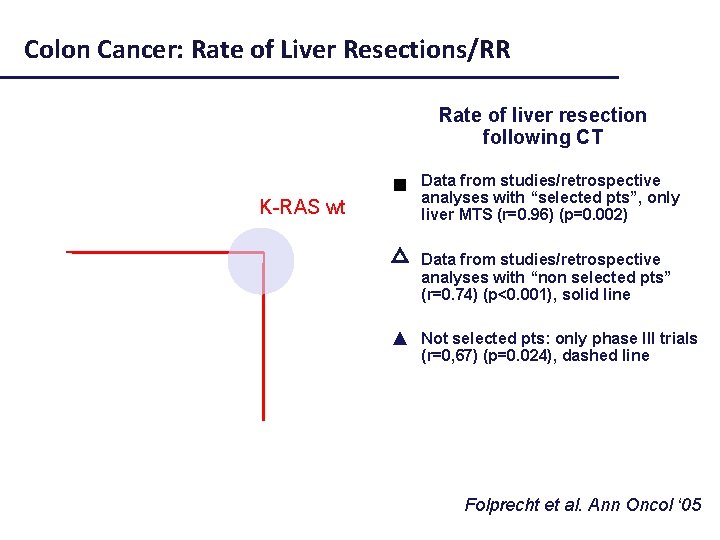
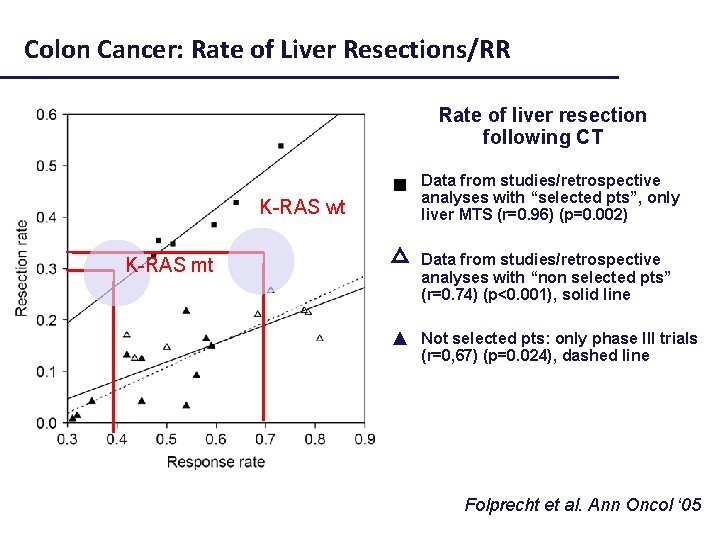
Colon Cancer: Rate of Liver Resections/RR Rate of liver resection following CT Data from studies/retrospective analyses with “selected pts”, only liver MTS (r=0. 96) (p=0. 002) Selected pts (liver mets) Not selected pts △ Data from studies/retrospective analyses with “non selected pts” (r=0. 74) (p<0. 001), solid line ▲ Not selected pts: only phase III trials (r=0, 67) (p=0. 024), dashed line Folprecht et al. Ann Oncol ‘ 05

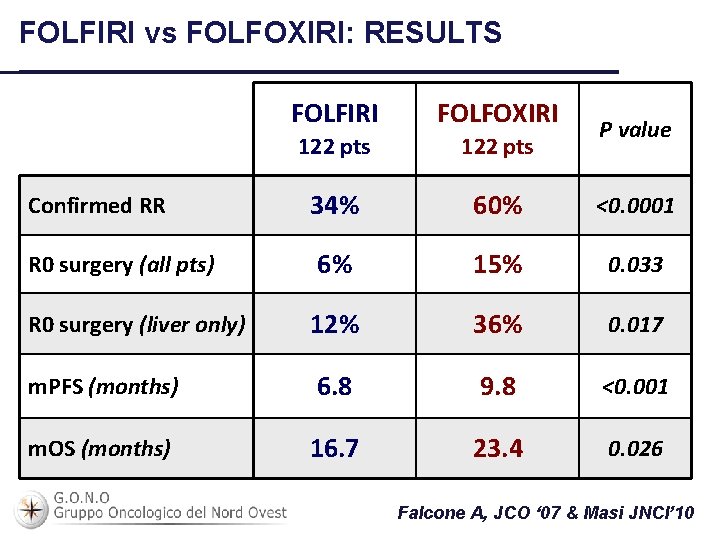
FOLFIRI vs FOLFOXIRI: RESULTS FOLFIRI FOLFOXIRI 122 pts Confirmed RR 34% 60% <0. 0001 R 0 surgery (all pts) 6% 15% 0. 033 R 0 surgery (liver only) 12% 36% 0. 017 m. PFS (months) 6. 8 9. 8 <0. 001 m. OS (months) 16. 7 23. 4 0. 026 P value Falcone A, JCO ‘ 07 & Masi JNCI’ 10

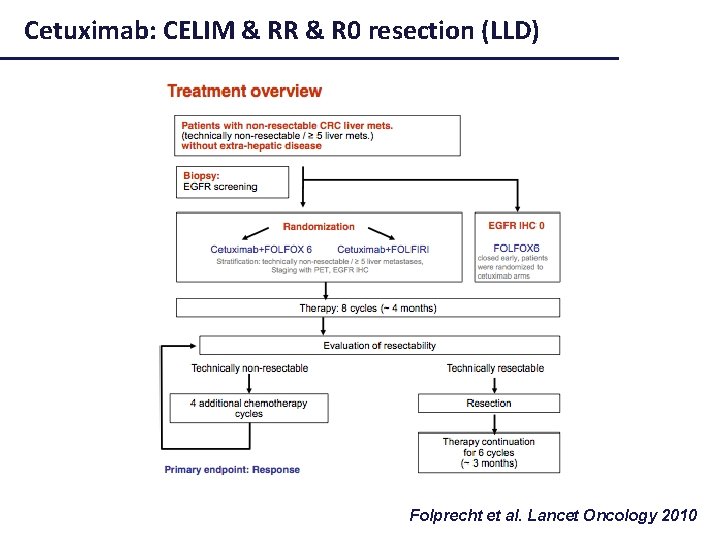
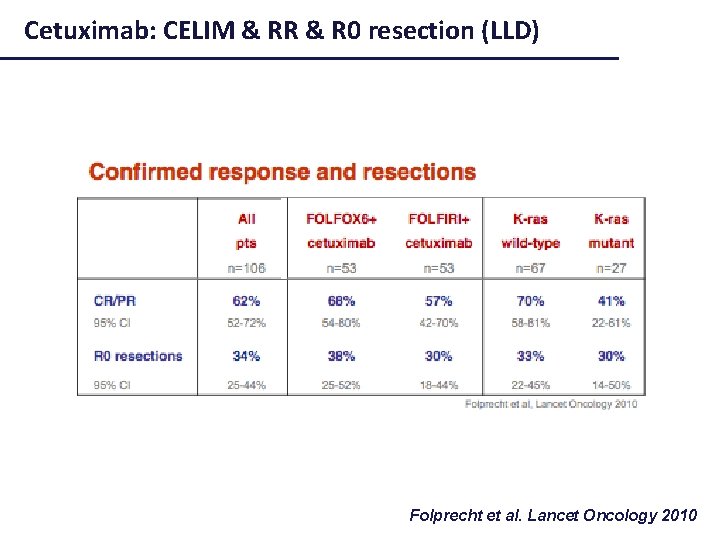
Cetuximab: CELIM & RR & R 0 resection (LLD) Folprecht et al. Lancet Oncology 2010

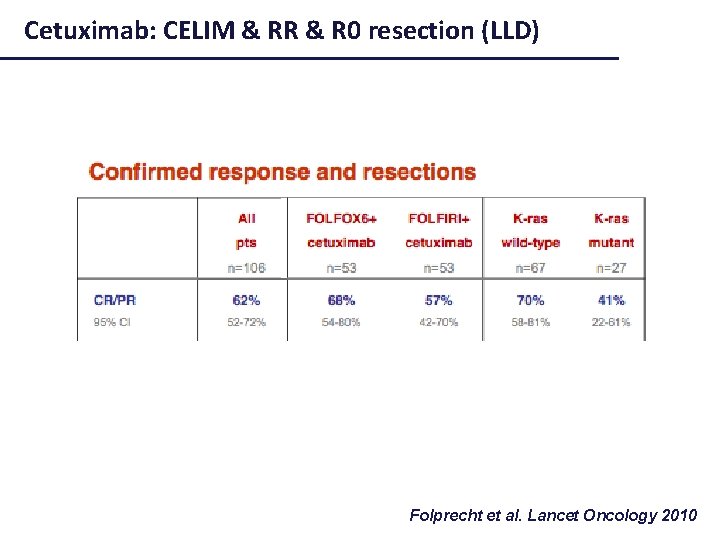
Cetuximab: CELIM & RR & R 0 resection (LLD) Folprecht et al. Lancet Oncology 2010

Colon Cancer: Rate of Liver Resections/RR Rate of liver resection following CT Data from studies/retrospective analyses with “selected pts”, only liver MTS (r=0. 96) (p=0. 002) K-RAS wt Not selected pts △ Data from studies/retrospective analyses with “non selected pts” (r=0. 74) (p<0. 001), solid line ▲ Not selected pts: only phase III trials (r=0, 67) (p=0. 024), dashed line Folprecht et al. Ann Oncol ‘ 05

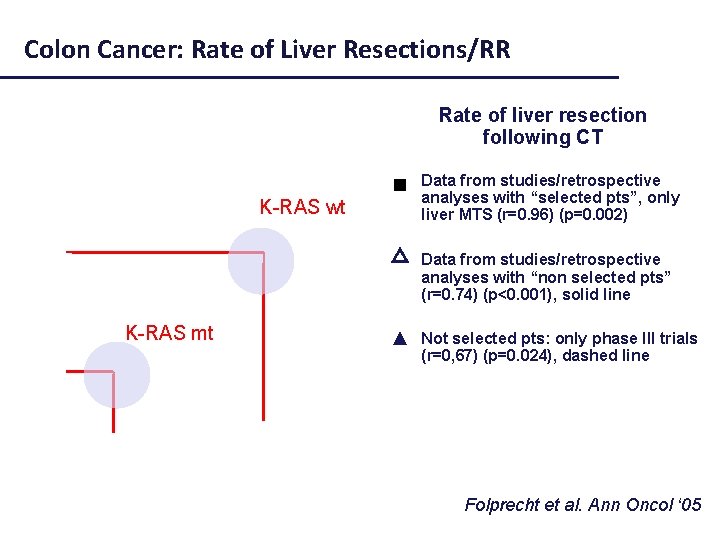
Colon Cancer: Rate of Liver Resections/RR Rate of liver resection following CT Selected pts (liver mets) Data from studies/retrospective analyses with “selected pts”, only liver MTS (r=0. 96) (p=0. 002) K-RAS wt K-RAS mt Not selected pts △ Data from studies/retrospective analyses with “non selected pts” (r=0. 74) (p<0. 001), solid line ▲ Not selected pts: only phase III trials (r=0, 67) (p=0. 024), dashed line Folprecht et al. Ann Oncol ‘ 05

Cetuximab: CELIM & RR & R 0 resection (LLD) Folprecht et al. Lancet Oncology 2010

Colon Cancer: Rate of Liver Resections/RR Rate of liver resection following CT Selected pts (liver mets) Data from studies/retrospective analyses with “selected pts”, only liver MTS (r=0. 96) (p=0. 002) K-RAS wt K-RAS mt Not selected pts △ Data from studies/retrospective analyses with “non selected pts” (r=0. 74) (p<0. 001), solid line ▲ Not selected pts: only phase III trials (r=0, 67) (p=0. 024), dashed line Folprecht et al. Ann Oncol ‘ 05

Response Rate in anti-EGFR trials Loupakis F, Bria E et al. Cancer 2011

Response Rate in BEVACIZUMAB trials Wagner et al. Cochrane Review ‘ 09

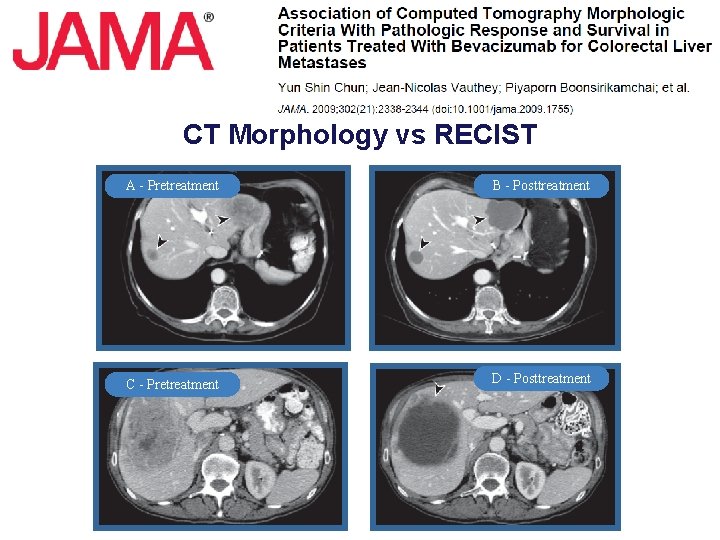
CT Morphology vs RECIST A - Pretreatment B - Posttreatment C - Pretreatment D - Posttreatment

CT Morphology vs Response on BEV l RECIST to Determine 234 pts with CRC liver mets treated with chemo + BEV − 50 pts underwent hepatic resection l Three blinded radiologists evaluated response of liver mets according to − Standard RECIST criteria − Novel CT morphology criteria Computer Tomographic Tumor Characteristics Morphology group Overall Attenuation Tumor-Liver Interface Pheripheral Rim of Enhancement 3 Heterogeneous III defined May be present 2 Mixed Variable If initially present, partially resolved 1 Homogeneous and hypoattenuating Sharp If initially present, completely resolved Adapted from Chun YS, et al. JAMA 2009; 302(21): 2338 -44.

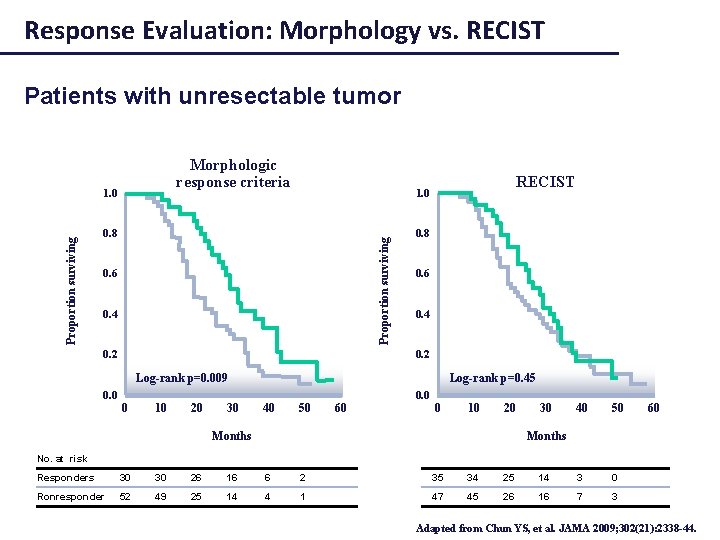
Response Evaluation: Morphology vs. RECIST Patients with unresectable tumor Morphologic response criteria RECIST 1. 0 0. 8 Proportion surviving 1. 0 0. 6 0. 4 0. 2 0. 8 0. 6 0. 4 0. 2 Log-rank p=0. 009 Log-rank p=0. 45 0. 0 0 10 20 30 40 50 60 0 10 20 Months 30 40 50 60 Months No. at risk Responders 30 30 26 16 6 2 35 34 25 14 3 0 Ronresponder 52 49 25 14 4 1 47 45 26 16 7 3 Adapted from Chun YS, et al. JAMA 2009; 302(21): 2338 -44.

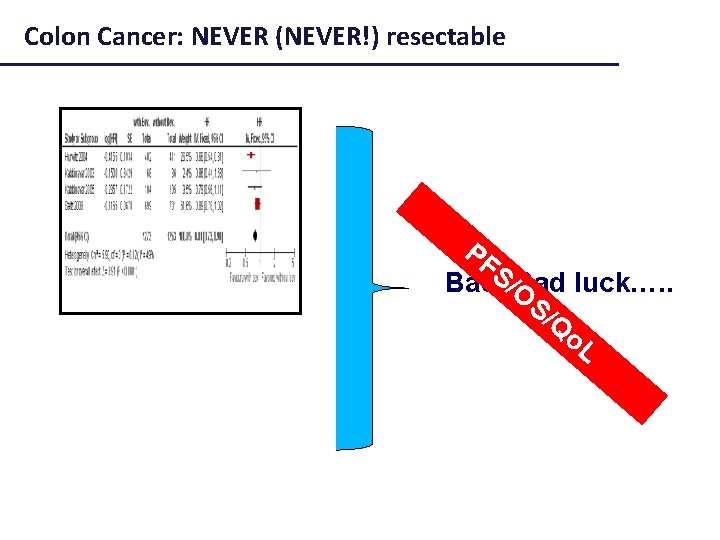
Colon Cancer: NEVER (NEVER!) resectable PF S Bad, /OBad luck…. . S/ Q o. L

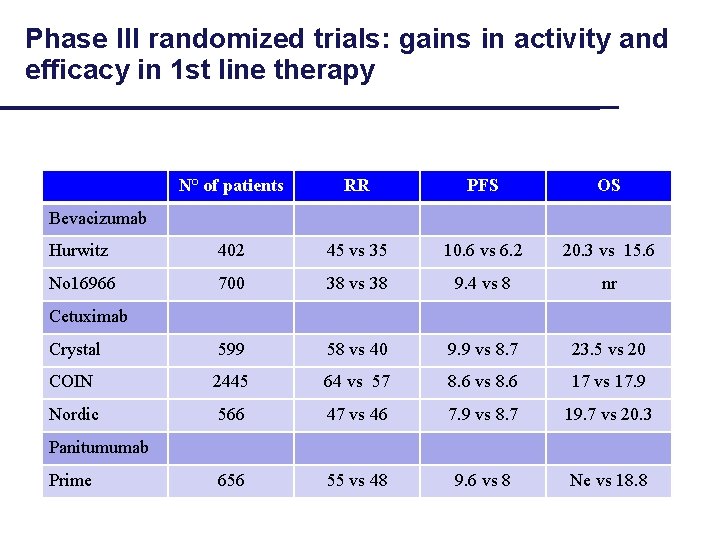
Phase III randomized trials: gains in activity and efficacy in 1 st line therapy N° of patients RR PFS OS Hurwitz 402 45 vs 35 10. 6 vs 6. 2 20. 3 vs 15. 6 No 16966 700 38 vs 38 9. 4 vs 8 nr Crystal 599 58 vs 40 9. 9 vs 8. 7 23. 5 vs 20 COIN 2445 64 vs 57 8. 6 vs 8. 6 17 vs 17. 9 Nordic 566 47 vs 46 7. 9 vs 8. 7 19. 7 vs 20. 3 656 55 vs 48 9. 6 vs 8 Ne vs 18. 8 Bevacizumab Cetuximab Panitumumab Prime

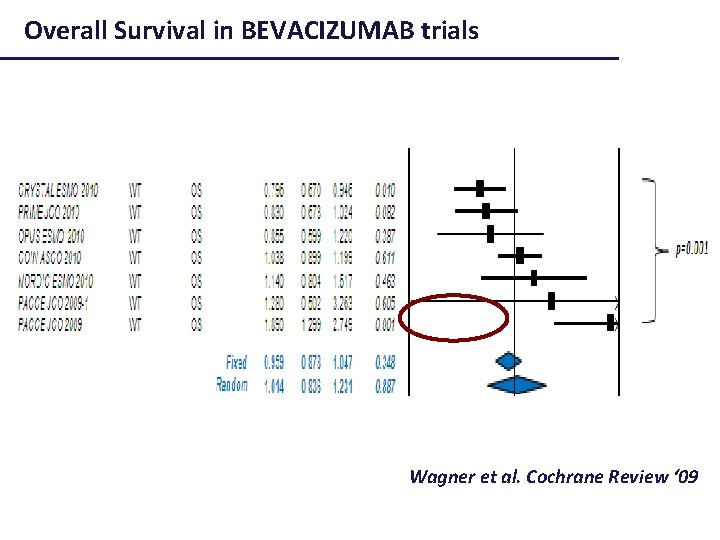
Overall Survival in BEVACIZUMAB trials Wagner et al. Cochrane Review ‘ 09

Overall Survival in anti-EGFRs trials Loupakis, Bria E et al. Cancer 2011

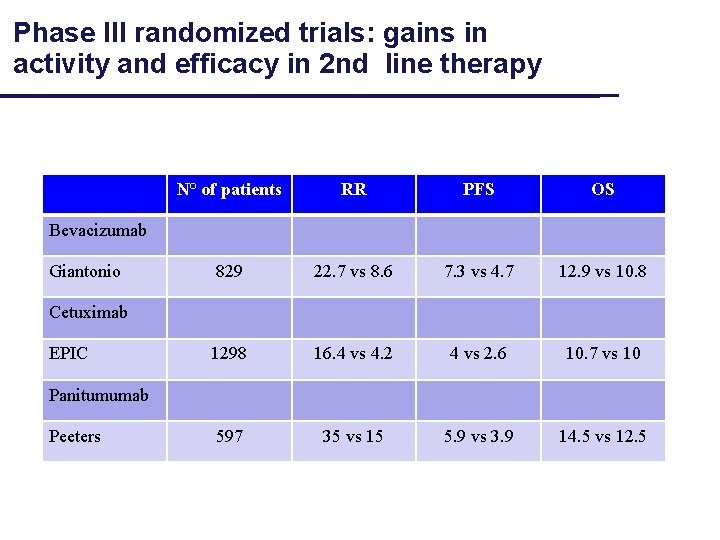
Phase III randomized trials: gains in activity and efficacy in 2 nd line therapy N° of patients RR PFS OS 829 22. 7 vs 8. 6 7. 3 vs 4. 7 12. 9 vs 10. 8 1298 16. 4 vs 4. 2 4 vs 2. 6 10. 7 vs 10 597 35 vs 15 5. 9 vs 3. 9 14. 5 vs 12. 5 Bevacizumab Giantonio Cetuximab EPIC Panitumumab Peeters

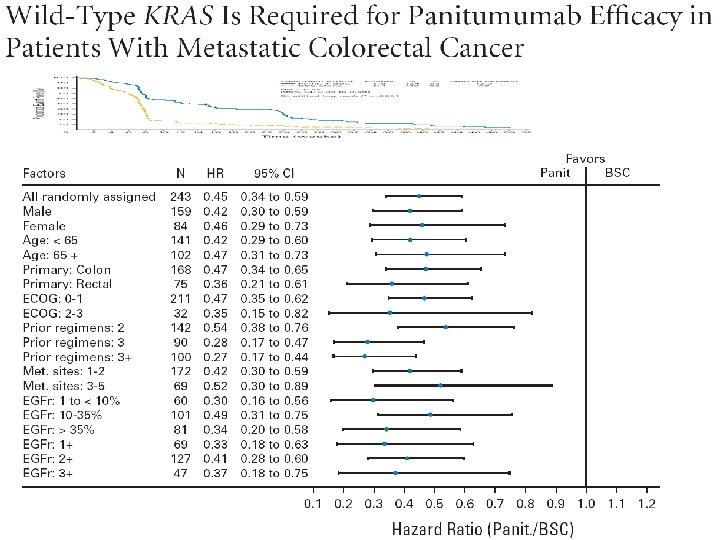
Amado JCO 2008

Amado JCO 2008

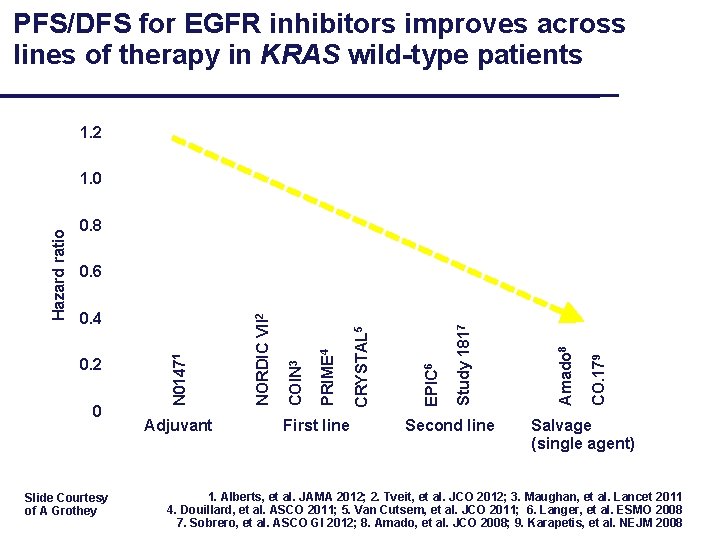
PFS/DFS for EGFR inhibitors improves across lines of therapy in KRAS wild-type patients 1. 2 0. 8 Slide Courtesy of A Grothey Adjuvant First line Second line CO. 179 Amado 8 Study 1817 EPIC 6 CRYSTAL 5 0 PRIME 4 0. 2 COIN 3 0. 4 NORDIC VII 2 0. 6 N 01471 Hazard ratio 1. 0 Salvage (single agent) 1. Alberts, et al. JAMA 2012; 2. Tveit, et al. JCO 2012; 3. Maughan, et al. Lancet 2011 4. Douillard, et al. ASCO 2011; 5. Van Cutsem, et al. JCO 2011; 6. Langer, et al. ESMO 2008 7. Sobrero, et al. ASCO GI 2012; 8. Amado, et al. JCO 2008; 9. Karapetis, et al. NEJM 2008

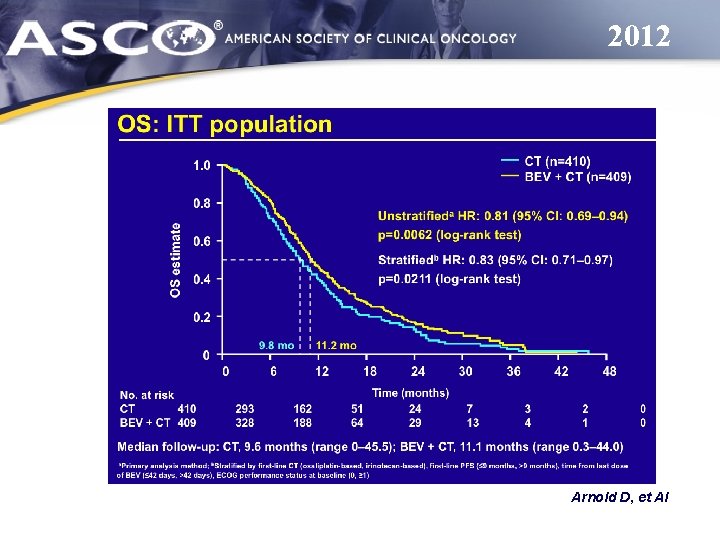
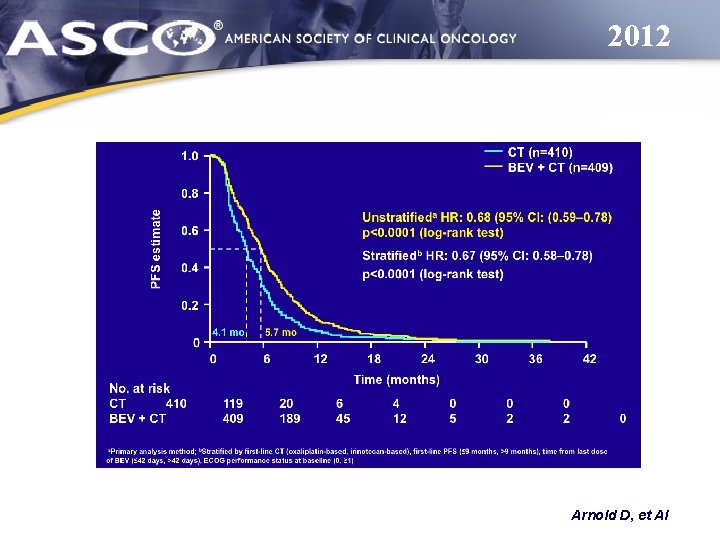
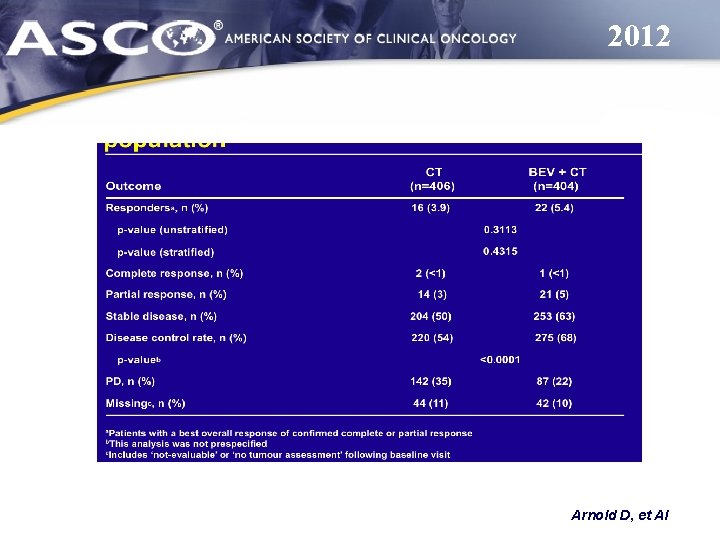
2012 Arnold D, et Al

2012 Arnold D, et Al

2012 Arnold D, et Al

2012 Arnold D, et Al

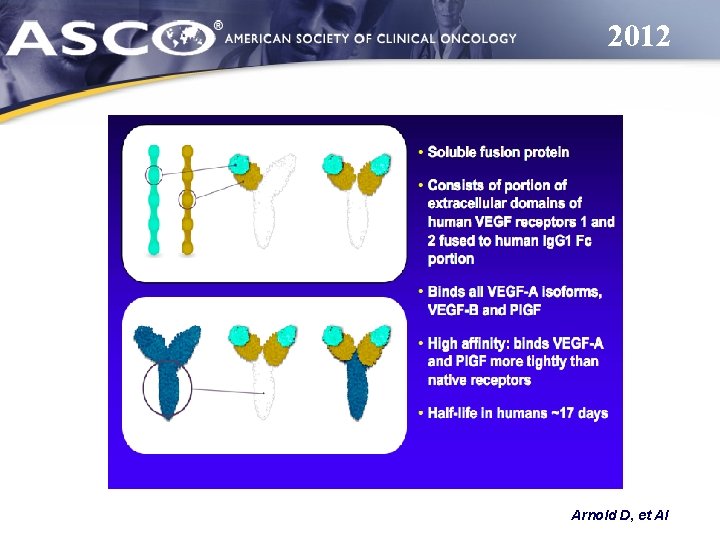
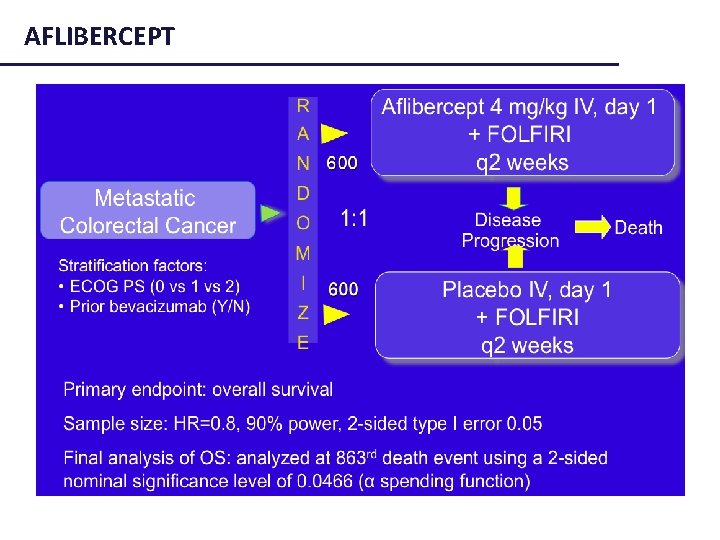
AFLIBERCEPT

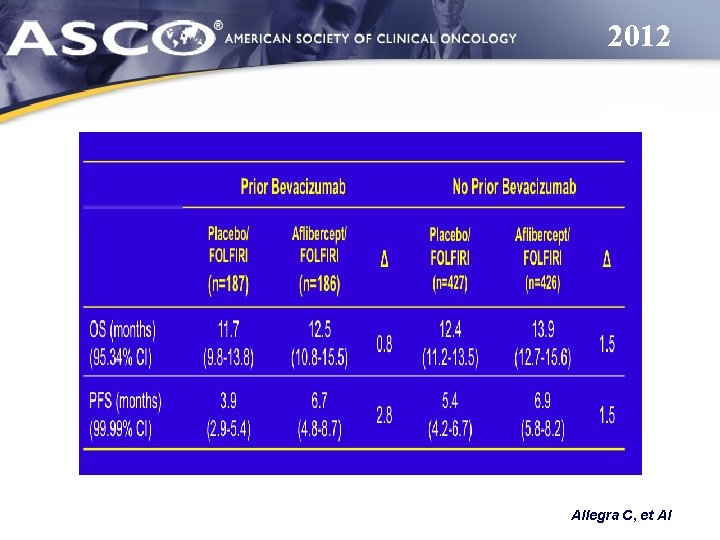
2012 Allegra C, et Al

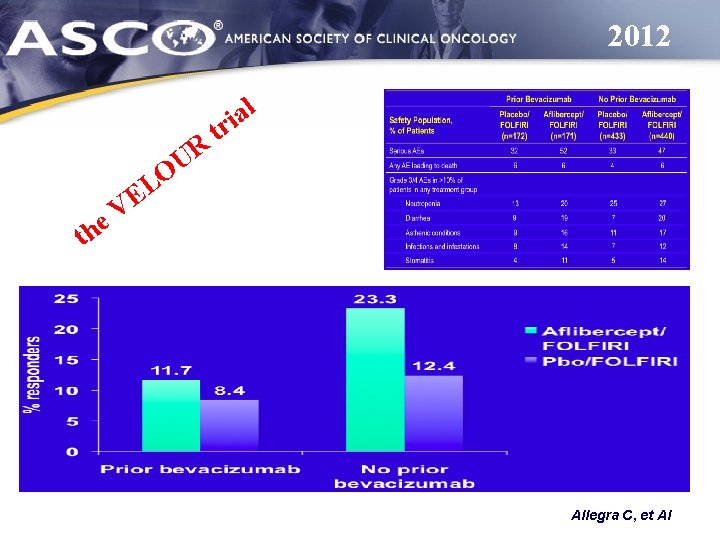
2012 R U O e h t l a ri t L E V Allegra C, et Al

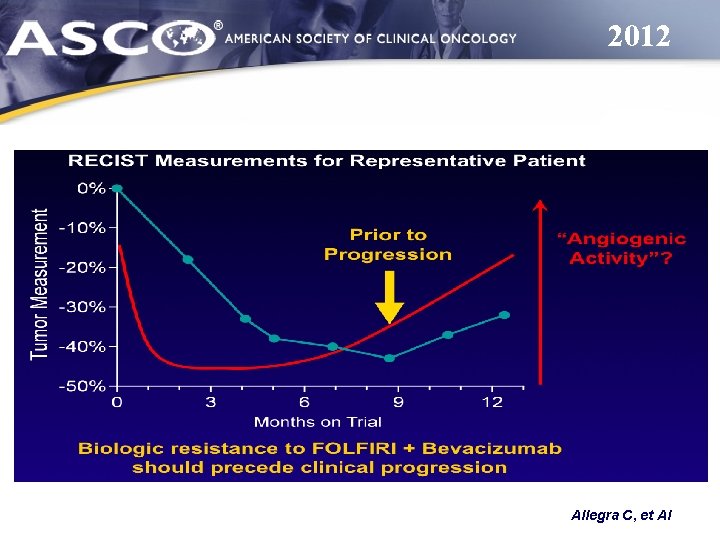
2012 Allegra C, et Al

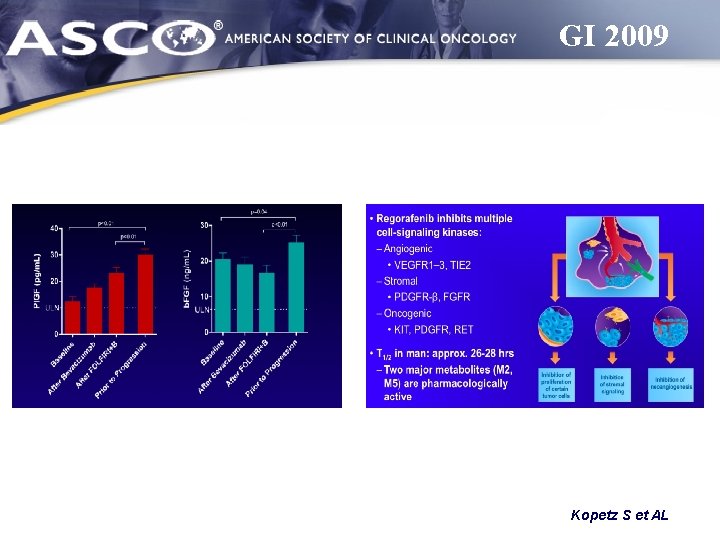
GI 2009 Kopetz S et AL

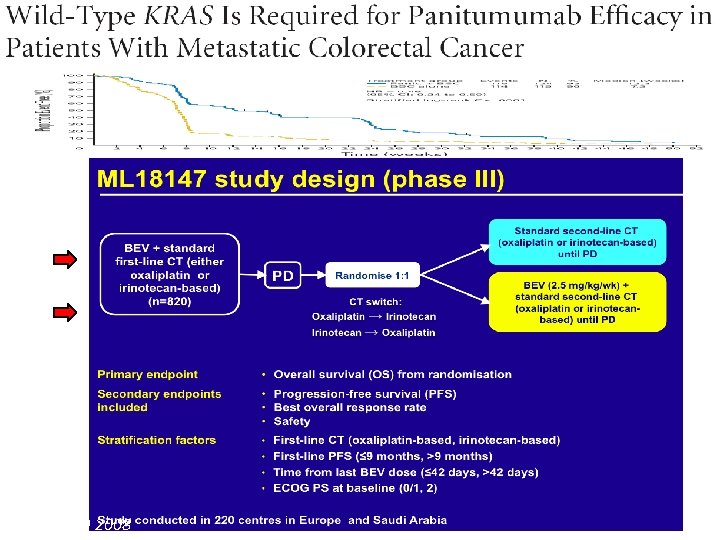
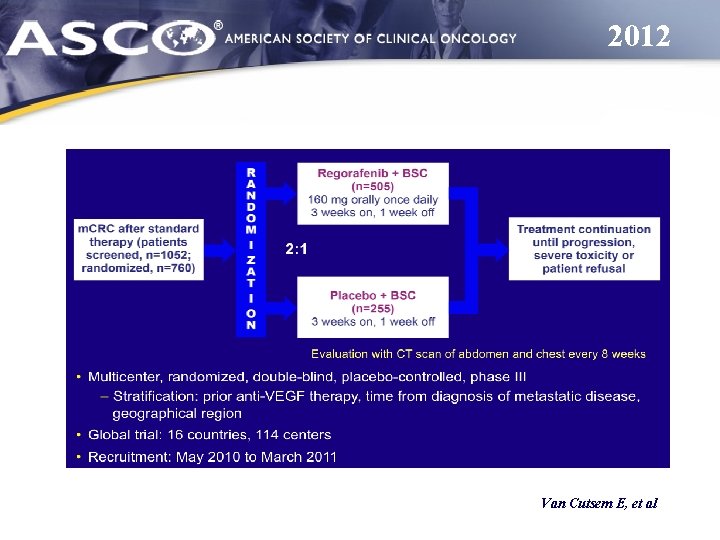
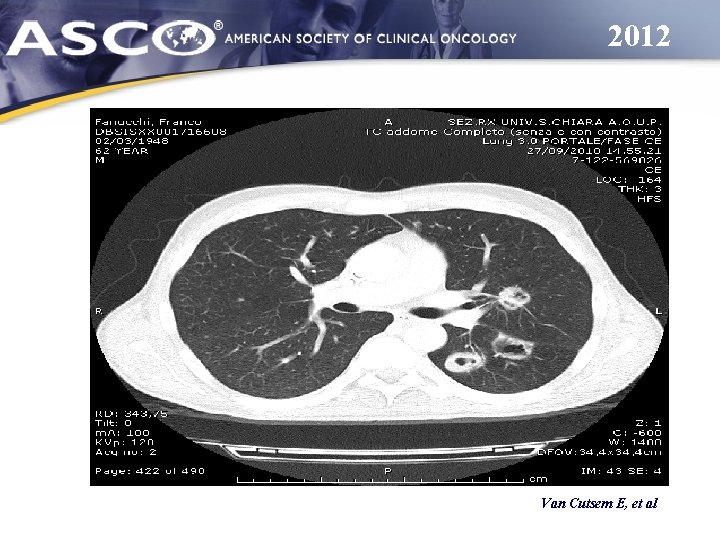
2012 Van Cutsem E, et al

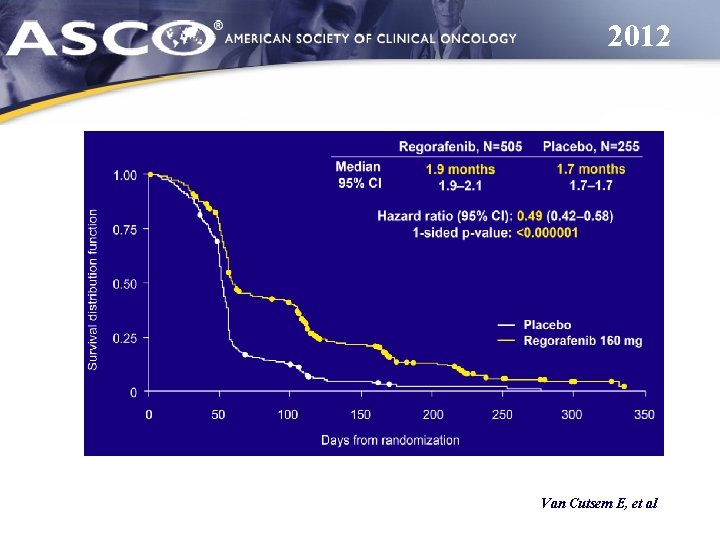
2012 Van Cutsem E, et al

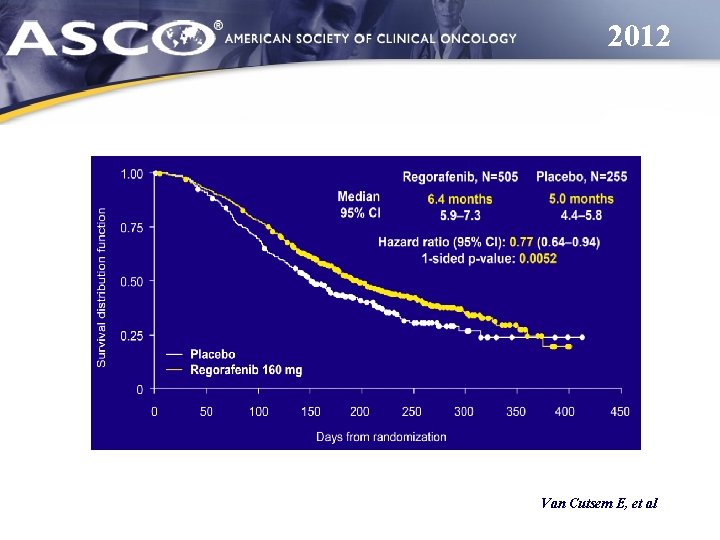
2012 Van Cutsem E, et al

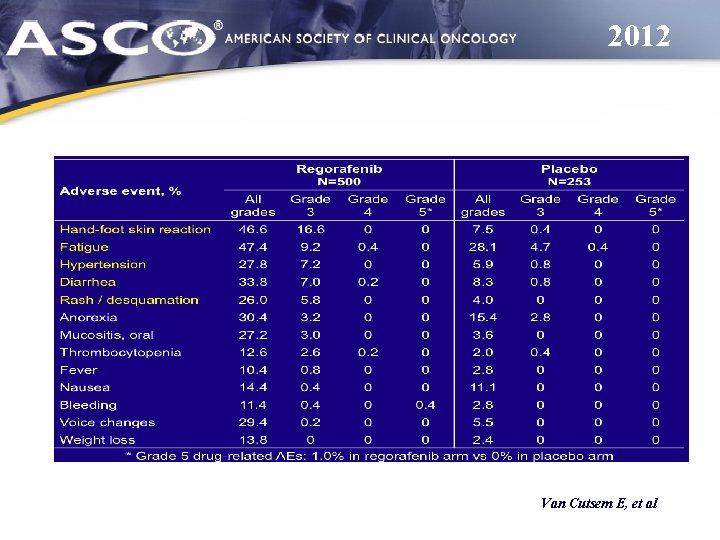
2012 Van Cutsem E, et al

2012 Van Cutsem E, et al

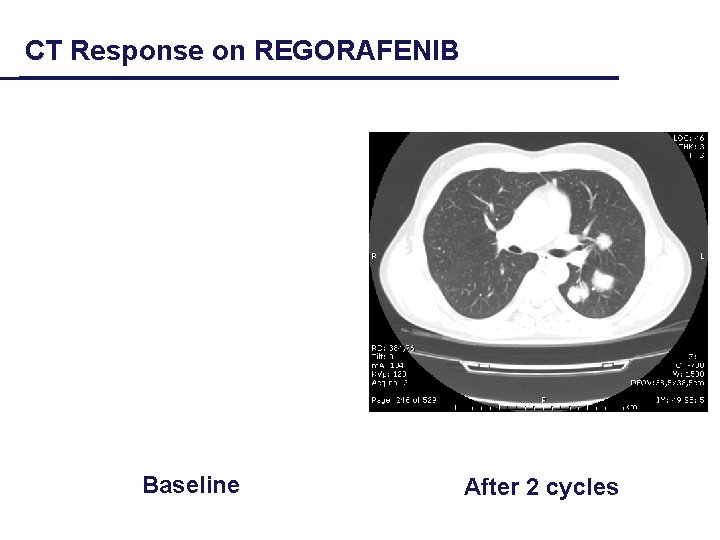
CT Response on REGORAFENIB Baseline After 2 cycles

Bittoni, Giampieri et al, CROH 2012

Bittoni, Giampieri et al, CROH 2012
 Scartozzi oncologo
Scartozzi oncologo Carmelo clinica
Carmelo clinica Atlas medica cartella clinica
Atlas medica cartella clinica Master oncologia infermieri
Master oncologia infermieri Oncologia sondrio
Oncologia sondrio Oncologia alghero
Oncologia alghero Ritardo diagnostico in oncologia risarcimento
Ritardo diagnostico in oncologia risarcimento Geosigmeto
Geosigmeto Dental campus ancona
Dental campus ancona Guido cacciapuoti wikipedia
Guido cacciapuoti wikipedia My family by george ancona
My family by george ancona Arcidiocesi di ancona
Arcidiocesi di ancona Sezione polizia stradale ancona
Sezione polizia stradale ancona Ristorantino dlf ancona
Ristorantino dlf ancona Ancona
Ancona Villa favorita ancona
Villa favorita ancona Mercatone emmezeta
Mercatone emmezeta Principle of arts
Principle of arts Proposal highlights
Proposal highlights Highlights from the book of isaiah
Highlights from the book of isaiah Highlights memorandum
Highlights memorandum What to expect in work immersion
What to expect in work immersion Investment highlights
Investment highlights The passage highlights……
The passage highlights…… Key highlights
Key highlights Highlights from the book of isaiah
Highlights from the book of isaiah Moto medica
Moto medica Equipe médica
Equipe médica Quien invento el esfigmomanómetro
Quien invento el esfigmomanómetro Unife scuole di specializzazione
Unife scuole di specializzazione Kalmia materia medica
Kalmia materia medica Ejemplos de auditoría médica
Ejemplos de auditoría médica Dott losavio neurologo
Dott losavio neurologo Materia medica graphites
Materia medica graphites Academia nacional de educacion medica
Academia nacional de educacion medica Lex artis medica
Lex artis medica Son los dos tipos de receta médica sicad
Son los dos tipos de receta médica sicad Avaliação médica dano corporal
Avaliação médica dano corporal Que es rp en receta médica
Que es rp en receta médica Master fisica medica uv
Master fisica medica uv Modelo de prescrição médica
Modelo de prescrição médica Um poliedro convexo tem 12 vertices e 18 arestas
Um poliedro convexo tem 12 vertices e 18 arestas Asterias rubens breast cancer
Asterias rubens breast cancer Academia nacional de educación médica
Academia nacional de educación médica Ant tart materia medica
Ant tart materia medica Juan carlos arango barrientos
Juan carlos arango barrientos Materia medica reloaded
Materia medica reloaded Documentos para apelar licencia medica en compin
Documentos para apelar licencia medica en compin Diceologia
Diceologia Dott greggio gabriele
Dott greggio gabriele Iatrogenia
Iatrogenia Vocacion medica
Vocacion medica 1.ingenieramédicaprogramadoraperiodistahijastra
1.ingenieramédicaprogramadoraperiodistahijastra Gestin medica
Gestin medica Specialista in fisica medica
Specialista in fisica medica Receta cuantificada veterinaria requisitos
Receta cuantificada veterinaria requisitos Art. 8 legge gelli
Art. 8 legge gelli Bayer prodotti
Bayer prodotti Domus medica
Domus medica Podoflemmatite
Podoflemmatite Hitória
Hitória Hiperaldosteronismo
Hiperaldosteronismo