Radiation Exposure and Health Radiation Energy that travels
































- Slides: 65

Radiation Exposure and Health Radiation: Energy that travels in the form of waves or high-speed particles It occurs naturally in sunlight and sound waves Man-made radiation is used in X-rays, nuclear weapons, nuclear power plants and cancer treatment

Everything that's living has some amount of radiation coming from it — a very small amount Radiation And Radioactive Material Is A Part Of Nature Plus there's radiation in the ground and the air Harmful radiation is ionizing radiation Bananas have a lot of potassium. And a small amount of potassium naturally is called potassium 40, which is radioactive NON-IONIZING radiation includes everything like radio waves and visible light and the microwave

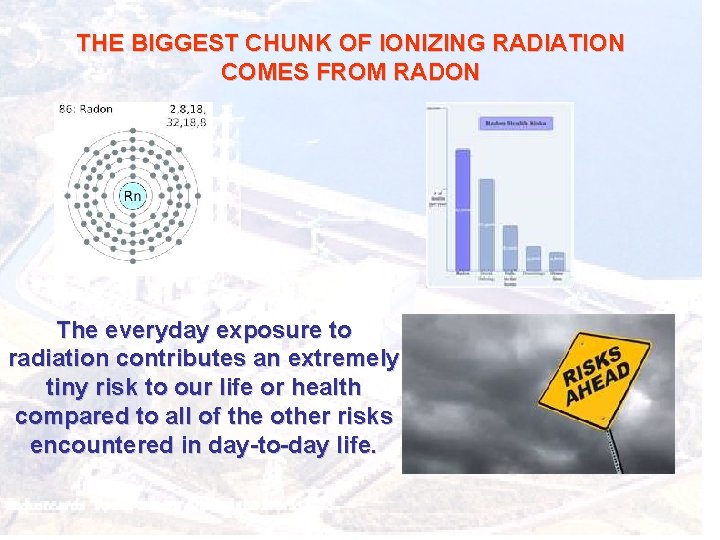
THE BIGGEST CHUNK OF IONIZING RADIATION COMES FROM RADON The everyday exposure to radiation contributes an extremely tiny risk to our life or health compared to all of the other risks encountered in day-to-day life.

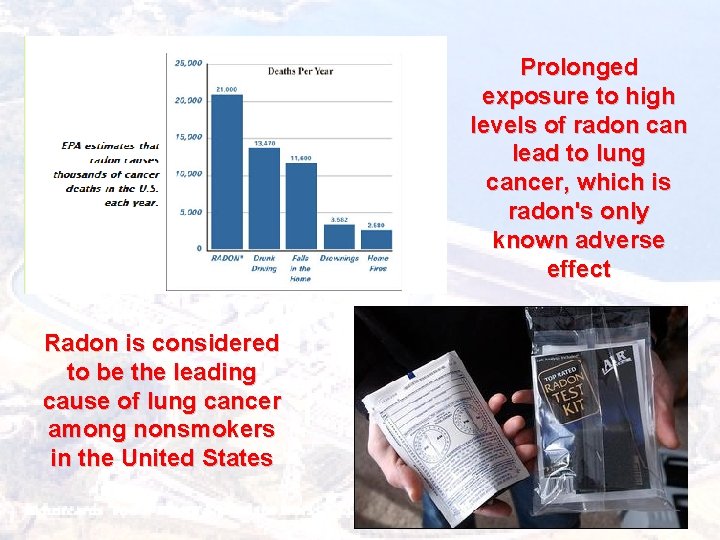
Prolonged exposure to high levels of radon can lead to lung cancer, which is radon's only known adverse effect Radon is considered to be the leading cause of lung cancer among nonsmokers in the United States

High radon areas in the U. S. tend to be in the northern section of the country due to the types of soils found there, (Zone One) States with high concentrations of radon include Iowa, North Dakota, South Dakota, Maine, Minnesota, Illinois, Kansas, Montana, Idaho, Wyoming, Colorado, Pennsylvania, and Virginia Moderate radon levels include Alaska, Arizona, Nevada, Missouri, Utah, and Wisconsin. Significant parts of California, Oregon, and most of New England (Zone Two)

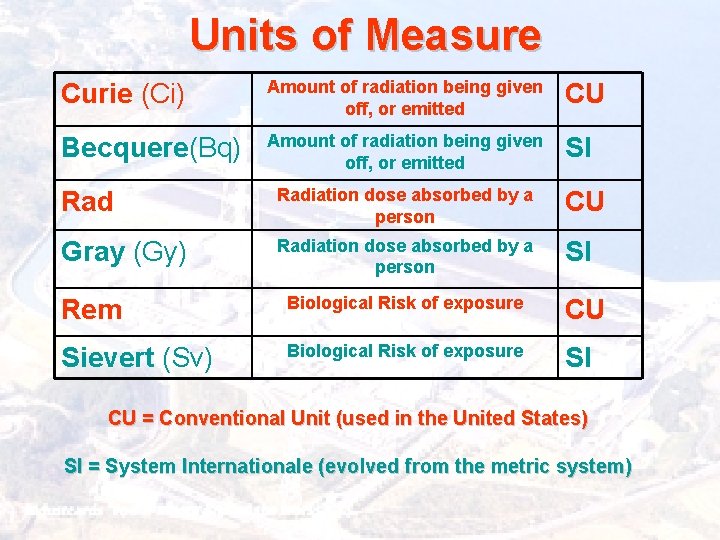
Units of Measure Curie (Ci) Amount of radiation being given off, or emitted CU Becquere(Bq) Amount of radiation being given off, or emitted SI Radiation dose absorbed by a person CU Gray (Gy) Radiation dose absorbed by a person SI Rem Biological Risk of exposure CU Sievert (Sv) Biological Risk of exposure SI CU = Conventional Unit (used in the United States) SI = System Internationale (evolved from the metric system)

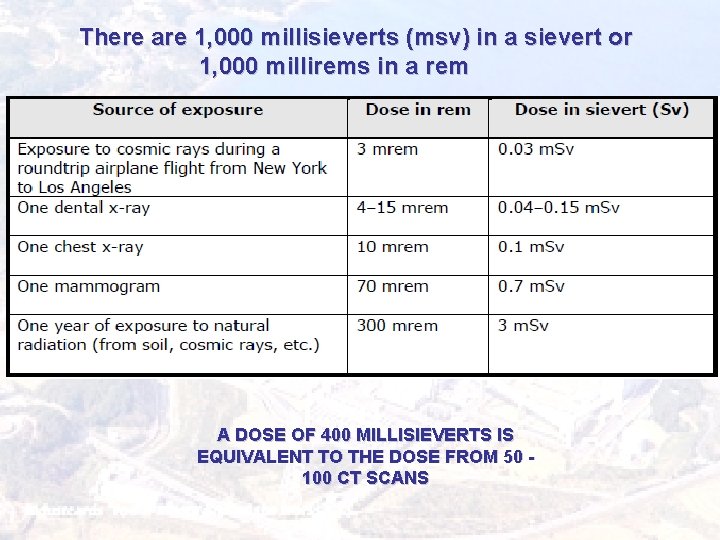
There are 1, 000 millisieverts (msv) in a sievert or 1, 000 millirems in a rem Measures A DOSE OF 400 MILLISIEVERTS IS EQUIVALENT TO THE DOSE FROM 50 100 CT SCANS


Ionizing Radiation Most Common – Alpha • A positively charged particle made up of two neutrons and two protons • Can be stopped by thin layers of light materials, such as a sheet of paper, and pose no direct or external radiation threat • Can pose a serious health threat if ingested or inhaled – Beta • An electron or positron emitted by certain radioactive nuclei • Beta particles can be stopped by aluminum • Can be lethal depending on the amount received • Also pose a serious internal radiation threat if inhaled or ingested

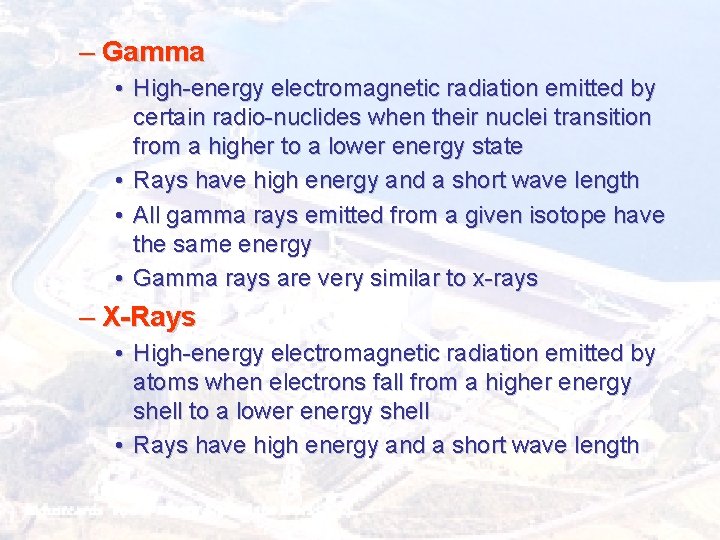
– Gamma • High-energy electromagnetic radiation emitted by certain radio-nuclides when their nuclei transition from a higher to a lower energy state • Rays have high energy and a short wave length • All gamma rays emitted from a given isotope have the same energy • Gamma rays are very similar to x-rays – X-Rays • High-energy electromagnetic radiation emitted by atoms when electrons fall from a higher energy shell to a lower energy shell • Rays have high energy and a short wave length


KINDS OF HEALTH EFFECTS The amount and duration of radiation exposure affects the severity or type of health effect Two Broad Categories Stochastic: Associated with long-term exposure Low-level (chronic) Increased levels of exposure make these health effects more likely to occur but do not influence the type or severity of the effect Cancer, changes in DNA Changes in DNA are called mutations Teratogenic mutations are caused by exposure of the fetus in the uterus Genetic mutations are passed on to offspring

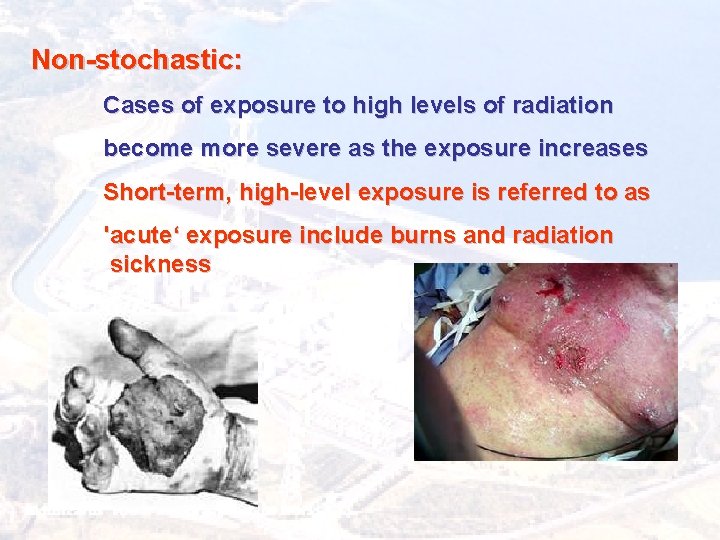
Non-stochastic: Cases of exposure to high levels of radiation become more severe as the exposure increases Short-term, high-level exposure is referred to as 'acute‘ exposure include burns and radiation sickness

RADIATION SICKNESS “radiation poisoning” Can cause premature aging and death If the dose is fatal, death usually occurs within two months The symptoms of radiation sickness include: Nausea Weakness Hair loss Skin burns Diminished organ function People exposed to high levels of radiation are also at greatest risk of infection, so they need to be kept in isolation wards in sterile environments to protect them from bacteria and viruses

IS ANY AMOUNT OF RADIATION SAFE? EPA makes the conservative (cautious) assumption that any increase in radiation exposure is accompanied by an increased risk of stochastic effects.

CHILDREN AND RADIATION • More sensitive to radiation than adults • More cells dividing and a greater opportunity for radiation to disrupt the process • Fetuses are also highly sensitive to radiation

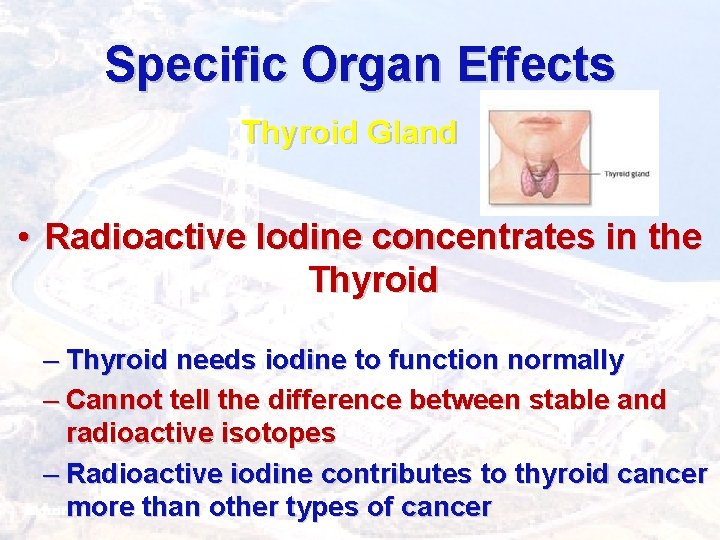
Specific Organ Effects Thyroid Gland • Radioactive Iodine concentrates in the Thyroid – Thyroid needs iodine to function normally – Cannot tell the difference between stable and radioactive isotopes – Radioactive iodine contributes to thyroid cancer more than other types of cancer

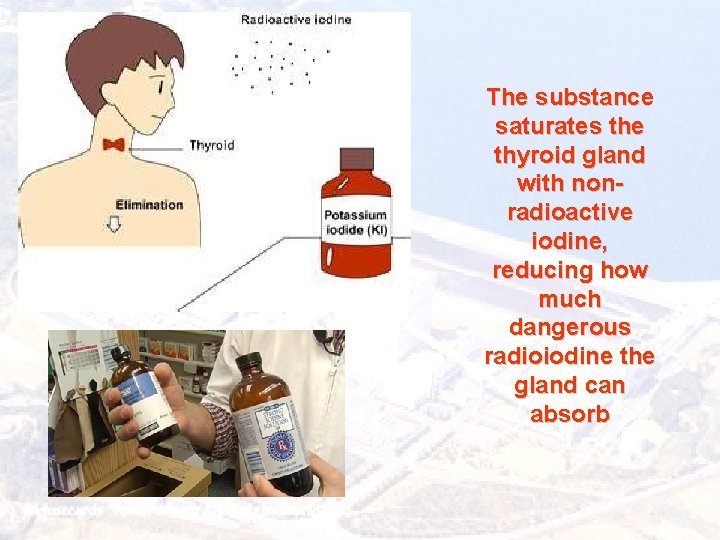
The substance saturates the thyroid gland with nonradioactive iodine, reducing how much dangerous radioiodine the gland can absorb

Bones and Teeth • Calcium, strontium-90 and radium-226 have similar chemical properties • Strontium and Radium in the body tend to collect in these calcium rich areas • They contribute to bone cancer

Teratogenic Mutations Exposure of Fetuses • • Smaller head or brain size Poorly formed eyes Abnormally slow growth Mental retardation Genetic effects About fifty severe hereditary effects will occur in a group of one million live-born children whose parents were both exposed to one rem. About one hundred twenty severe hereditary effects would occur in all descendants.

Exposure Treatment External particles of radioactive material can be washed away If the thyroid is contaminated, it can be treated with potassium iodide, which counters some of radiation's effects Drugs are available that increase white blood-cell production to counter any damage that may have occurred to the bone marrow, and to reduce the risk of further infections due to immune-system damage. There also specific drugs that can help to reduce the damage to internal organs caused by radioactive particles

Can Radioactive Particles Be Transmitted From Person To Person YES, IN TWO WAYS Material that attaches to dust or other small particles and settles on skin or clothing can be transferred during physical contact An individual who is internally contaminated can emit particles in urine, blood or sweat, and coming into contact with these fluids can transmit exposure.

EPA EXPOSURE LIMITS About 100 mrem per year from all sources In the U. S. , the Nuclear Regulatory Commission restricts workers to 50 millisieverts of radiation exposure per year Staying indoors and sealing your house is a way to prevent ingesting or carrying around any radioactive material Because iodine has a half-life of just over a week, any grasses, crops or animals exposed to it should be cleared of any residual material in a matter of weeks The pathway for radioactive iodine to get into human beings is via milk. The iodine hits the ground, gets on the grass, cows eat the grass, and it is concentrated in the milk, and people drink the milk. "

References http: //www. epa. gov/rpdweb 00/understand/health_effects. html http: //www. nlm. nih. gov/medlineplus/radiationexposure. html http: //www. kqed. org/news/story/2011/03/30/48777/everyday_radiation_ exposure_is_a_tiny_health_risk? source=npr&category=health http: //healthland. time. com/2011/03/15/japans-next-nightmare-healthproblems-from-radiation-exposure/ http: //www. bbc. co. uk/news/health-12722435 http: //www. abelard. org/briefings/ionising-radiation. php#introduction http: //www. bt. cdc. gov/radiation/pdf/measurement. pdf

Radiation may be absorbed by the medium it passes through. Radiation can kill living cells or change the nature of living cells. All living things contain living cells. We have many different types of cells which perform different functions including: ü ü ü Skin cells. Red blood cells (they transport oxygen around the body) White blood cells (they fight infection). Nerve cells. Muscle cells. Brain cells.

The Effects of Ionising Radiation Ionising radiation can kill or change the nature of living cells. The effects of the damage inflicted by the ionising radiation may: ü be severe and cause immediate effects, or ü not become apparent for a long time. The biological effect of radiation depends on: ü The type of radiation. ü The type of body tissue or body organ that absorbs the radiation. ü The total amount of energy absorbed.

Short-Term Effects of Radiation Short-term effects usually occur when there’s a large amount of exposure to radiation.

WW 2 – Hiroshima and Nagasaki During the Second World War, two atomic bombs were dropped on Hiroshima and Nagasaki in Japan. Those people who survived the blast were exposed to a large dose of radiation. Such doses caused severe damage to cells all over the body, especially in the skin, blood, bone tissue and gut. Many of these people died within a few weeks. Those people who were exposed to a smaller dose recovered from such immediate effects.

Chernobyl Nuclear Power Station There was also a huge nuclear accident at the Chernobyl Nuclear Power station in the former USSR in 1986. Workers there were carrying out experiments on the reactor rods which caused fires to start. A number of firemen were exposed to very large amounts of radiation and 30 people died as a result. The damage to the power station was extensive but the radiation effects over a wide area were considerable.

Chernobyl Nuclear Power Station ü 135 000 people were removed from an area within a radius of 30 km. ü The smoke and radioactive debris reached a height of 1200 m and travelled across Russia, Poland Scandinavia. ü A cloud of material from the accident reached the UK and, with heavy rain, there was material deposited on parts of north Wales, Cumbria and Scotland. This caused certain farm animals (e. g. lambs) to be banned from sale as they had absorbed radiation from the grass.

Long-Term Effects of Radiation These effects take longer to become apparent and can be caused by much lower levels of radiation. One of the most important long-term effects of radiation is that of cancer in various parts of the body. Uranium miners tended to get lung cancer due to breathing in gases which emitted alpha particles. People who painted the dials of clocks with luminous paint developed one cancer from using their lips to make points on the brushes.

Exposure to ionising radiation does not necessarily cause cancer The mechanisms for cancer occurring are poorly understood at the moment. One theory is that the ionising radiation affects the DNA material within us – our genetic make-up. Our DNA contains genetic instructions which control the operation and reproduction of the cells. If ionisations caused by ionising radiations alter these instructions in the DNA, there is a chance that cancer will develop. Genetic damage can be caused to cells by radiation, including cells which are involved in reproduction.

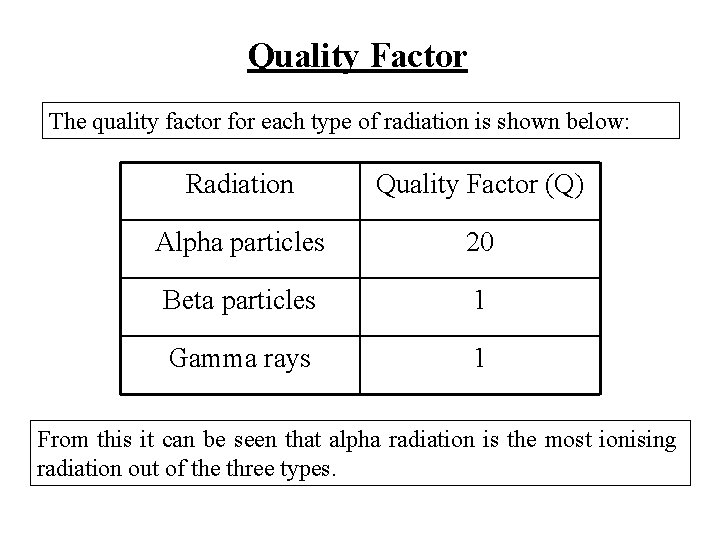
Quality Factor Different types of radiation have different effects on living cells. Even though the same type of tissue may receive the same dose, the biological effects of different radiations will be different. To take this into account, a quality factor is assigned to all types of radiation. The quality factor, Q, allows the effects that different radiations have on living cells to be compared.

Quality Factor The quality factor for each type of radiation is shown below: Radiation Quality Factor (Q) Alpha particles 20 Beta particles 1 Gamma rays 1 From this it can be seen that alpha radiation is the most ionising radiation out of the three types.

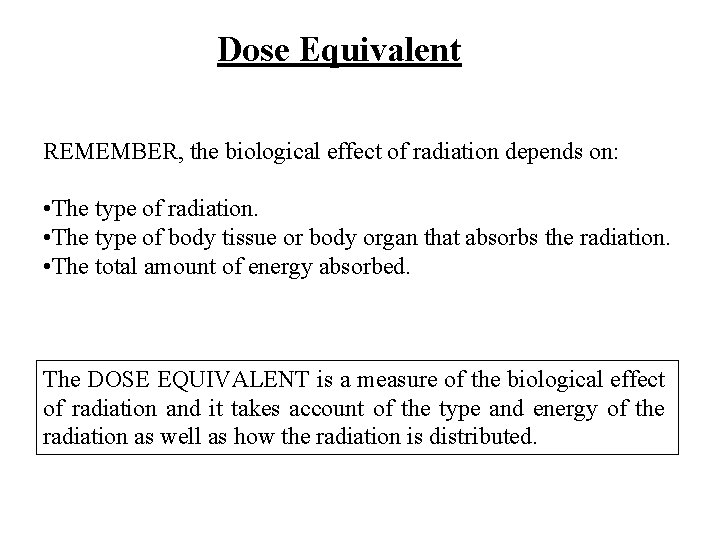
Dose Equivalent REMEMBER, the biological effect of radiation depends on: • The type of radiation. • The type of body tissue or body organ that absorbs the radiation. • The total amount of energy absorbed. The DOSE EQUIVALENT is a measure of the biological effect of radiation and it takes account of the type and energy of the radiation as well as how the radiation is distributed.

The DOSE EQUIVALENT is measured in sieverts (Sv). Because 1 Sv is a very large dose of radiation which could only happen as a result of a very serious nuclear accident or explosion, doses are given in millisieverts (m. Sv) or microsieverts ( Sv).

Suppose that 100 people all receive a dose equivalent of 1 Sv spread over the whole body. It is estimated that, of the 100 people on average 4 of them would eventually die as a result of the radiation. But precisely who would die, or when they would die, or what illness they would die of, cannot be predicted.

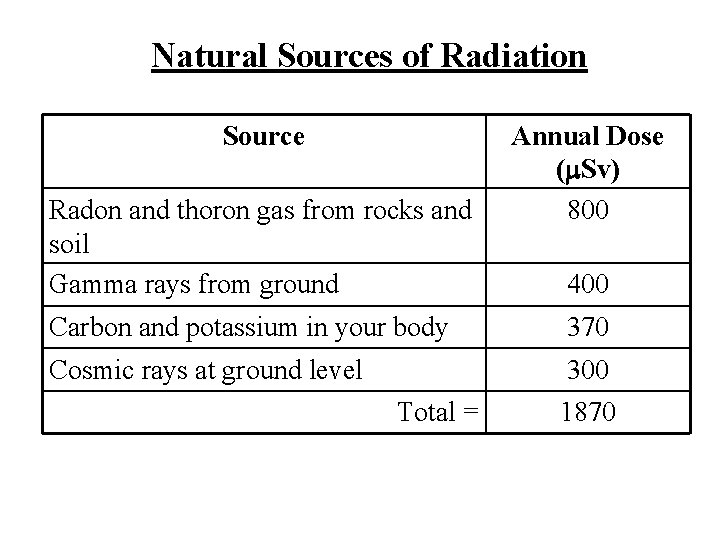
Background Radiation is all around us! Background radiation is radiation that is naturally occurring.

Natural Sources of Radiation Source Radon and thoron gas from rocks and soil Gamma rays from ground Annual Dose ( Sv) 800 400 Carbon and potassium in your body 370 Cosmic rays at ground level 300 Total = 1870

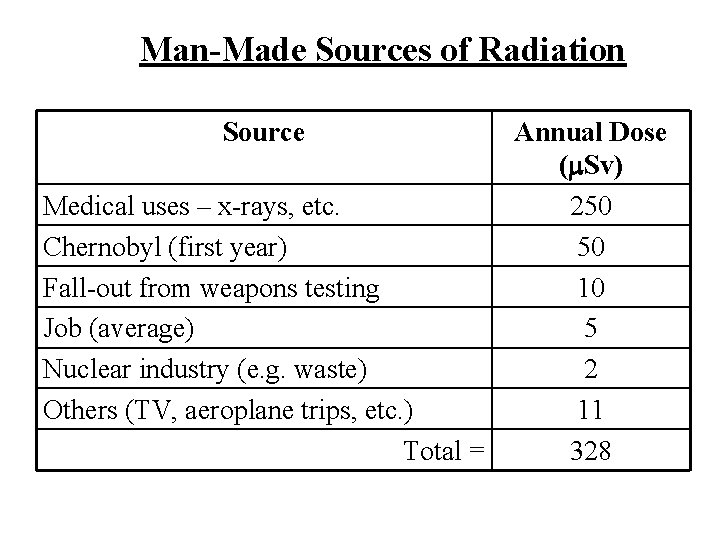
Man-Made Sources of Radiation Source Medical uses – x-rays, etc. Chernobyl (first year) Fall-out from weapons testing Job (average) Nuclear industry (e. g. waste) Others (TV, aeroplane trips, etc. ) Total = Annual Dose ( Sv) 250 50 10 5 2 11 328

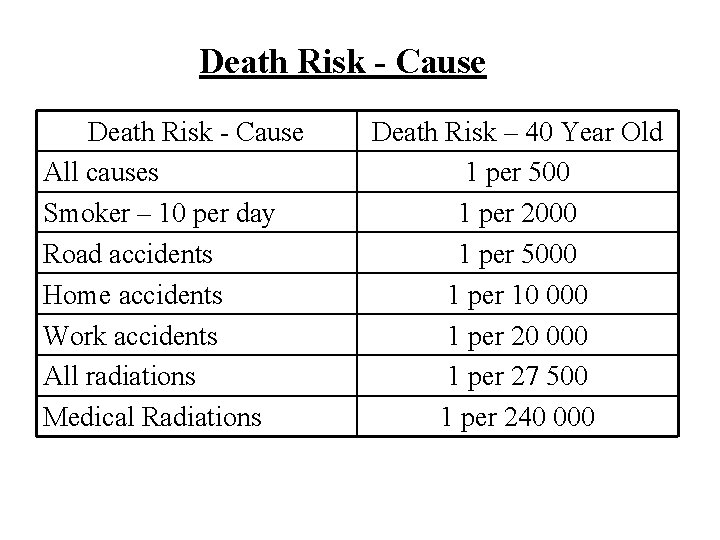
Death Risk - Cause All causes Smoker – 10 per day Road accidents Home accidents Work accidents All radiations Medical Radiations Death Risk – 40 Year Old 1 per 500 1 per 2000 1 per 5000 1 per 10 000 1 per 27 500 1 per 240 000

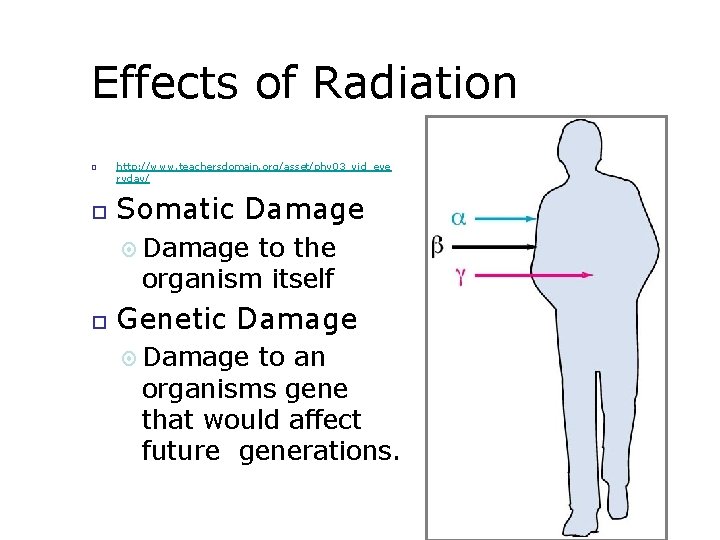
Effects of Radiation http: //www. teachersdomain. org/asset/phy 03_vid_eve ryday/ Somatic Damage to the organism itself Genetic Damage to an organisms gene that would affect future generations.

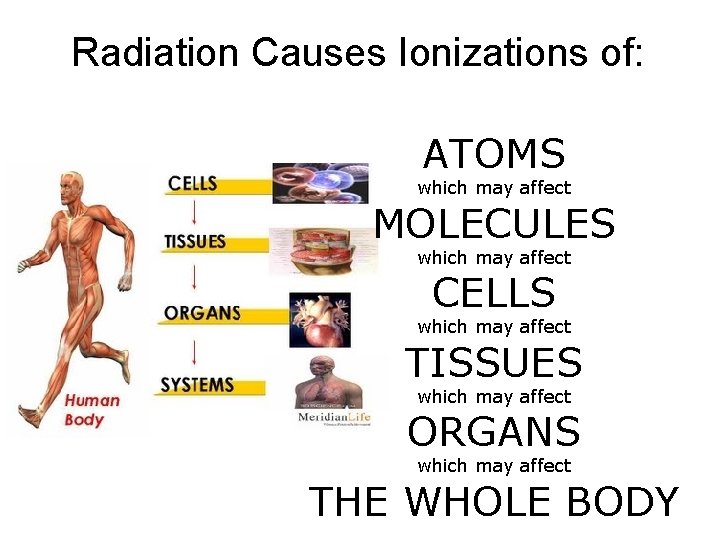
Radiation Causes Ionizations of: ATOMS which may affect MOLECULES which may affect CELLS which may affect TISSUES which may affect ORGANS which may affect THE WHOLE BODY


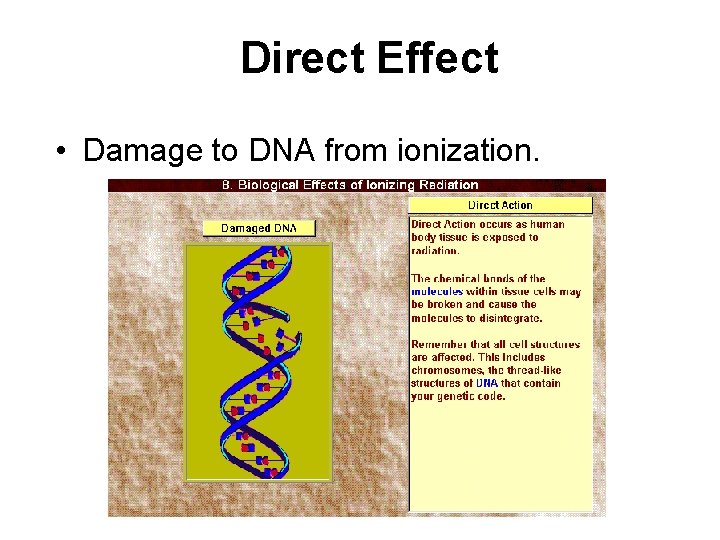
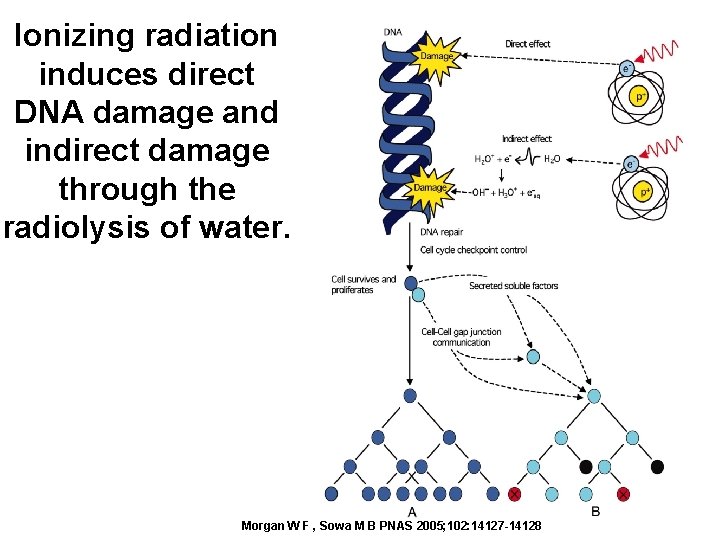
Direct Effect • Damage to DNA from ionization.

Indirect Effect • Radiation interacting with the water in a cell, rather then the DNA. – Could lead to the braking of bonds in the water molecule that could lead to toxic substances.

Ionizing radiation induces direct DNA damage and indirect damage through the radiolysis of water. Morgan W F , Sowa M B PNAS 2005; 102: 14127 -14128


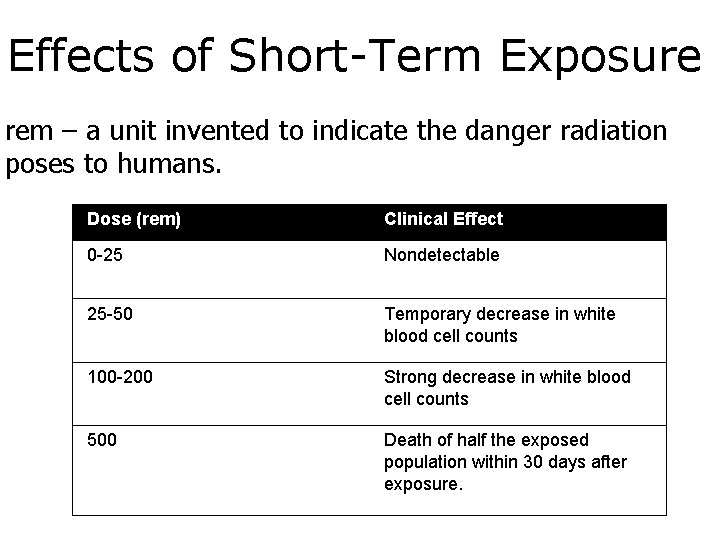
Effects of Short-Term Exposure rem – a unit invented to indicate the danger radiation poses to humans. Dose (rem) Clinical Effect 0 -25 Nondetectable 25 -50 Temporary decrease in white blood cell counts 100 -200 Strong decrease in white blood cell counts 500 Death of half the exposed population within 30 days after exposure.

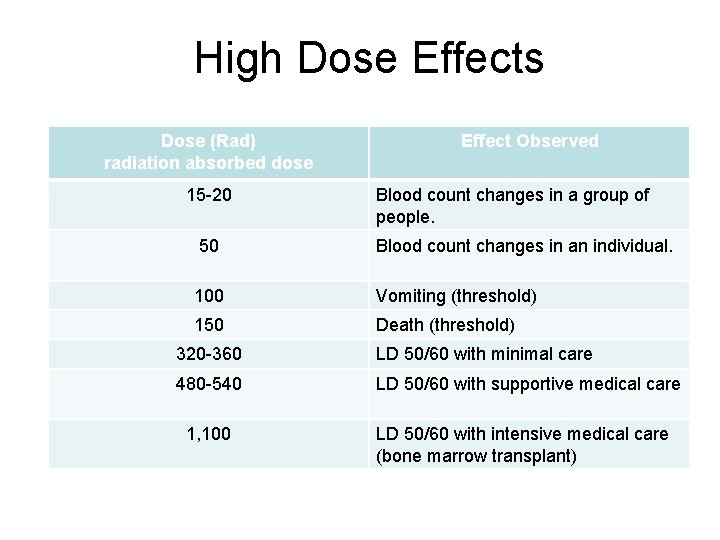
High Dose Effects Dose (Rad) radiation absorbed dose 15 -20 Effect Observed Blood count changes in a group of people. 50 Blood count changes in an individual. 100 Vomiting (threshold) 150 Death (threshold) 320 -360 LD 50/60 with minimal care 480 -540 LD 50/60 with supportive medical care 1, 100 LD 50/60 with intensive medical care (bone marrow transplant)


More than 2, 000 rad: Death is a certainty. At doses above 5, 000 rad, the central nervous system (brain and muscles) can no longer control the body functions, including breathing and blood circulation. Everything happens very quickly. Death occurs within days or hours. Nothing can be done, and medical care is for comfort only. 1, 000 to 2, 000 rad: The probability of death increases to 100% within one to two 2000 rad weeks. The initial symptoms appear immediately. A few days later, things get very bad, very quickly since the gastrointestinal system is destroyed. Once the GI system ceases to function, nothing can be done, and medical care is for comfort only. 150 to 1, 100 rad: Severe blood changes will be noted and symptoms appear immediately. Approximately two weeks later, some of those exposed may die. At 300 -500 rad, up to one half of the people exposed will die within 30 days without intensive medical attention. Death is due to the destruction of the blood forming organs. Without white blood cells, infection is likely. At the lower end of the dose range, isolation, antibiotics, and transfusions may provide the bone marrow with time to generate new blood cells, and full recovery is possible. At the upper end of the dose range, a bone marrow transplant may be required to produce new blood cells. 50 to 150 rad: Slight blood changes including temporary drop in production of new blood cells will be noted and likely symptoms of nausea, fatigue and vomiting for one or two days. 5 to 50 rad: Slight blood changes may be detected by medical evaluation Less than 5 rad: No immediate observable effects


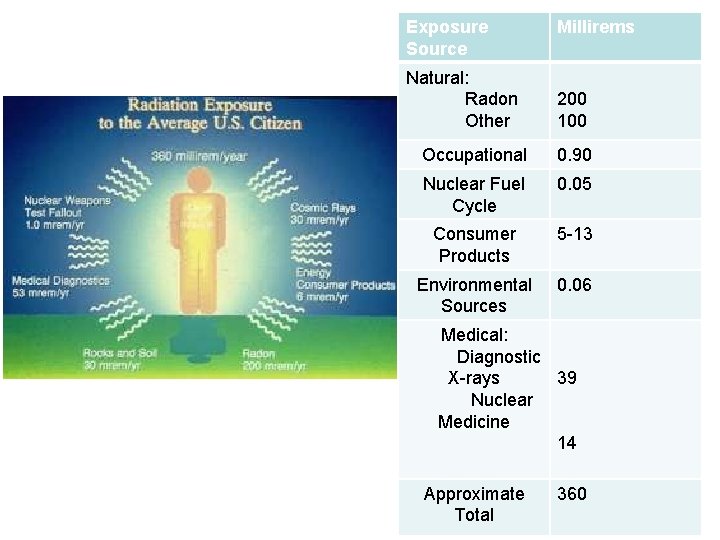
Exposure Source Natural: Radon Other Millirems 200 100 Occupational 0. 90 Nuclear Fuel Cycle 0. 05 Consumer Products 5 -13 Environmental Sources 0. 06 Medical: Diagnostic X-rays 39 Nuclear Medicine 14 Approximate Total 360

Industry q q Used to measure thickness of materials and to detect defects in metals and materials. Power space craft. Clean toxic pollutants. Improve food production.

Everyday Uses Smoke Detectors Rely on tiny radioactive source to sound the alarm when it sense smoke form a fire. Nonstick pans Treated with radiation to ensure that the coating sticks to the surface.

Everyday Uses Watches and Clocks Ceramic materials (for example, tiles, pottery) often contain elevated levels of naturally occurring uranium, thorium, and/or potassium. Glassware Modern watches and clocks sometimes use a small quantity of hydrogen-3 (tritium) or promethium-147 as a source of light. Older (for example, pre-1970) watches and clocks used radium-226 as a source of light. Antique glassware with a yellow or greenish color, can contain easily detectable quantities of uranium. Cosmetics, Hair Products, Contact Lenses Sterilized with radiation to remove irritants and allergens.

Everyday Uses Gas Lantern Mantles While it is less common than it once was, some brands of gas lantern mantles incorporate thorium-232. Antique Radioactive Curative Claims In the past, primarily 1920 through 1950, a wide range of radioactive products were sold as cure-alls. For example, radium-containing pills, pads, solutions, and devices designed to add radon to drinking water.

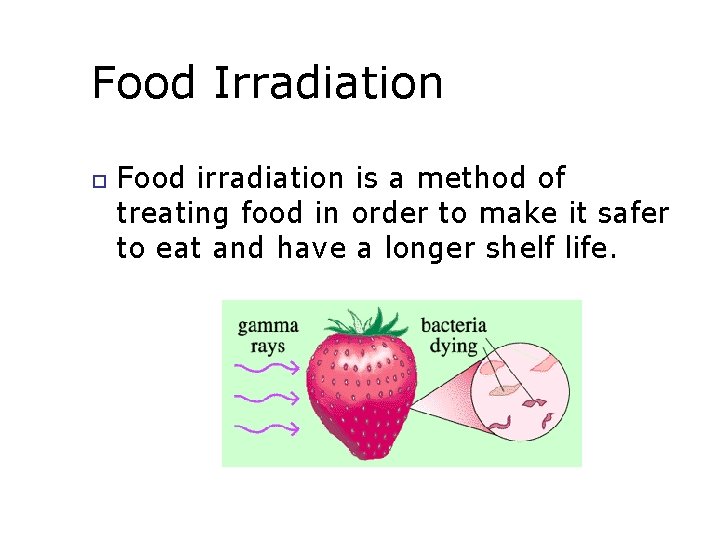
Food Irradiation Food irradiation is a method of treating food in order to make it safer to eat and have a longer shelf life.

Medical Uses

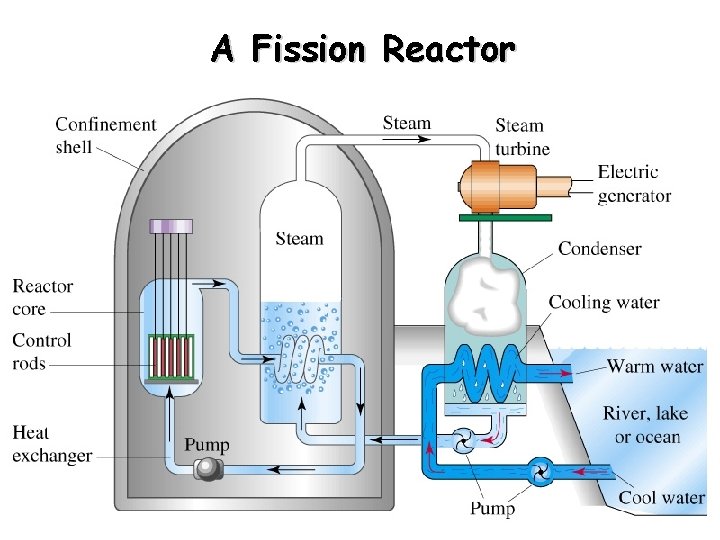
Nuclear Power Nuclear reactors are devices that control fission reactions producing new substances and energy. Steam is created from the heat (energy) produced. The steam turns the turbines to produce electric energy.

A Fission Reactor

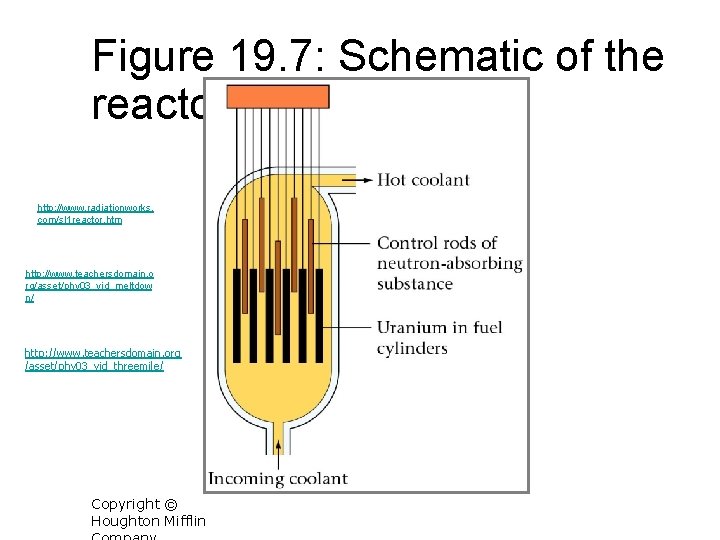
Figure 19. 7: Schematic of the reactor core. http: //www. radiationworks. com/sl 1 reactor. htm http: //www. teachersdomain. o rg/asset/phy 03_vid_meltdow n/ http: //www. teachersdomain. org /asset/phy 03_vid_threemile/ Copyright © Houghton Mifflin 19 -63

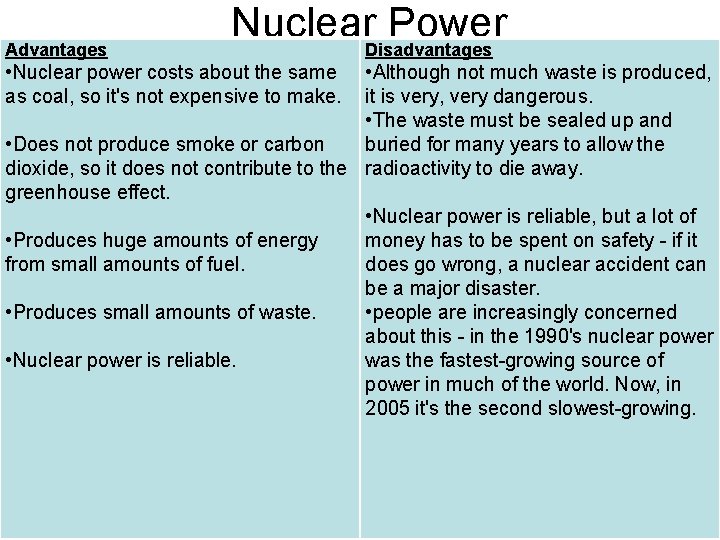
Advantages Nuclear. Disadvantages Power • Nuclear power costs about the same as coal, so it's not expensive to make. • Although not much waste is produced, it is very, very dangerous. • The waste must be sealed up and • Does not produce smoke or carbon buried for many years to allow the dioxide, so it does not contribute to the radioactivity to die away. greenhouse effect. • Nuclear power is reliable, but a lot of • Produces huge amounts of energy money has to be spent on safety - if it from small amounts of fuel. does go wrong, a nuclear accident can be a major disaster. • Produces small amounts of waste. • people are increasingly concerned about this - in the 1990's nuclear power • Nuclear power is reliable. was the fastest-growing source of power in much of the world. Now, in 2005 it's the second slowest-growing.

Weapons A nuclear weapon is an explosive device that derives its destructive force from nuclear reactions, either fission or a combination of fission and fusion.
 Managing economic exposure and translation exposure
Managing economic exposure and translation exposure Economic exposure
Economic exposure Managing economic exposure and translation exposure
Managing economic exposure and translation exposure Operating exposure adalah
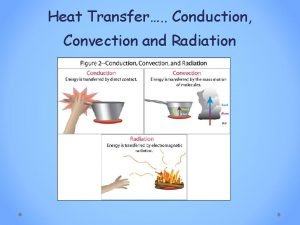
Operating exposure adalah Does heat travel in a straight line
Does heat travel in a straight line Phân độ lown ngoại tâm thu
Phân độ lown ngoại tâm thu Block nhĩ thất độ 2 mobitz 1
Block nhĩ thất độ 2 mobitz 1 Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Walmart thất bại ở nhật
Walmart thất bại ở nhật Tìm vết của mặt phẳng
Tìm vết của mặt phẳng Hãy nói thật ít để làm được nhiều
Hãy nói thật ít để làm được nhiều Tôn thất thuyết là ai
Tôn thất thuyết là ai Gây tê cơ vuông thắt lưng
Gây tê cơ vuông thắt lưng Sau thất bại ở hồ điển triệt
Sau thất bại ở hồ điển triệt Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Sound energy
Sound energy Sound is a longitudinal wave
Sound is a longitudinal wave Is a form of energy that travels in a straight line
Is a form of energy that travels in a straight line Mechanical wave
Mechanical wave What form of energy travels through space
What form of energy travels through space Lifewave antenna
Lifewave antenna Health and social component 3
Health and social component 3 Nature of exposure and risk
Nature of exposure and risk Measuring and managing economic exposure
Measuring and managing economic exposure Exposure attention comprehension acceptance and retention
Exposure attention comprehension acceptance and retention Measuring and managing operating exposure
Measuring and managing operating exposure Access to medical and exposure records
Access to medical and exposure records Enquiry letter to travel agency
Enquiry letter to travel agency Gulliver's travels test
Gulliver's travels test Gulliver's travels book 3 chapter 5
Gulliver's travels book 3 chapter 5 High heels and low heels in gulliver's travels
High heels and low heels in gulliver's travels Jonathan swift biography
Jonathan swift biography An object travels back and forth along a straight line
An object travels back and forth along a straight line Phantom tours and travels pvt ltd
Phantom tours and travels pvt ltd Distinguish between education and health services
Distinguish between education and health services Chapter 3 health wellness and health disparities
Chapter 3 health wellness and health disparities Health propaganda definition
Health propaganda definition Chapter 1 understanding health and wellness lesson 2
Chapter 1 understanding health and wellness lesson 2 Understanding health and wellness
Understanding health and wellness Section 2 describing energy (continued)
Section 2 describing energy (continued) Primary energy and secondary energy
Primary energy and secondary energy Disadvantages of conventional energy
Disadvantages of conventional energy Helmholtz free energy
Helmholtz free energy Renewable energy and energy efficiency partnership
Renewable energy and energy efficiency partnership Kinetic energy example
Kinetic energy example Types of potential energy
Types of potential energy Potential energy
Potential energy Kinetic energy and potential energy formula
Kinetic energy and potential energy formula Eroei
Eroei Whmis with ghs stands for
Whmis with ghs stands for Risk exposure index
Risk exposure index Unit 15:3 providing first aid for bleeding and wounds
Unit 15:3 providing first aid for bleeding and wounds Interoceptive exposure
Interoceptive exposure Slut award
Slut award The traditional three-exposure hypothesis is based on:
The traditional three-exposure hypothesis is based on: Pengendalian kebisingan
Pengendalian kebisingan Examples of altruism
Examples of altruism Ideally insurable risk
Ideally insurable risk Faulty
Faulty Garcia effect
Garcia effect Exposure therapy irvine
Exposure therapy irvine Exposure sheet animation
Exposure sheet animation Navigator photoshop
Navigator photoshop Negative exposure assessment
Negative exposure assessment