SCLC Dott Andrea Ardizzoni UOC Oncologia Medica Small










- Slides: 31

SCLC Dott. Andrea Ardizzoni UOC Oncologia Medica

Small Cell Lung Cancer (SCLC) • Reduced incidence (from 25 -30 to 13 -15%) • Still accounts for ~ 20 -25, 000 deaths yearly • Strongly associated with cigarette smoking • Neuroendocrine features • High frequency of TP 53 and RB gene mutations • Highly aggressive • Often metastasized at the time of diagnosis • Highly responsive to CT and RT • High rate of early relapse/PD • Poor cure-rate (10 -20%) and overall prognosis (MS 9 -12 months)

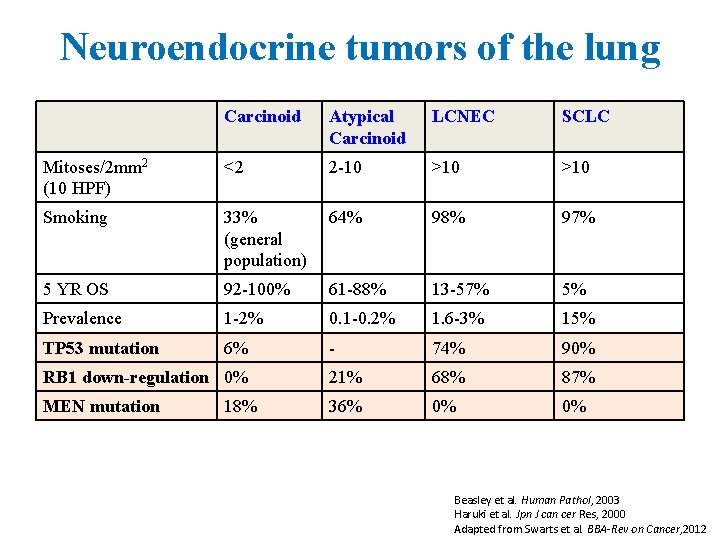
Neuroendocrine tumors of the lung Carcinoid Atypical Carcinoid LCNEC SCLC Mitoses/2 mm 2 (10 HPF) <2 2 -10 >10 Smoking 33% (general population) 64% 98% 97% 5 YR OS 92 -100% 61 -88% 13 -57% 5% Prevalence 1 -2% 0. 1 -0. 2% 1. 6 -3% 15% TP 53 mutation 6% - 74% 90% RB 1 down-regulation 0% 21% 68% 87% MEN mutation 36% 0% 0% 18% Beasley et al. Human Pathol, 2003 Haruki et al. Jpn J can cer Res, 2000 Adapted from Swarts et al. BBA-Rev on Cancer, 2012

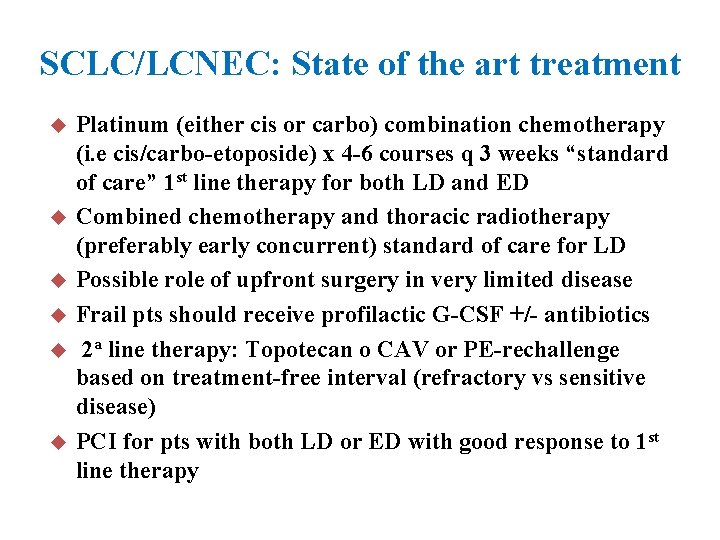
SCLC/LCNEC: State of the art treatment u u u Platinum (either cis or carbo) combination chemotherapy (i. e cis/carbo-etoposide) x 4 -6 courses q 3 weeks “standard of care” 1 st line therapy for both LD and ED Combined chemotherapy and thoracic radiotherapy (preferably early concurrent) standard of care for LD Possible role of upfront surgery in very limited disease Frail pts should receive profilactic G-CSF +/- antibiotics 2 a line therapy: Topotecan o CAV or PE-rechallenge based on treatment-free interval (refractory vs sensitive disease) PCI for pts with both LD or ED with good response to 1 st line therapy


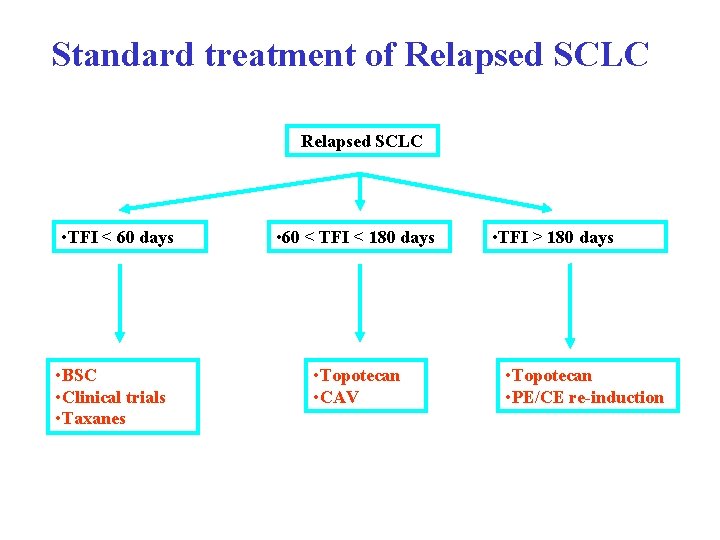
Standard treatment of Relapsed SCLC • TFI < 60 days • BSC • Clinical trials • Taxanes • 60 < TFI < 180 days • Topotecan • CAV • TFI > 180 days • Topotecan • PE/CE re-induction

SCLC/LCNEC: What’s new in 2015 • • Molecular profiling Novel agents for relapsed disease Role of TRT in ED Role of surgery

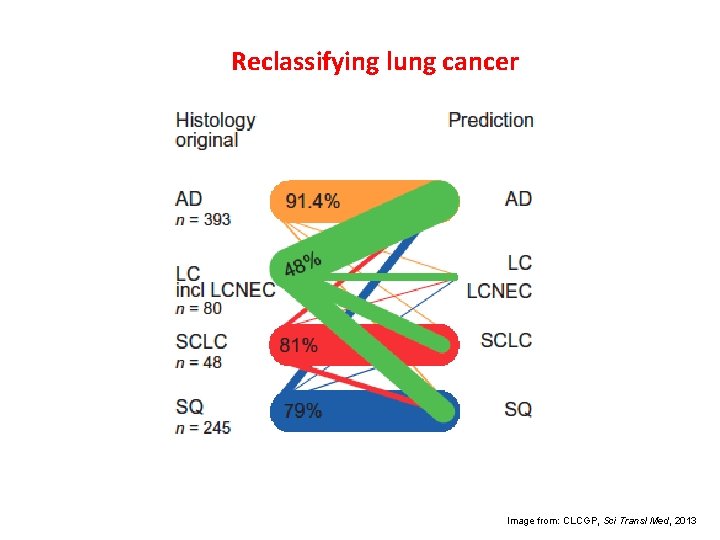
Reclassifying lung cancer Image from: CLCGP, Sci Transl Med, 2013

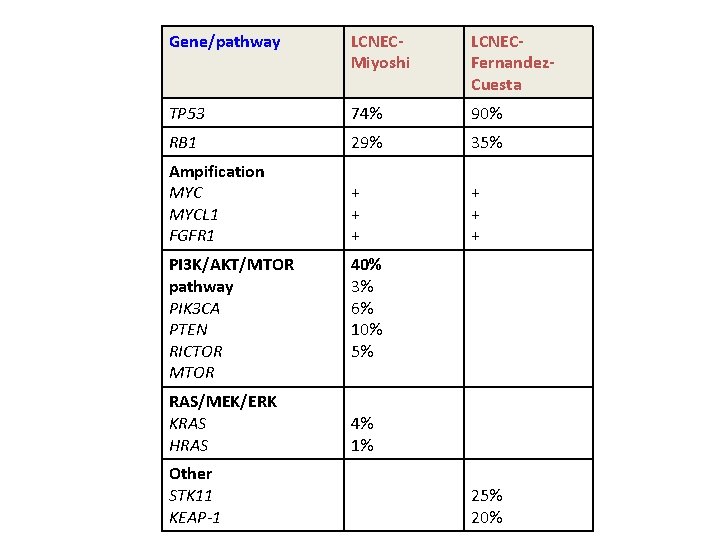
Gene/pathway LCNECMiyoshi LCNECFernandez. Cuesta TP 53 74% 90% RB 1 29% 35% Ampification MYCL 1 FGFR 1 + + + PI 3 K/AKT/MTOR pathway PIK 3 CA PTEN RICTOR MTOR RAS/MEK/ERK KRAS HRAS Other STK 11 KEAP-1 40% 3% 6% 10% 5% 4% 1% 25% 20%

Pembrolizumab for Extensive Stage SCLC: Efficacy and Relationship With PD-L 1 Expression Patrick A. Ott, 1 Elena Elez, 2 Sandrine Hiret, 3 Dong-Wan Kim, 4 Rebecca A. Moss, 1 Tammy Winser, 5 Sanatan Saraf, 5 Marisa Dolled-Filhart, 5 Jonathan Cheng, 5 Bilal Piperdi, 5 Janice M. Mehnert 6 1 Dana-Farber Cancer Institute, Boston, MA, USA; 2 Vall d’Hebron Institute of Oncology, Barcelona, Spain; René Gauducheau, Nantes, France; 4 Seoul National University Hospital, Seoul, Republic of Korea; 5 Merck & Co. , Inc. , Kenilworth, NJ, USA; 6 Rutgers Cancer Institute of New Jersey, New Brunswick, NJ, USA 3 ICO 3285 – Patrick A. Ott

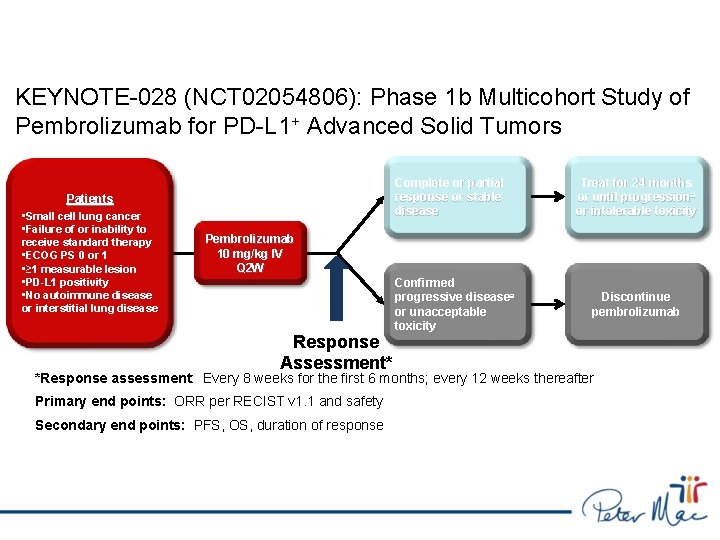
KEYNOTE-028 (NCT 02054806): Phase 1 b Multicohort Study of Pembrolizumab for PD-L 1+ Advanced Solid Tumors Complete or partial response or stable disease Patients • Small cell lung cancer • Failure of or inability to receive standard therapy • ECOG PS 0 or 1 • ≥ 1 measurable lesion • PD-L 1 positivity • No autoimmune disease or interstitial lung disease Treat for 24 months or until progression a or intolerable toxicity Pembrolizumab 10 mg/kg IV Q 2 W Response Assessment* Confirmed progressive diseasea or unacceptable toxicity Discontinue pembrolizumab *Response assessment: Every 8 weeks for the first 6 months; every 12 weeks thereafter Primary end points: ORR per RECIST v 1. 1 and safety Secondary end points: PFS, OS, duration of response a. If clinically stable, patients are to remain on pembrolizumab until progressive disease is confirmed on a second scan performed ≥ 4 weeks later.

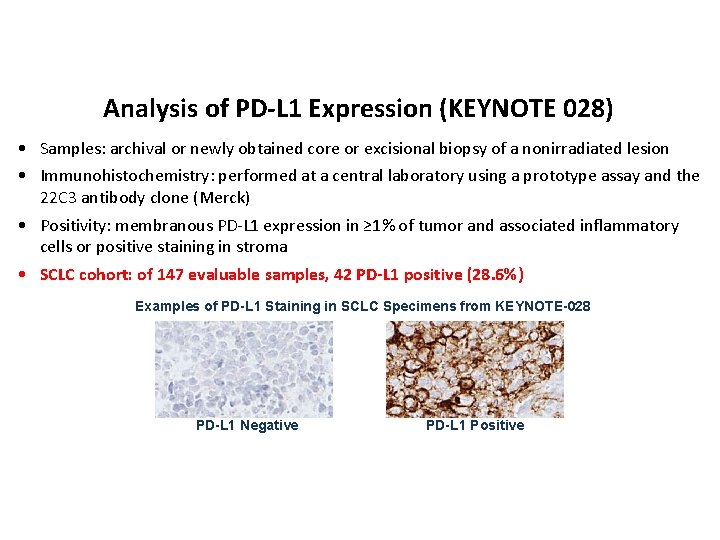
Analysis of PD-L 1 Expression (KEYNOTE 028) • Samples: archival or newly obtained core or excisional biopsy of a nonirradiated lesion • Immunohistochemistry: performed at a central laboratory using a prototype assay and the 22 C 3 antibody clone (Merck) • Positivity: membranous PD-L 1 expression in ≥ 1% of tumor and associated inflammatory cells or positive staining in stroma • SCLC cohort: of 147 evaluable samples, 42 PD-L 1 positive (28. 6%) Examples of PD-L 1 Staining in SCLC Specimens from KEYNOTE-028 PD-L 1 Negative PD-L 1 Positive

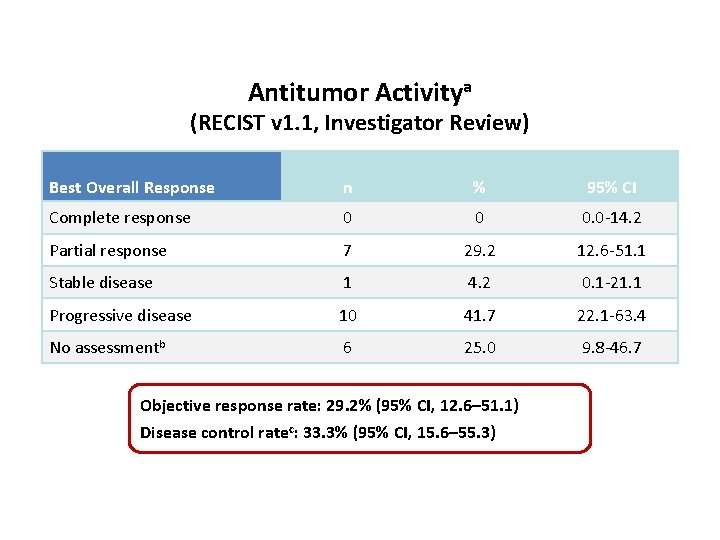
Antitumor Activitya (RECIST v 1. 1, Investigator Review) Best Overall Response n % 95% CI Complete response 0 0 0. 0 -14. 2 Partial response 7 29. 2 12. 6 -51. 1 Stable disease 1 4. 2 0. 1 -21. 1 Progressive disease 10 41. 7 22. 1 -63. 4 No assessmentb 6 25. 0 9. 8 -46. 7 Objective response rate: 29. 2% (95% CI, 12. 6– 51. 1) a. Both Disease control ratec: 33. 3% (95% CI, 15. 6– 55. 3) confirmed and unconfirmed responses are included. Response was assessed by RECIST v 1. 1 per investigator review. b. Includes patients who died or discontinued for clinical progression before the first imaging assessment (n = 3) or who had not reached the first imaging assessment at data cutoff (n = 3). c. Patients with CR, PR, or SD of any duration. Data cutoff date: June 24, 2015.

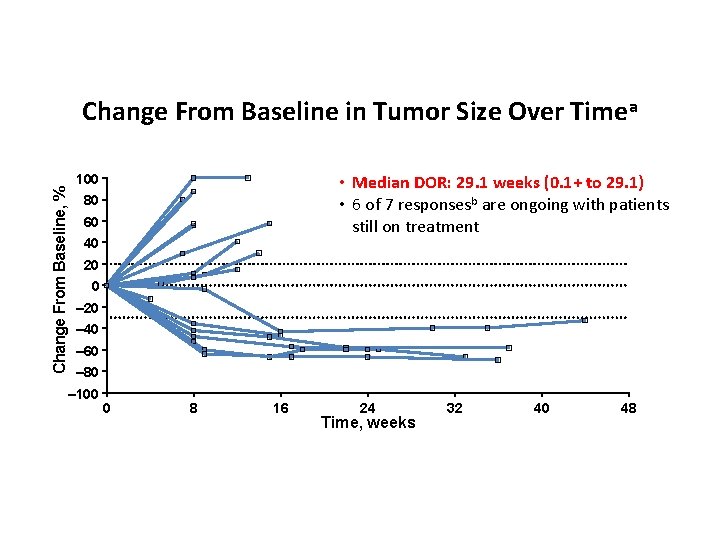
Change From Baseline, % Change From Baseline in Tumor Size Over Timea 100 • Median DOR: 29. 1 weeks (0. 1+ to 29. 1) • 6 of 7 responsesb are ongoing with patients still on treatment 80 60 40 20 0 – 20 – 40 – 60 – 80 – 100 a. Includes 0 8 16 24 Time, weeks 32 patients with ≥ 1 evaluable postbaseline tumor assessment (n = 18). Response was assessed by RECIST v 1. 1 per investigator review. b. Includes confirmed and unconfirmed responses. Data cutoff date: June 24, 2015. 40 48

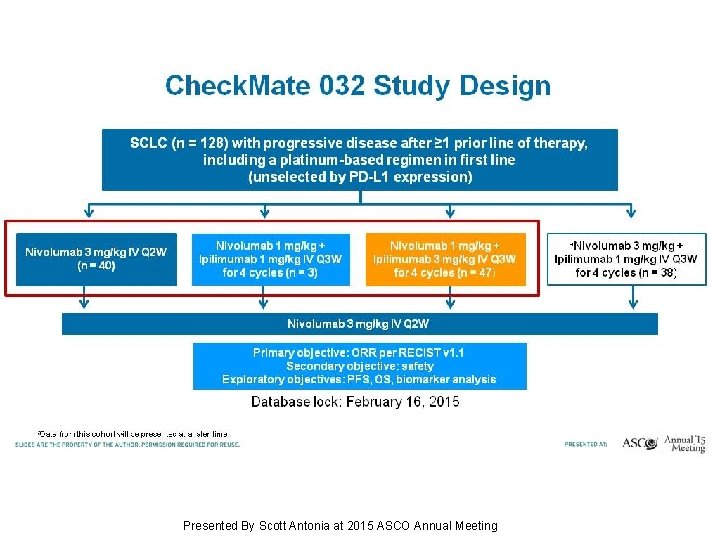
Check. Mate 032 Study Design Presented By Scott Antonia at 2015 ASCO Annual Meeting

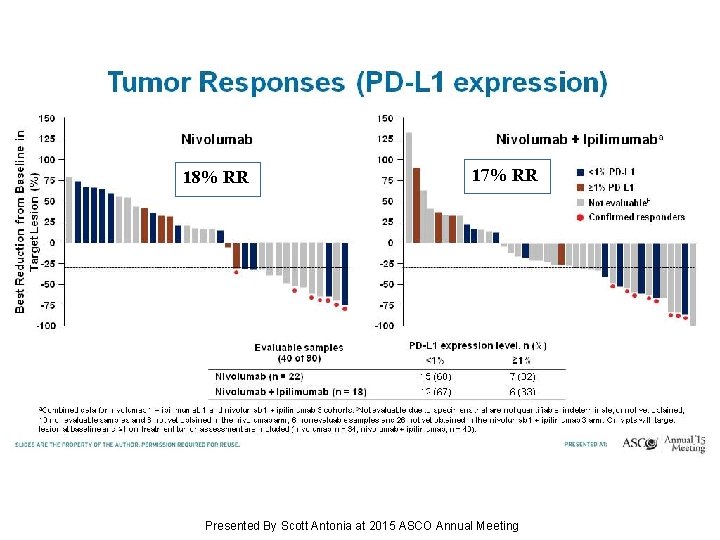
17% RR 18% RR Tumor Responses (PD-L 1 expression) Presented By Scott Antonia at 2015 ASCO Annual Meeting


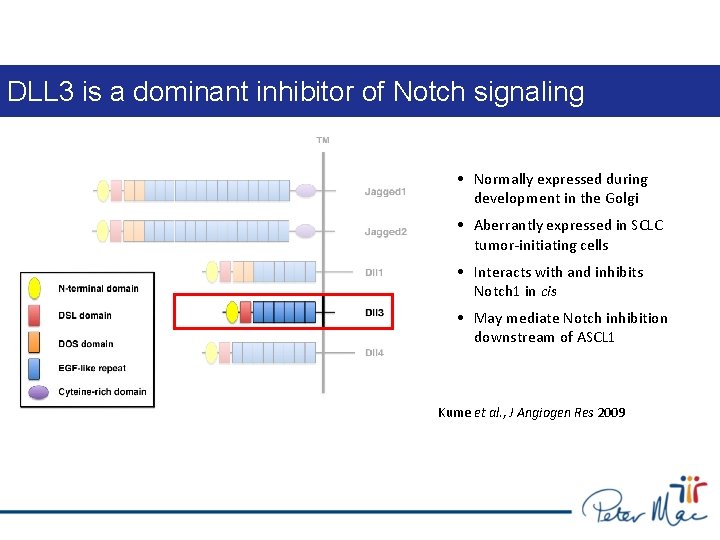
DLL 3 is a dominant inhibitor of Notch signaling • Normally expressed during development in the Golgi • Aberrantly expressed in SCLC tumor-initiating cells • Interacts with and inhibits Notch 1 in cis • May mediate Notch inhibition downstream of ASCL 1 Kume et al. , J Angiogen Res 2009

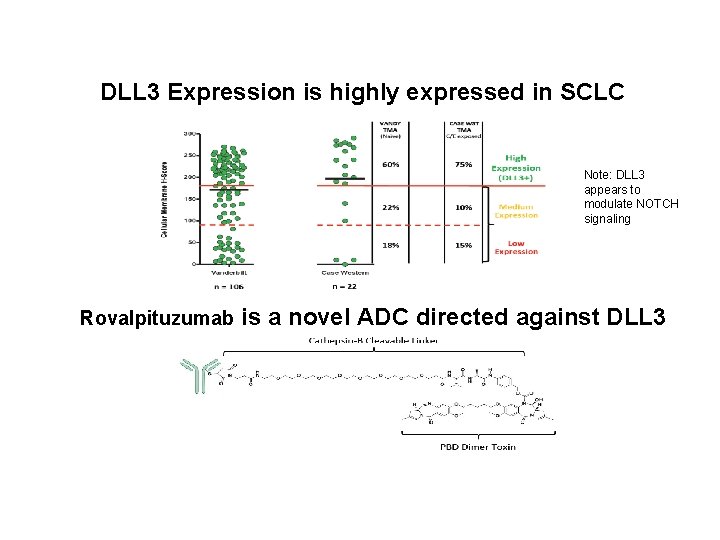
DLL 3 Expression is highly expressed in SCLC Note: DLL 3 appears to modulate NOTCH signaling Rovalpituzumab is a novel ADC directed against DLL 3



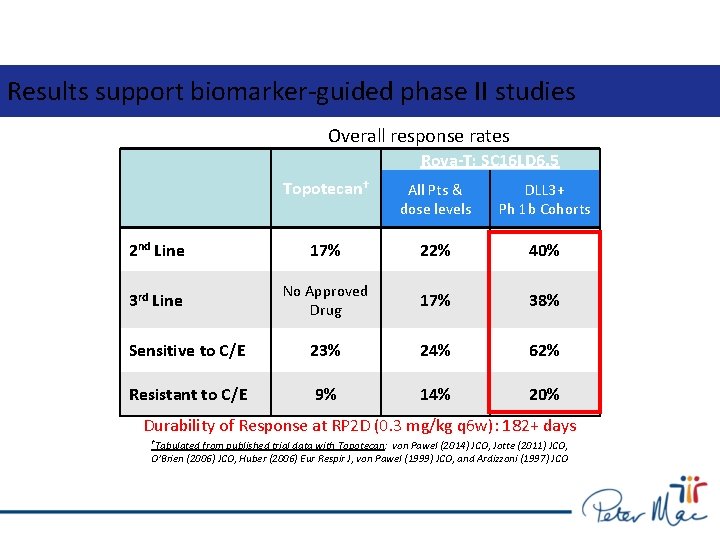
Results support biomarker-guided phase II studies Overall response rates Rova-T; SC 16 LD 6. 5 Topotecan† All Pts & dose levels DLL 3+ Ph 1 b Cohorts 2 nd Line 17% 22% 40% 3 rd Line No Approved Drug 17% 38% Sensitive to C/E 23% 24% 62% Resistant to C/E 9% 14% 20% Durability of Response at RP 2 D (0. 3 mg/kg q 6 w): 182+ days †Tabulated from published trial data with Topotecan: von Pawel (2014) JCO, Jotte (2011) JCO, O’Brien (2006) JCO, Huber (2006) Eur Respir J, von Pawel (1999) JCO, and Ardizzoni (1997) JCO

Which Patients with ES-SCLC Should Receive Thoracic Radiotherapy Routinely? Ben Slotman, Corinne Faivre-Finn, Harm Van Tinteren, John Praag, Joost Knegjens, Sherif El Sharouni, Matthew Hatton, Astrid Keijser, Suresh Senan Ben Slotman Professor and Chair Department of Radiation Oncology VUmc, Amsterdam, The Netherlands

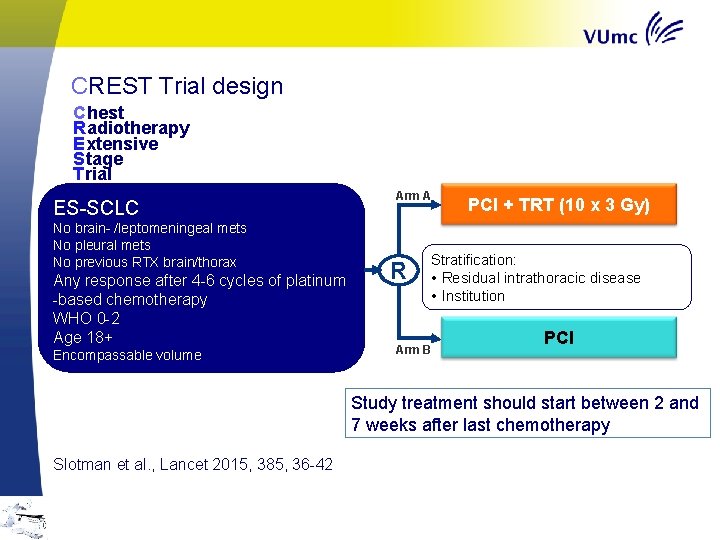
CREST Trial design C hest R adiotherapy E xtensive S tage T rial ES-SCLC No brain- /leptomeningeal mets No pleural mets No previous RTX brain/thorax Any response after 4 -6 cycles of platinum -based chemotherapy WHO 0 -2 Age 18+ Encompassable volume Arm A R PCI + TRT (10 x 3 Gy) Stratification: • Residual intrathoracic disease • Institution Arm B PCI Study treatment should start between 2 and 7 weeks after last chemotherapy Slotman et al. , Lancet 2015, 385, 36 -42

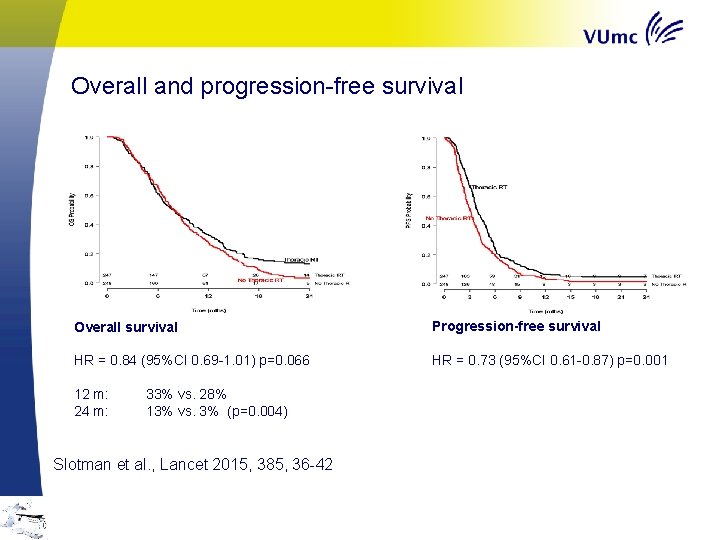
Overall and progression-free survival Overall survival Progression-free survival HR = 0. 84 (95%CI 0. 69 -1. 01) p=0. 066 HR = 0. 73 (95%CI 0. 61 -0. 87) p=0. 001 12 m: 24 m: 33% vs. 28% 13% vs. 3% (p=0. 004) Slotman et al. , Lancet 2015, 385, 36 -42

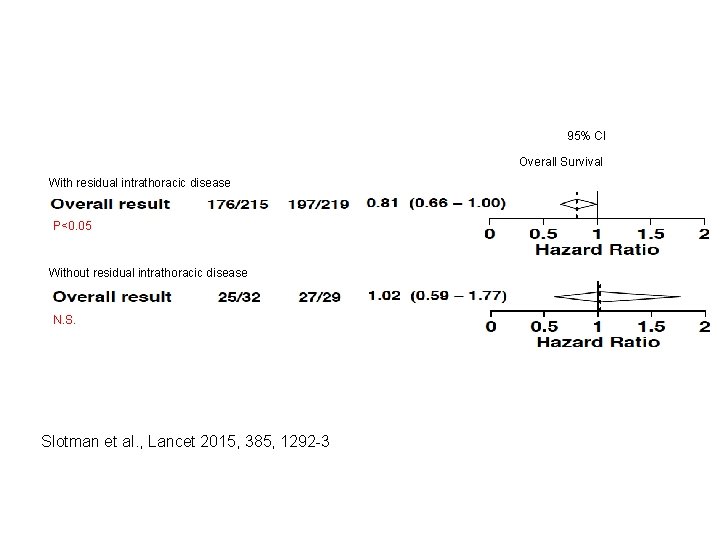
95% CI Overall Survival With residual intrathoracic disease P<0. 05 Overall survival Without residual intrathoracic disease N. S. Slotman et al. , Lancet 2015, 385, 1292 -3

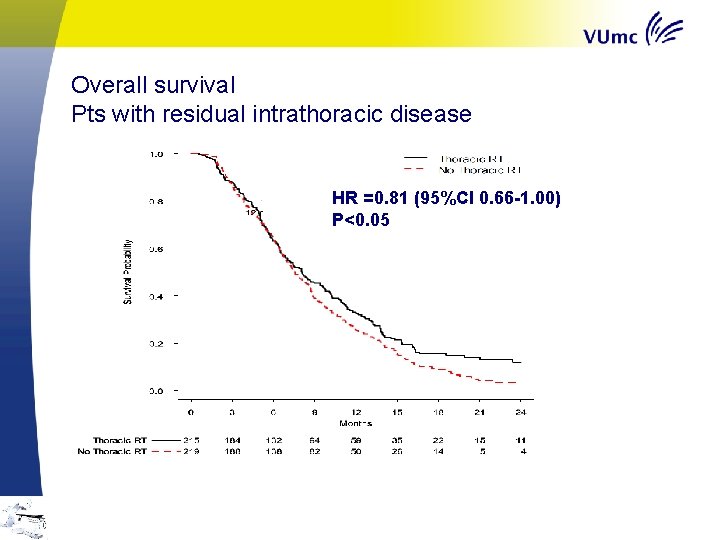
Overall survival Pts with residual intrathoracic disease HR =0. 81 (95%CI 0. 66 -1. 00) P<0. 05

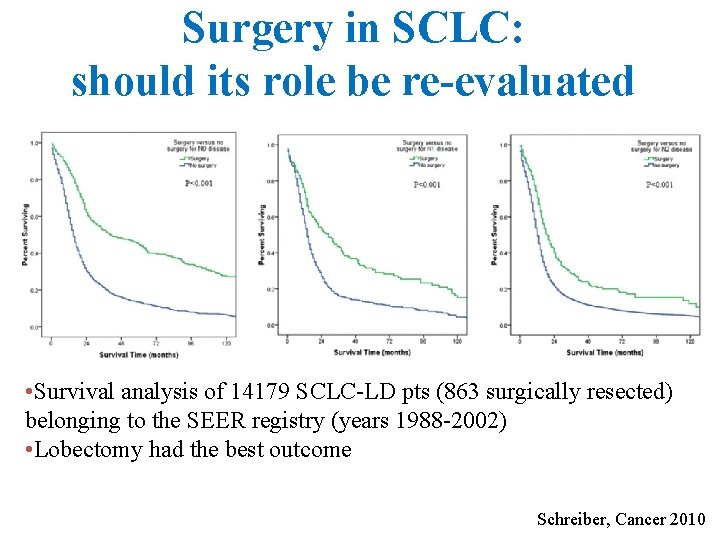
Surgery in SCLC: should its role be re-evaluated • Survival analysis of 14179 SCLC-LD pts (863 surgically resected) belonging to the SEER registry (years 1988 -2002) • Lobectomy had the best outcome Schreiber, Cancer 2010

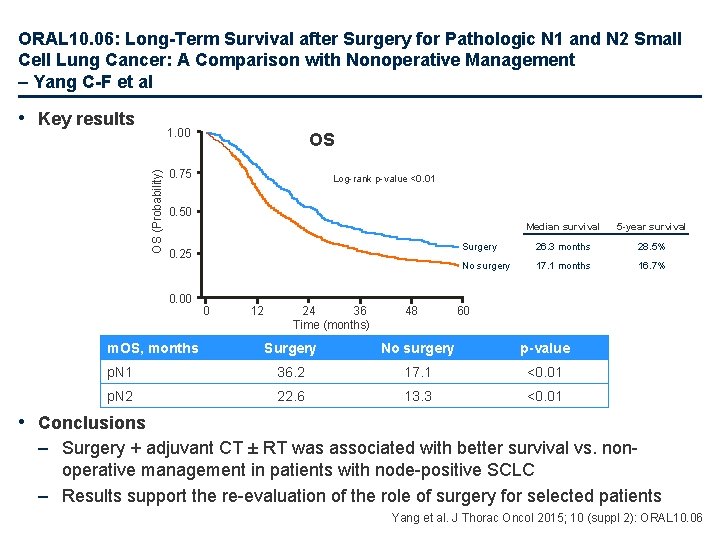
ORAL 10. 06: Long-Term Survival after Surgery for Pathologic N 1 and N 2 Small Cell Lung Cancer: A Comparison with Nonoperative Management – Yang C-F et al Study objective • To test whether or not surgery, in the setting of modern adjuvant therapies, offers a survival advantage among patients with node-positive SCLC compared with nonoperative management Study design • Patients were identified between 2003 and 2011 from the National Cancer Data Base: – Patients had to have p. T 1– 2 N 1– 2 M 0 SCLC – All patients underwent non-operative management (CT ± RT ≥ 45 Gy) or surgery (with adjuvant CT ± RT ≥ 45 Gy) – Patients with a history of unrelated malignancy and palliative-intent treatment were excluded • Data were assessed using Kaplan-Meier analyses and propensity score matching Yang et al. J Thorac Oncol 2015; 10 (suppl 2): ORAL 10. 06

ORAL 10. 06: Long-Term Survival after Surgery for Pathologic N 1 and N 2 Small Cell Lung Cancer: A Comparison with Nonoperative Management – Yang C-F et al • Key results OS (Probability) 1. 00 OS 0. 75 Log-rank p-value <0. 01 0. 50 0. 25 0. 00 m. OS, months 0 12 24 36 Time (months) 48 Median survival 5 -year survival Surgery 26. 3 months 28. 5% No surgery 17. 1 months 16. 7% 60 Surgery No surgery p-value p. N 1 36. 2 17. 1 <0. 01 p. N 2 22. 6 13. 3 <0. 01 • Conclusions – Surgery + adjuvant CT ± RT was associated with better survival vs. nonoperative management in patients with node-positive SCLC – Results support the re-evaluation of the role of surgery for selected patients Yang et al. J Thorac Oncol 2015; 10 (suppl 2): ORAL 10. 06

SCLC/LCNEC: What’s new in 2015 Conclusions • No practice-changing new data • Thorough molecular profiling of SCLC/LCNEC with possible druggable target identified • Promising novel agents for relapsed disease (immune check-point inhibitors • Possible role of TRT in selected cases of ED • Renewed interest for surgery in LD (including N+) 31
 Andrea ardizzoni
Andrea ardizzoni Reconstruction venn diagram
Reconstruction venn diagram Oncologia sondrio
Oncologia sondrio Oncologia alghero
Oncologia alghero Ritardo diagnostico in oncologia risarcimento
Ritardo diagnostico in oncologia risarcimento Master infermieristica oncologica
Master infermieristica oncologica Eduportal uoc gr
Eduportal uoc gr Office uoc
Office uoc Apertium uoc
Apertium uoc Girolamo pelaia
Girolamo pelaia Trò chơi âm nhạc
Trò chơi âm nhạc Ghế xoay là chi tiết có ren gì
Ghế xoay là chi tiết có ren gì Sơ đồ khối tìm ước chung lớn nhất
Sơ đồ khối tìm ước chung lớn nhất Polygon uoc
Polygon uoc Derecho en la uoc
Derecho en la uoc Asterias rubens breast cancer
Asterias rubens breast cancer Diceologia medica
Diceologia medica Academia nacional de educacion medica
Academia nacional de educacion medica Unife scuole di specializzazione
Unife scuole di specializzazione Que es la lex artis
Que es la lex artis Materia medica reloaded
Materia medica reloaded Slido o que é
Slido o que é Ricorso 696 bis responsabilità medica modello
Ricorso 696 bis responsabilità medica modello Modelo de prescrição médica
Modelo de prescrição médica Atlas medica cartella clinica
Atlas medica cartella clinica Ingenieramédicaprogramadoraperiodistahijastra
Ingenieramédicaprogramadoraperiodistahijastra Ricetta bianca esempio
Ricetta bianca esempio Fisica master
Fisica master Lisis terminologia medica
Lisis terminologia medica Receta cuantificada veterinaria requisitos
Receta cuantificada veterinaria requisitos Vocacion medicina
Vocacion medicina Clinica las carmelitas en soyapango telefono
Clinica las carmelitas en soyapango telefono





















































