Risk Assessment of the Impact of Lethality Standards
































- Slides: 48

Risk Assessment of the Impact of Lethality Standards on Salmonellosis from Ready-to-eat Meat and Poultry Products Greg Paoli Decisionalysis Risk Consultants, Inc. March 24, 2005

Outline Scope of Risk Assessment n Overview of Risk Estimation Process n Review of Key Assumptions n Review of Risk Estimates n Uncertainty and Limitations n Summary n 2

Risk Management Question: n This presentation deals with the question below: n The proposed RTE rule has a minimum lethality performance standard of a 6. 5 -log reduction of Salmonella spp. in meat for all categories (cooked, fermented, salt-cured and dried). What would be the public health impact of alternative lethality standards of 5. 0 and 6. 5/7. 0 -log reductions of Salmonella? 3

Scope of the Risk Assessment n Estimation of the number of cases of salmonellosis resulting from salmonellae in contaminated raw materials that survive lethality treatments applied to ready-to-eat (RTE) meat and poultry products (MPPs) n Addresses 16 RTE meat and poultry product categories 4

Scope of Risk Assessment n Does not include: n Illnesses caused by other pathogens n n n Does not include: n n n Examples: E. coli O 157: H 7, Campylobacter jejuni, Listeria monocytogenes This is primarily a technical limitation post-lethality product contamination after the lethality step, by food preparers or at any other stage acute process failures … since the risk from these events is not sensitive to the level of the lethality standard 5

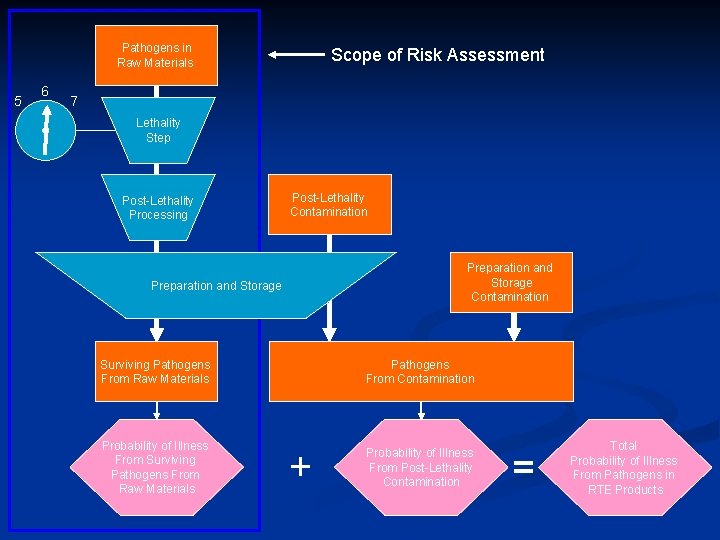
Pathogens in Raw Materials 5 6 Scope of Risk Assessment 7 Lethality Step Post-Lethality Processing Post-Lethality Contamination Preparation and Storage Surviving Pathogens From Raw Materials Probability of Illness From Surviving Pathogens From Raw Materials Pathogens From Contamination + Probability of Illness From Post-Lethality Contamination = Total Probability of Illness From Pathogens in RTE Products

Scope Issues n Validation data are not available n n FSIS sampling program could provide an upperbound on estimates of contamination levels Product categories are not exhaustive But, they are representative of a diverse set of important, high-volume product categories n They span key lethality processes: cooked, fermented, dried and salt-cured n 7

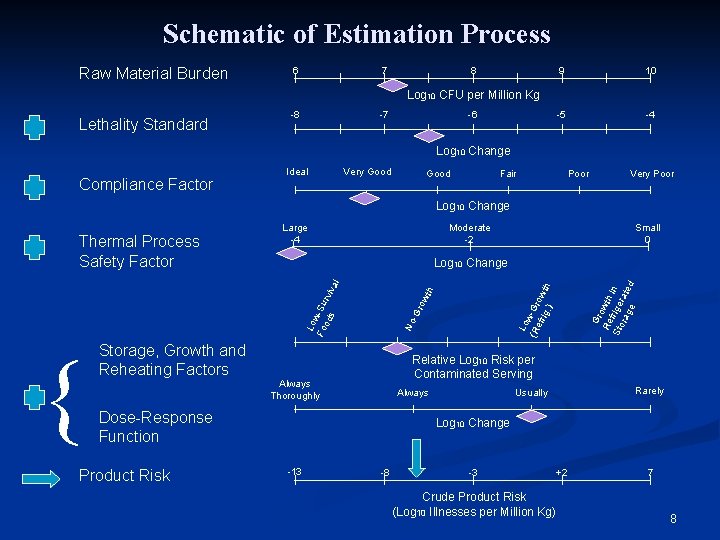
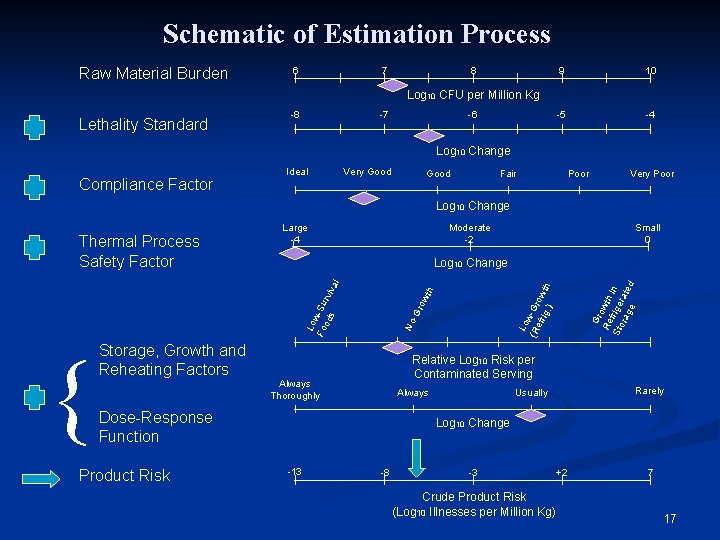
Schematic of Estimation Process Raw Material Burden 6 7 8 9 10 -5 -4 Log 10 CFU per Million Kg Lethality Standard -8 -7 -6 Log 10 Change Compliance Factor Ideal Very Good Fair Poor Very Poor Log 10 Change Moderate -2 Gr o Re wth f r Sto ige in rag rate d e Relative Log 10 Risk per Contaminated Serving Always Thoroughly Rarely Usually Always Dose-Response Function Product Risk Lo w (R -Gr efr o ig. wth ) No -G row th Lo w Fo -Su od r s viv { Storage, Growth and Reheating Factors Small 0 Log 10 Change al Thermal Process Safety Factor Large -4 Log 10 Change -13 -8 -3 Crude Product Risk (Log 10 Illnesses per Million Kg) +2 7 8

Overview of Risk Estimation Process Stage 1 n Develop representative product categories n Assign raw material streams to product categories n Estimate the expected number of organisms in raw materials, for a given mass of product 9

Overview of Risk Estimation Process Stage 2 Apply lethality treatment at a prescribed level n Adjust lethality treatment based on compliance n Apply thermal process safety factors, if any n This provides an estimate of the number of surviving organisms in a given mass of product 10

Overview of Risk Estimation Process Stage 3 Estimate the growth of the organism population during storage at retail and in home, if any n Apply heat treatment by preparer, if any n This provides a distribution of the number of consumed organisms in servings 11

Overview of Risk Estimation Process Stage 4 n Apply a dose-response relationship to convert the distribution of ingested doses to the probability of illness This provides an estimate of the expected number of cases of salmonellosis for a given mass of product 12

Overview of Risk Estimation Process Stage 5 n Apply the amount of consumption of each product category, in a year This provides an estimate of the expected number of cases of salmonellosis in a year for each product category, and in total 13

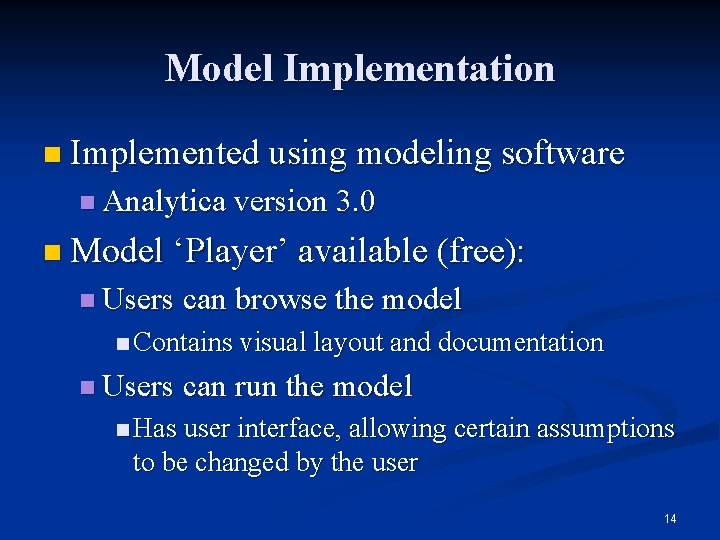
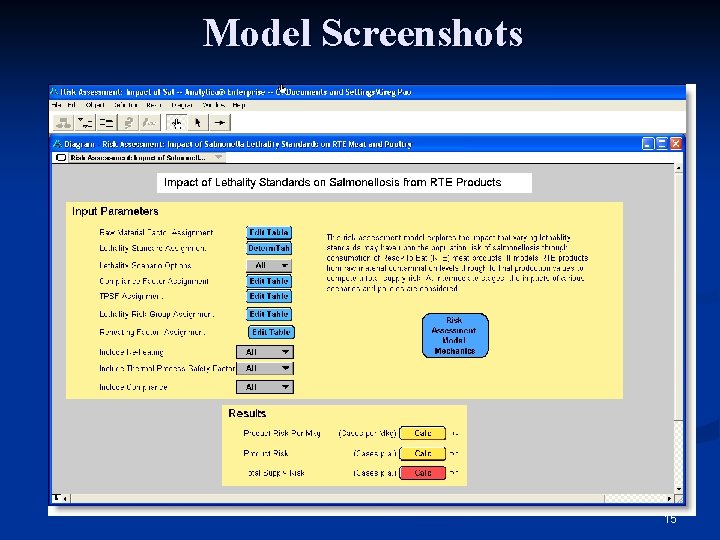
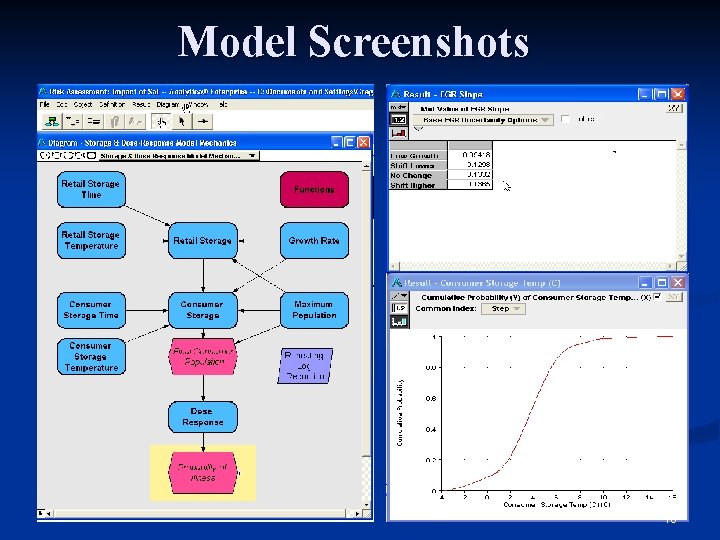
Model Implementation n Implemented using modeling software n Analytica version 3. 0 n Model ‘Player’ available (free): n Users can browse the model n Contains visual layout and documentation n Users can run the model n Has user interface, allowing certain assumptions to be changed by the user 14

Model Screenshots 15

Model Screenshots 16

Schematic of Estimation Process Raw Material Burden 6 7 8 9 10 -5 -4 Log 10 CFU per Million Kg Lethality Standard -8 -7 -6 Log 10 Change Compliance Factor Ideal Very Good Fair Poor Very Poor Log 10 Change Moderate -2 Gr o Re wth f r Sto ige in rag rate d e Relative Log 10 Risk per Contaminated Serving Always Thoroughly Rarely Usually Always Dose-Response Function Product Risk Lo w (R -Gr efr o ig. wth ) No -G row th Lo w Fo -Su od r s viv { Storage, Growth and Reheating Factors Small 0 Log 10 Change al Thermal Process Safety Factor Large -4 Log 10 Change -13 -8 -3 Crude Product Risk (Log 10 Illnesses per Million Kg) +2 7 17

Review of Key Assumptions n Types of assumptions n Designation of product categories n Data selection and treatment n Estimation methods and simplifications n Reasoned assumptions in the presence of data and theory gaps 18

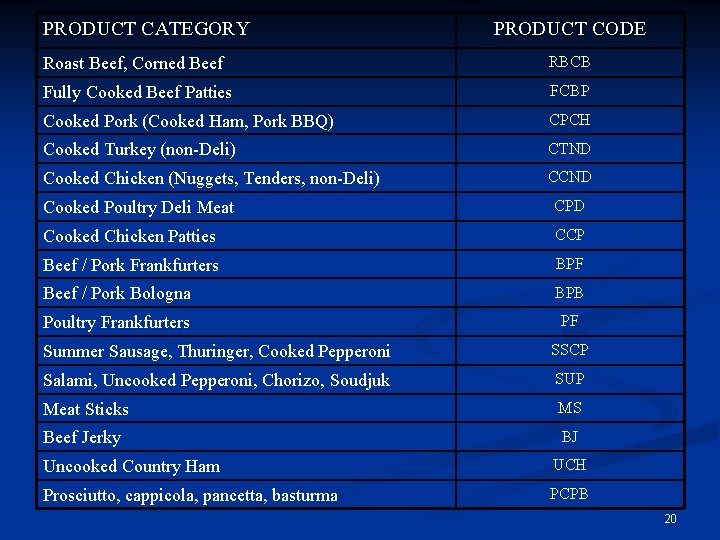
Product Categories n Designed to be compatible with: Risk management requirements n Data sources (e. g. , production, consumption) n Distinctions important to risk estimation n Manageable number of categories n n Coverage of major products in all four lethality categories n Cooked, fermented, dried and salt-cured 19

PRODUCT CATEGORY PRODUCT CODE Roast Beef, Corned Beef RBCB Fully Cooked Beef Patties FCBP Cooked Pork (Cooked Ham, Pork BBQ) CPCH Cooked Turkey (non-Deli) CTND Cooked Chicken (Nuggets, Tenders, non-Deli) CCND Cooked Poultry Deli Meat CPD Cooked Chicken Patties CCP Beef / Pork Frankfurters BPF Beef / Pork Bologna BPB Poultry Frankfurters PF Summer Sausage, Thuringer, Cooked Pepperoni SSCP Salami, Uncooked Pepperoni, Chorizo, Soudjuk SUP Meat Sticks MS Beef Jerky BJ Uncooked Country Ham UCH Prosciutto, cappicola, pancetta, basturma PCPB 20

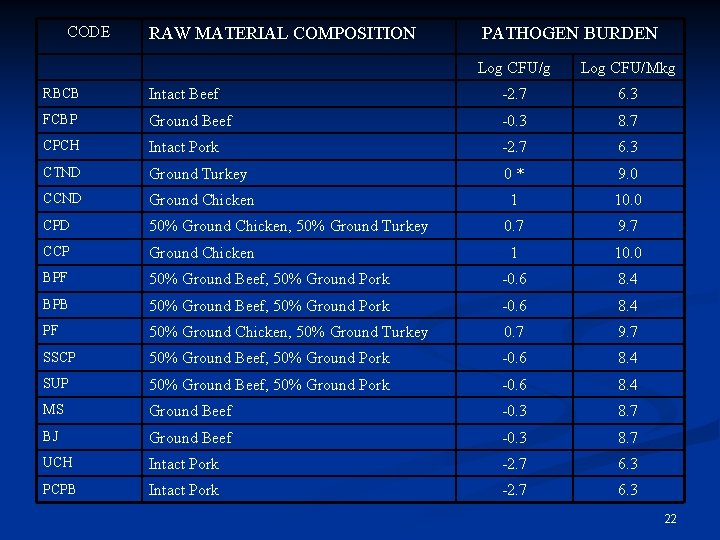
Raw Material Pathogen Burden Used FSIS Microbiological Baseline Surveys n Estimate the expected number of salmonellae in a given mass of raw materials (per gram, per million kilograms) n Separate estimates made for: n beef, pork, chicken, turkey n Both ground and intact for each n 21

CODE RAW MATERIAL COMPOSITION PATHOGEN BURDEN Log CFU/g Log CFU/Mkg RBCB Intact Beef -2. 7 6. 3 FCBP Ground Beef -0. 3 8. 7 CPCH Intact Pork -2. 7 6. 3 CTND Ground Turkey 0* 9. 0 CCND Ground Chicken 1 10. 0 CPD 50% Ground Chicken, 50% Ground Turkey 0. 7 9. 7 CCP Ground Chicken 1 10. 0 BPF 50% Ground Beef, 50% Ground Pork -0. 6 8. 4 BPB 50% Ground Beef, 50% Ground Pork -0. 6 8. 4 PF 50% Ground Chicken, 50% Ground Turkey 0. 7 9. 7 SSCP 50% Ground Beef, 50% Ground Pork -0. 6 8. 4 SUP 50% Ground Beef, 50% Ground Pork -0. 6 8. 4 MS Ground Beef -0. 3 8. 7 BJ Ground Beef -0. 3 8. 7 UCH Intact Pork -2. 7 6. 3 PCPB Intact Pork -2. 7 6. 3 22

Lethality Treatments n Expressed in units of ‘log reductions’ This is the base-10 logarithm of the reduction factor n A 5 -log reduction means the population will be reduced by, on average, 5 factors of 10, or 100, 000 n Equivalently, we could say that each organism has a 1 in 100, 000 chance of survival of the process n If a million organisms (6 -logs) were subjected to a “ 5 -log process” we would expect, on average, 10 survivors (1 -log). n 23

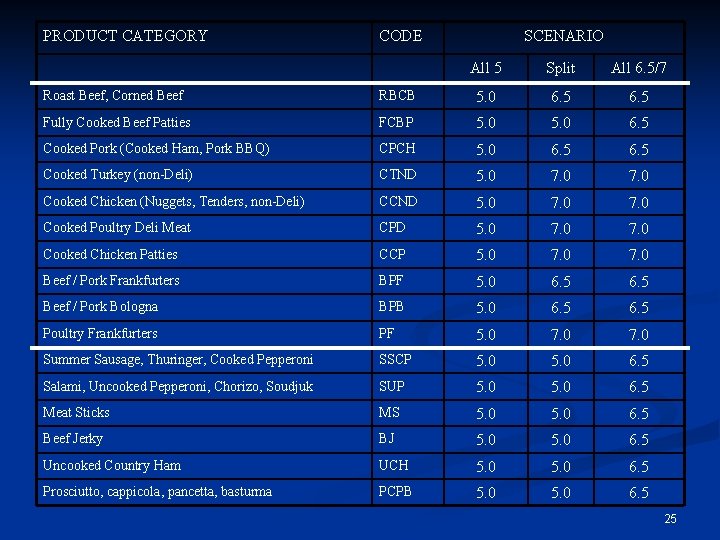
Lethality Scenarios Three alternate policy scenarios were requested: n “All 5 -log” n n “All 6. 5/7. 0 -log” n n n All products (except if they contain poultry) require 6. 5 -log if they contain poultry they require a 7. 0 log reduction. “Split” (Default) n n n All products require, at least, a 5 -log reduction All cooked products require 6. 5/7. 0 -log reductions; all other products require a 5. 0 -log reduction. One exception is fully-cooked beef patties which would require 5. 0 -log When not otherwise indicated, estimates refer to the “Split” scenario. 24

PRODUCT CATEGORY CODE SCENARIO All 5 Split All 6. 5/7 Roast Beef, Corned Beef RBCB 5. 0 6. 5 Fully Cooked Beef Patties FCBP 5. 0 6. 5 Cooked Pork (Cooked Ham, Pork BBQ) CPCH 5. 0 6. 5 Cooked Turkey (non-Deli) CTND 5. 0 7. 0 Cooked Chicken (Nuggets, Tenders, non-Deli) CCND 5. 0 7. 0 Cooked Poultry Deli Meat CPD 5. 0 7. 0 Cooked Chicken Patties CCP 5. 0 7. 0 Beef / Pork Frankfurters BPF 5. 0 6. 5 Beef / Pork Bologna BPB 5. 0 6. 5 Poultry Frankfurters PF 5. 0 7. 0 Summer Sausage, Thuringer, Cooked Pepperoni SSCP 5. 0 6. 5 Salami, Uncooked Pepperoni, Chorizo, Soudjuk SUP 5. 0 6. 5 Meat Sticks MS 5. 0 6. 5 Beef Jerky BJ 5. 0 6. 5 Uncooked Country Ham UCH 5. 0 6. 5 Prosciutto, cappicola, pancetta, basturma PCPB 5. 0 6. 5 25

Lethality Compliance Factors n Based on expert elicitation study (RTI, 2004) n n Full compliance results in some additional lethality n n n “What proportion of the producers of product Y achieve an X-log reduction. ” Assumes some overshoot of target Assumes all cooked products in full compliance with 6. 5/7. 0 Deviations from full compliance result in reduced lethality n Largest adjustment for fermented products in the 6. 5/7. 0 log scenario. 26

Thermal Process Safety Factors For a variety of reasons, processors may apply a process that yields a much smaller average probability of survival than is implied by the strict interpretation of being in compliance with the required lethality. n For example, in complying with a 7 -log reduction requirement, a process may actually achieve a mean probability of survival equivalent to an 11 -log reduction. n 27

Thermal Process Safety Factors n Selected reasons for safety factors: Product geometry and heat transfer to interior n Consumer preference for texture, ‘done-ness’ n Design and validation of processes with strains that are much more resistant than ‘average’ (e. g. Salmonella enterica serovar Senftenberg) n Motivated to achieve significant reductions of other pathogens, for example, E. coli O 157: H 7 n Where contamination is limited to the surface combined with intense heating to warm the inside n 28

Thermal Process Safety Factors n Estimation Challenge: n n We know they exist … and that they can have a large impact on the risk estimation process (for certain products). They may be simulated or known for certain products and processes, but we need to know the net impact across the whole industry that is producing a product. The industry-wide TPSF is strongly influenced by the proportion of processors having lower safety factors. Most data geared toward assuring compliance; they are not readily applied for estimation of product risk 29

Thermal Process Safety Factors n n Requires reasoned assumptions Implemented as having three possibilities: n n n Small 0 -log increment Medium 2 -log increment Large 4 -log increment n Each product category is assigned one of these three factors n Model allows adjustment and removal for sensitivity analysis. 30

Single Organism Assumption n n Survival of organisms (at the level of serving-sized pieces of meat) is modeled as a rare event. The implication is that, in each contaminated serving, we assume that there is only surviving organism (prior to considering growth). A further implication is that we can simplify the representation of the pathogen burden to the total number of organisms without concern for variations in density. Implications for Validation n Rare events, recognizable outbreaks not expected from this contamination pathway 31

Storage and Growth n Four scenarios are assigned to products: No-Growth, regardless of temperature n Low-Survival, a further 1 -log reduction during storage n Normal Growth, Refrigerated Storage n n minimum temperature is applied n Low Growth, Refrigerated Storage n half the growth rate of Normal Growth with minimum temperature applied 32

Storage and Growth Detailed growth modeling for diverse products, even within one product category would be very time and resource intensive. n Data and models to accommodate this variety in distinct products are relatively limited n n Growth and Low-Growth categories are modeled using a ‘square-root’ model, relating growth rate to temperature 33

Storage and Growth For products allowing growth … n Growth is modeled in two stages: retail and consumer storage n A variety of time and temperature distributions are provided for in the model to explore sensitivity, but only defaults are carried through to final calculations. n 34

Reheating n Three levels of lethality due to reheating are considered: n n None 0 -log (no change in population) Minimal 2 -log additional reduction Thorough 4 -log additional reduction Products are assigned to reheating pattern categories which specify the proportions of consumers applying each of the three levels above. n Never, Rarely, Usually, Always Thoroughly 35

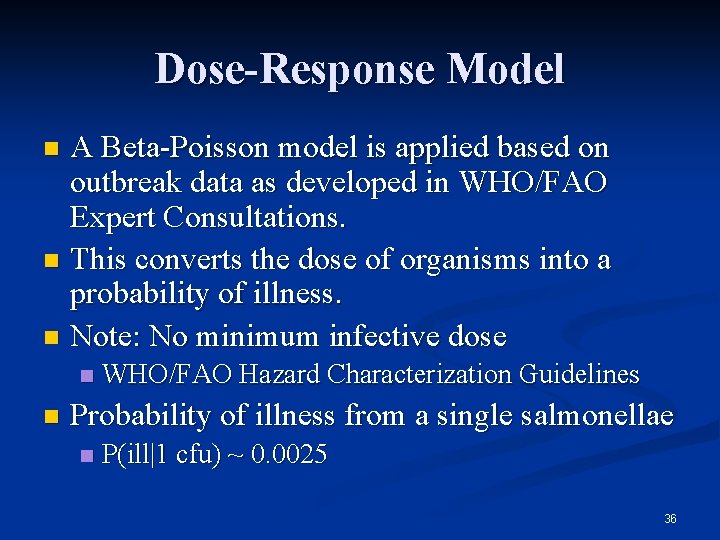
Dose-Response Model A Beta-Poisson model is applied based on outbreak data as developed in WHO/FAO Expert Consultations. n This converts the dose of organisms into a probability of illness. n Note: No minimum infective dose n n n WHO/FAO Hazard Characterization Guidelines Probability of illness from a single salmonellae n P(ill|1 cfu) ~ 0. 0025 36

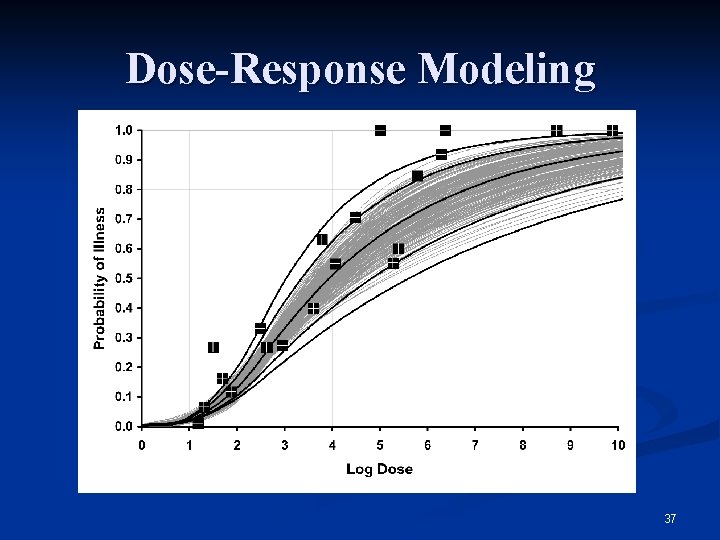
Dose-Response Modeling 37

Consumption Volumes For most product categories, based on Economic Census (Dept. of Commerce) n For a few product categories, based on a database product (EDEA, Environ Corp. ) containing CSFII data (ARS, USDA). n n n CSFII: Continuing Survey of Food Intake by Individuals Highly variable uncertainties n Quite high for smaller volume products 38

Risk Estimates n Main estimates (salmonellosis): n n Annual Cases per Million Kg of Product Class Total Cases due to All Product Classes Options for estimates n n Exclude Thermal Process Safety Factor Exclude Reheating Exclude Compliance Adjustment Assumptions for Behavior under Relaxed Lethality Standard n Baseline: industry relaxes lethality to lowered standard where applicable 39

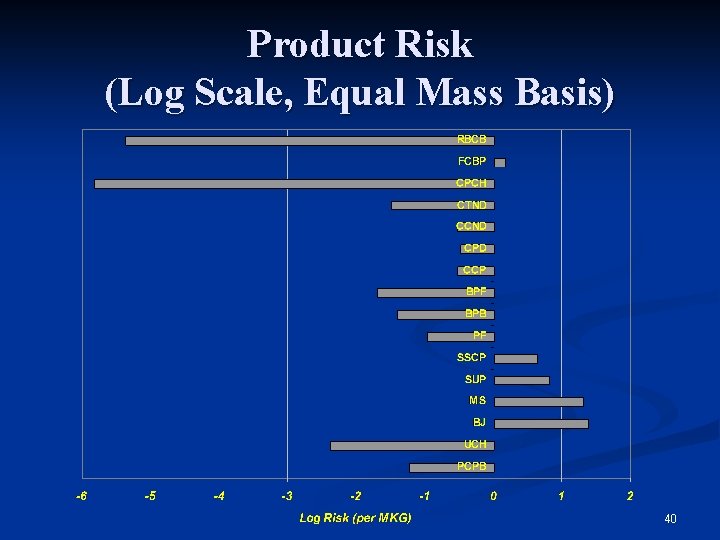
Product Risk (Log Scale, Equal Mass Basis) 40

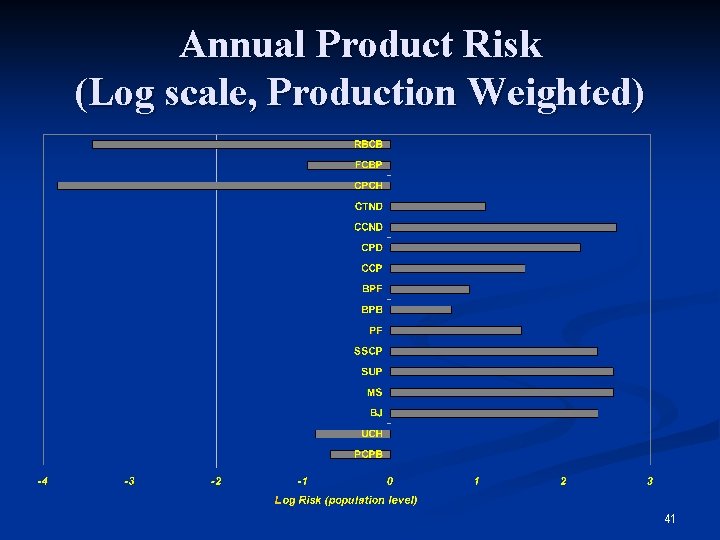
Annual Product Risk (Log scale, Production Weighted) 41

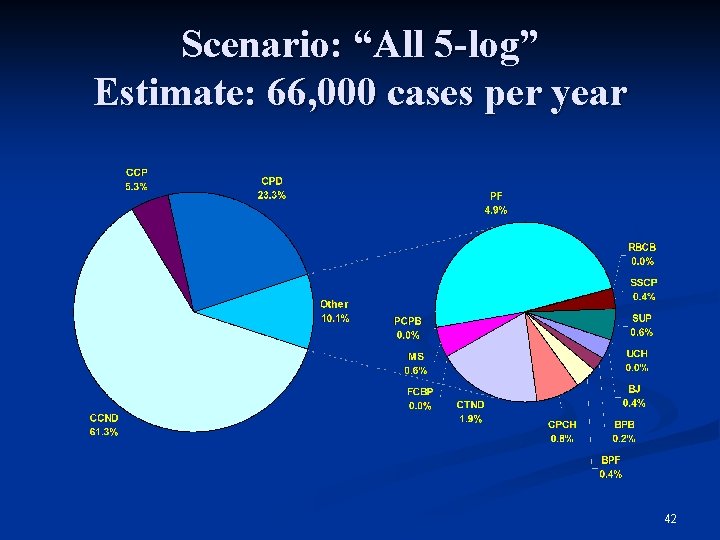
Scenario: “All 5 -log” Estimate: 66, 000 cases per year 42

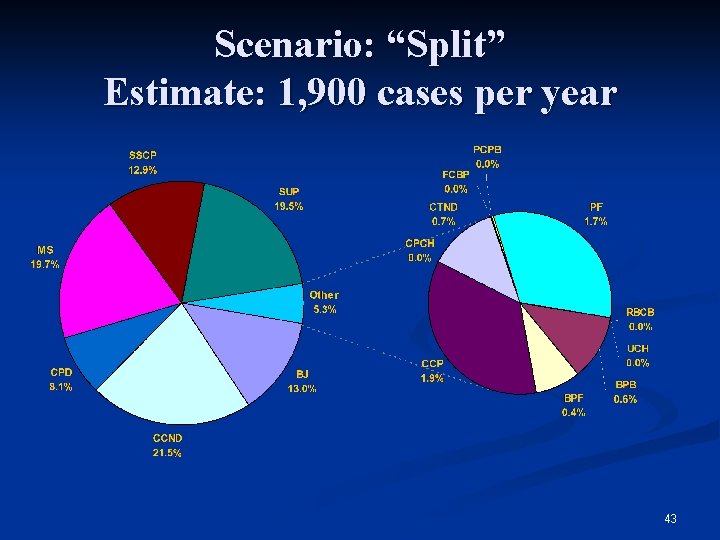
Scenario: “Split” Estimate: 1, 900 cases per year 43

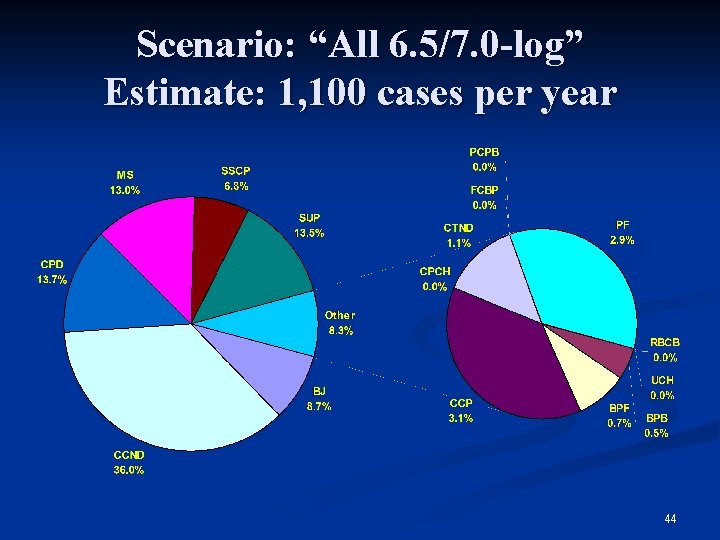
Scenario: “All 6. 5/7. 0 -log” Estimate: 1, 100 cases per year 44

Uncertainty and Limitations n Much of the uncertainty is not readily quantifiable. n Raw material pathogen burden ** n Uncertainty in compliance * n Thermal process safety factors **** n Storage and Growth ** n Reheating Impact * n Production Volumes ** n Categorizations ** n Dose-Response *** 45

Uncertainty and Limitations n Risk estimates presented should be considered to fall within a broad range of uncertainty. n They may be several factors of 10 smaller or larger. n Given this uncertainty, the relative ranking (or attribution of total risk) should be considered correspondingly uncertain. 46

Summary n The risk assessment provides policymakers with estimates of the impact of alternate lethality standards (5 -log, 6. 5 -log, 7 -log reductions) on the expected number of cases of salmonellosis, for 16 product categories. n Model software is designed to allow for exploration of the impact of alternate assumptions at numerous stages in the estimation process. n The risk assessment model and the report will be revised in response to public comment. 47

Thank you for your attention 48
 Hp
Hp Credit risk market risk operational risk
Credit risk market risk operational risk Probability impact risk
Probability impact risk Sustainable engineering ktu syllabus
Sustainable engineering ktu syllabus Experience assessment cipd
Experience assessment cipd Rapid environmental impact assessment in disaster
Rapid environmental impact assessment in disaster Traffic impact assessment
Traffic impact assessment Change impact assessment template
Change impact assessment template Eia
Eia Event impact assessment
Event impact assessment Difference between eia and environmental audit
Difference between eia and environmental audit Lmia online pilot
Lmia online pilot Mental wellbeing impact assessment
Mental wellbeing impact assessment Mental wellbeing impact assessment
Mental wellbeing impact assessment Regulatory impact assessment india
Regulatory impact assessment india Customer defined service standards
Customer defined service standards Risk projection in software engineering
Risk projection in software engineering Risk avoidance
Risk avoidance Relative risk calculation
Relative risk calculation Residual risk and secondary risk pmp
Residual risk and secondary risk pmp Ar = ir x cr x dr
Ar = ir x cr x dr Absolute risk vs relative risk
Absolute risk vs relative risk Activity sheet 2: stock market calculations
Activity sheet 2: stock market calculations Classification of risk
Classification of risk Pembelanjaan resiko
Pembelanjaan resiko The biggest risk is not taking any risk
The biggest risk is not taking any risk Key risk indicators for vendor management
Key risk indicators for vendor management Ir x cr x dr
Ir x cr x dr Business risk vs financial risk capital structure
Business risk vs financial risk capital structure Relative risk
Relative risk Risk map
Risk map Relative risk
Relative risk Hát kết hợp bộ gõ cơ thể
Hát kết hợp bộ gõ cơ thể Frameset trong html5
Frameset trong html5 Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Chó sói
Chó sói Tư thế worm breton
Tư thế worm breton Hát lên người ơi alleluia
Hát lên người ơi alleluia Môn thể thao bắt đầu bằng chữ f
Môn thể thao bắt đầu bằng chữ f Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công thức tiính động năng
Công thức tiính động năng Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Mật thư tọa độ 5x5
Mật thư tọa độ 5x5 101012 bằng
101012 bằng Phản ứng thế ankan
Phản ứng thế ankan Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật