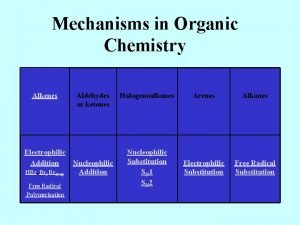
Organic chemistry Electrophilic addition rearrangement The mechanism of










- Slides: 21

Organic chemistry

Electrophilic addition: rearrangement The mechanism of electrophilic addition is consistent with the occurrence of rearrangements. If carbonium ions are intermediates in electrophilic addition, rearrangements are not only observed, but they occur according, to just the pattern that would be predicted.

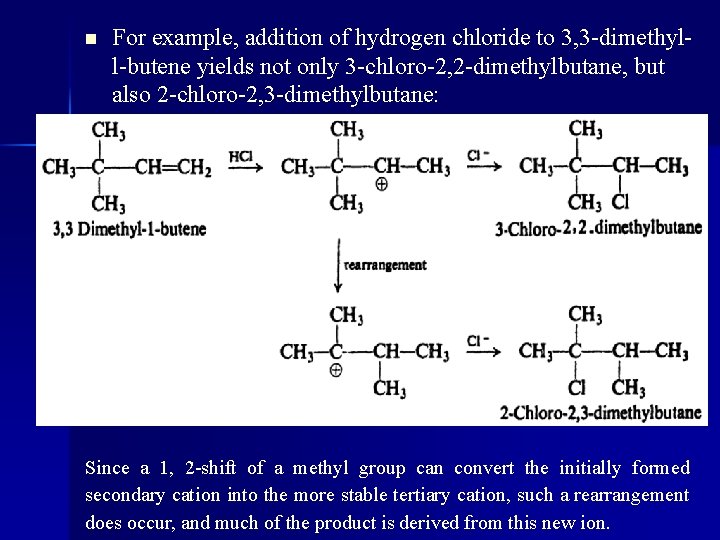
n For example, addition of hydrogen chloride to 3, 3 -dimethyll-butene yields not only 3 -chloro-2, 2 -dimethylbutane, but also 2 -chloro-2, 3 -dimethylbutane: Since a 1, 2 -shift of a methyl group can convert the initially formed secondary cation into the more stable tertiary cation, such a rearrangement does occur, and much of the product is derived from this new ion.

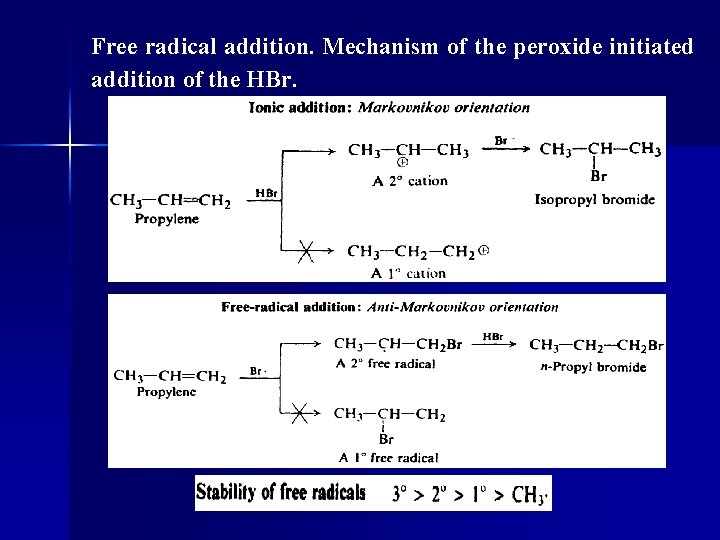
Free radical addition. Mechanism of the peroxide initiated addition of the HBr.

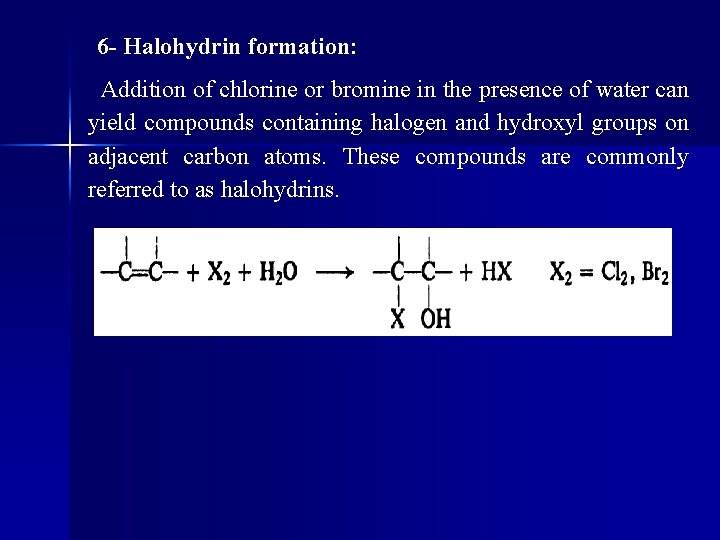
6 - Halohydrin formation: Addition of chlorine or bromine in the presence of water can yield compounds containing halogen and hydroxyl groups on adjacent carbon atoms. These compounds are commonly referred to as halohydrins.

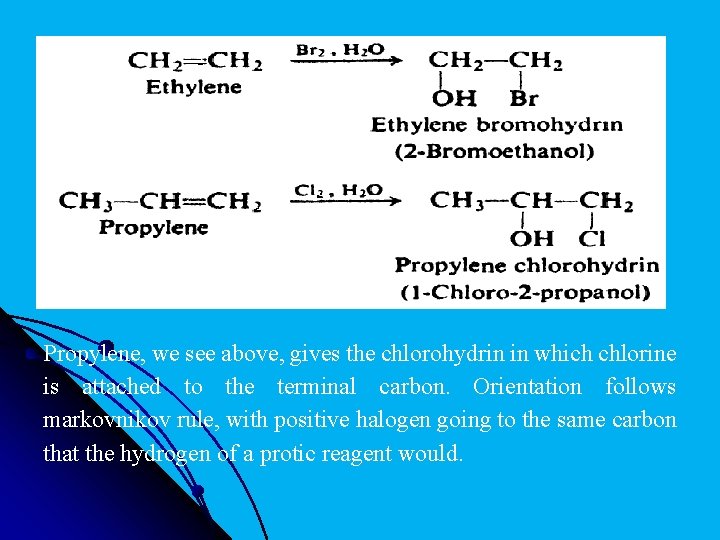
l Propylene, we see above, gives the chlorohydrin in which chlorine is attached to the terminal carbon. Orientation follows markovnikov rule, with positive halogen going to the same carbon that the hydrogen of a protic reagent would.

l 7 - Addition of alkenes. Dimerization: l Under proper conditions, isobutylene is converted by sulfuric or phosphoric acid into a mixture of two alkenes of molecular formula C 8 H 16. The two alkenes are isomers, and differ only in position of the double bond.

l Since the alkenes produced contain exactly twice the number of carbon and hydrogen atoms as the original isobutylene, they are known as (dimers (di = two, mer = part) of isobutylene, and the reaction is called dimerization.

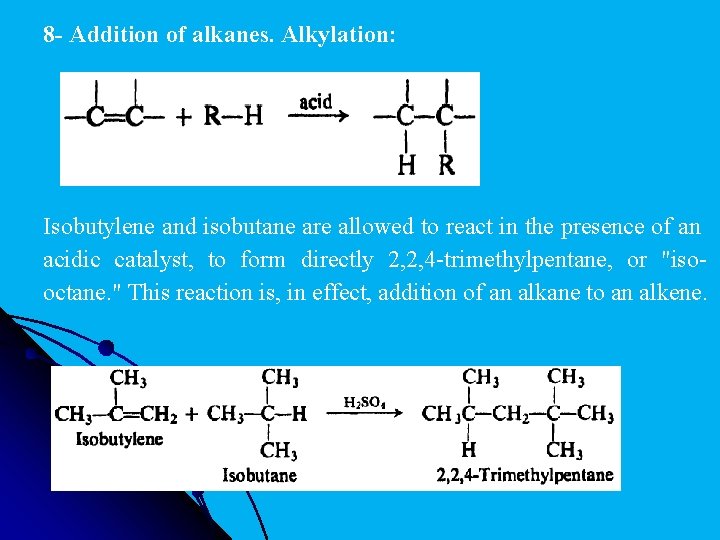
8 - Addition of alkanes. Alkylation: Isobutylene and isobutane are allowed to react in the presence of an acidic catalyst, to form directly 2, 2, 4 -trimethylpentane, or "isooctane. " This reaction is, in effect, addition of an alkane to an alkene.

l 9 - Oxymercuration-demercuration: Alkenes react with mercuric acetate in the presence of water to give hydroxyl-mercurial compounds which on reduction yield alcohols. The first stage, oxymercuration, involves addition to the carbon double bond of ─OH and ─Hg. OAc. Then, in demercuration the ─Hg. OAc is replaced by ─H. Oxymercuration-demercuration gives alcohols corresponding to markovnikov addition of water to the carbon─carbon double bond.

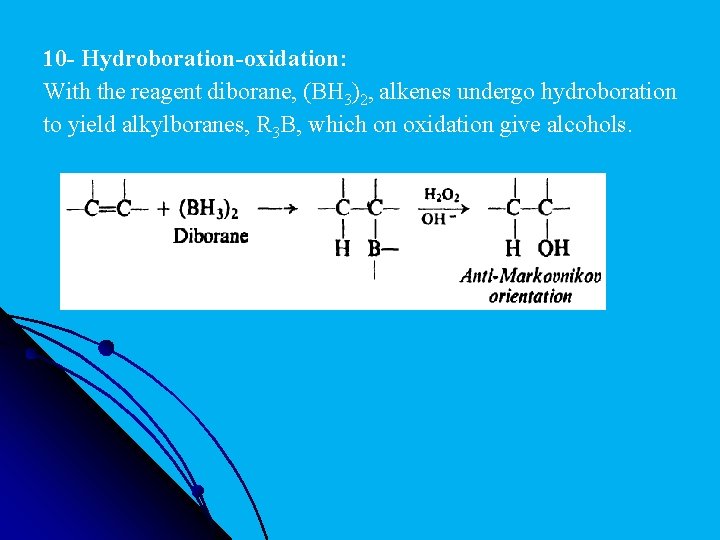
10 - Hydroboration-oxidation: With the reagent diborane, (BH 3)2, alkenes undergo hydroboration to yield alkylboranes, R 3 B, which on oxidation give alcohols.

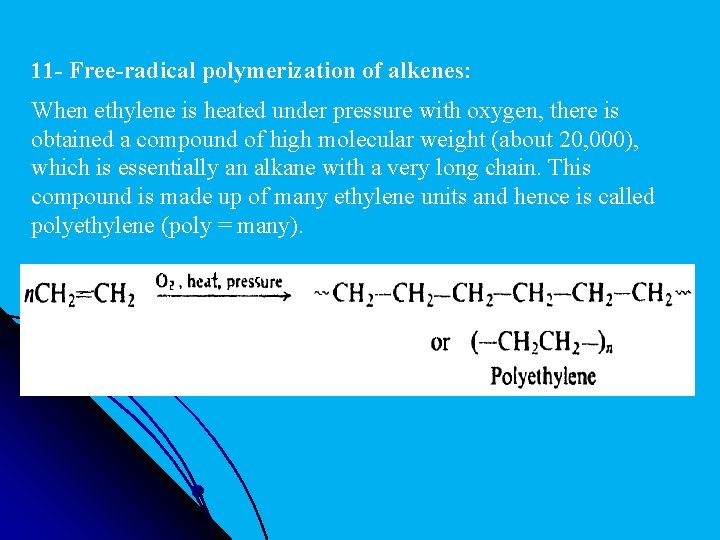
11 - Free-radical polymerization of alkenes: When ethylene is heated under pressure with oxygen, there is obtained a compound of high molecular weight (about 20, 000), which is essentially an alkane with a very long chain. This compound is made up of many ethylene units and hence is called polyethylene (poly = many).

The formation of polyethylene is a simple example of the process called polymerization: the joining together of many small molecules to make very large molecules. The compound composed of these very large molecules is called a polymer (Greek: poly + meros, many parts). The simple compounds from which polymers are made are called monomers (mono= one). Polymerization of substituted ethylenes yields compounds whose structures contain the long chain of polyethylene, with substituents attached at more or less regular intervals.

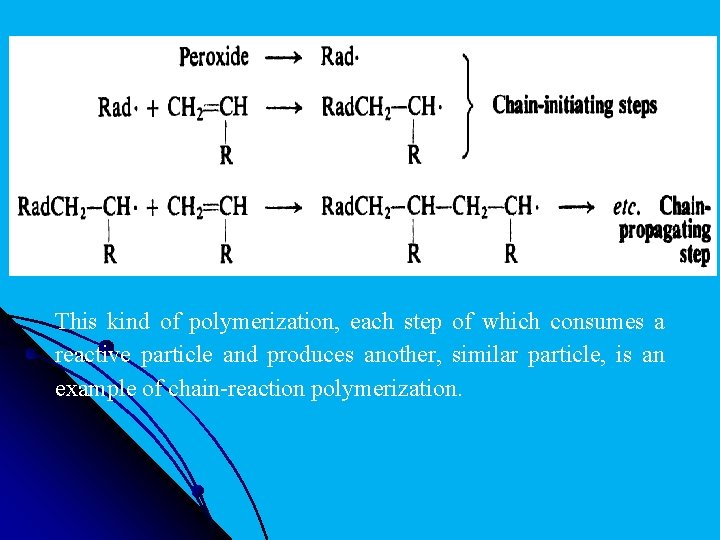
l Polymerization requires the presence of a small amount of an initiator. Among the commonest of these initiators are peroxides, which function by breaking down to form a free radical. This radical adds to a molecule of alkene, and in doing so generates another free radical. This radical adds to another molecule of alkene to generate a still . larger radical, which in turn adds to another molecule of alkene, and so on. Eventually the chain is terminated by steps, such as union of two radicals that consume but do not generate radicals.

l This kind of polymerization, each step of which consumes a reactive particle and produces another, similar particle, is an example of chain-reaction polymerization.

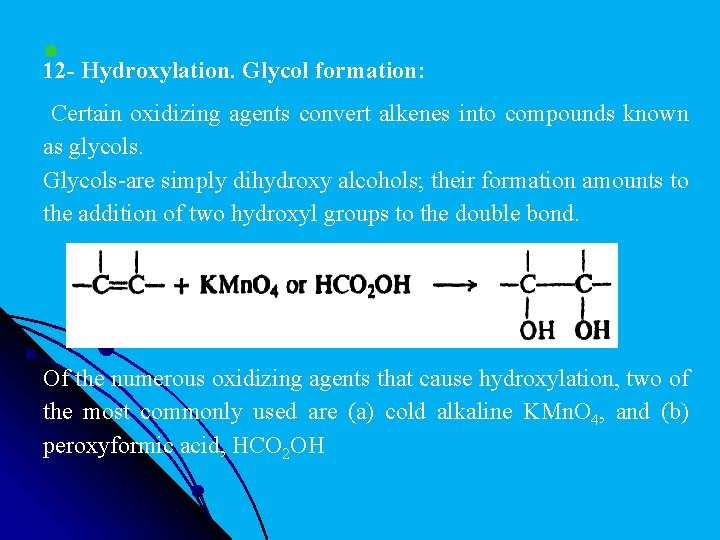
l 12 - Hydroxylation. Glycol formation: Certain oxidizing agents convert alkenes into compounds known as glycols. Glycols-are simply dihydroxy alcohols; their formation amounts to the addition of two hydroxyl groups to the double bond. Of the numerous oxidizing agents that cause hydroxylation, two of the most commonly used are (a) cold alkaline KMn. O 4, and (b) peroxyformic acid, HCO 2 OH

l

13 - Substitution Reactions Halogenations. Allylic substitution. We know that alkenes undergo addition of halogen at low temperatures and in the absence of light, and generally in the liquid phase: conditions that favor ionic reactions, or at least do not aid formation of radicals.

l If we wish to direct the attack of halogen to the alkyl portion of an alkene molecule, then, we choose conditions that are favorable for the free-radical reaction and unfavorable for the ionic reaction, at a temperature of 500 -600 o. C, a mixture of gaseous propylene and chlorine yields chiefly the substitution product, 3 -chloro-l-propene, known as allyl chloride (CH 2=CH─CH 2─ = allyl). Bromine behaves similarly.

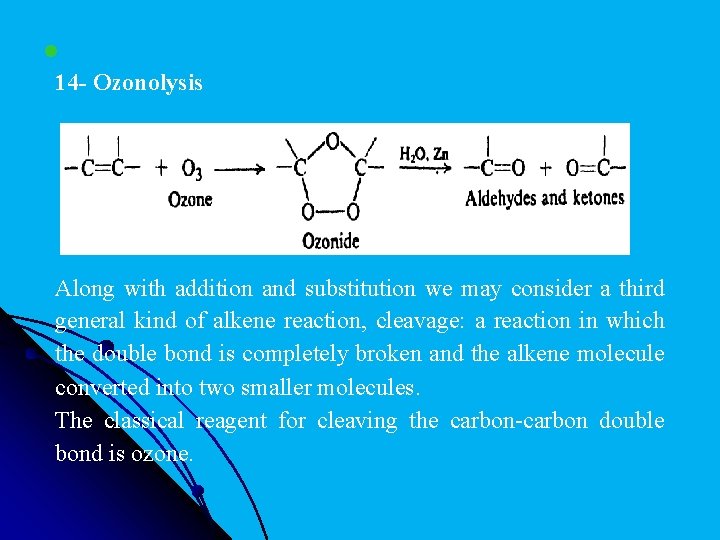
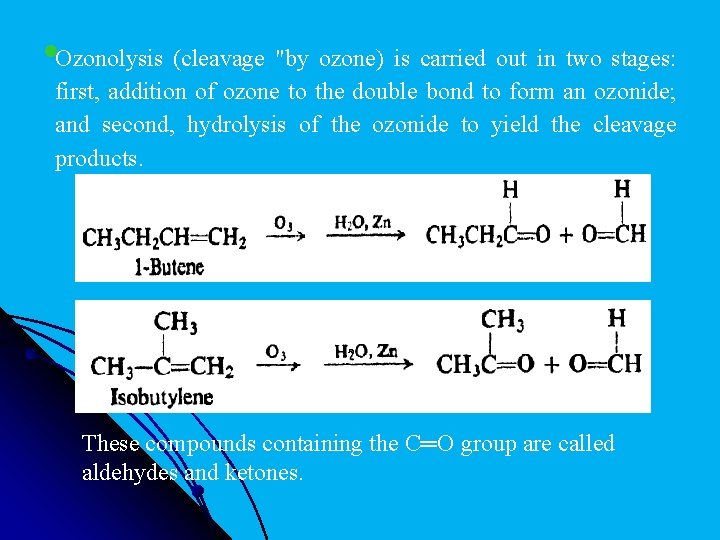
l 14 - Ozonolysis Along with addition and substitution we may consider a third general kind of alkene reaction, cleavage: a reaction in which the double bond is completely broken and the alkene molecule converted into two smaller molecules. The classical reagent for cleaving the carbon-carbon double bond is ozone.

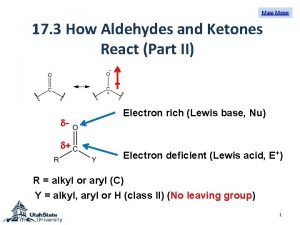
l. Ozonolysis (cleavage "by ozone) is carried out in two stages: first, addition of ozone to the double bond to form an ozonide; and second, hydrolysis of the ozonide to yield the cleavage products. These compounds containing the C═O group are called aldehydes and ketones.
 Ethene hbr
Ethene hbr Friedel crafts acylation of benzene
Friedel crafts acylation of benzene Functional groups ib chemistry
Functional groups ib chemistry Inorganic chemistry vs organic chemistry
Inorganic chemistry vs organic chemistry When do we use sine and cosine rule
When do we use sine and cosine rule Demjanov rearrangement
Demjanov rearrangement Alkene can be formed from carbonium ion by
Alkene can be formed from carbonium ion by Chemical reactions rearranging atoms worksheet answers
Chemical reactions rearranging atoms worksheet answers Wlr3 openreach
Wlr3 openreach Migratory aptitude is greater for
Migratory aptitude is greater for Arrange the sentences in correct order
Arrange the sentences in correct order Semipinacol rearrangement
Semipinacol rearrangement Diels alder reaction
Diels alder reaction Mclafferty rearrangement
Mclafferty rearrangement Enamine hydrolysis
Enamine hydrolysis Feedback inhibitor
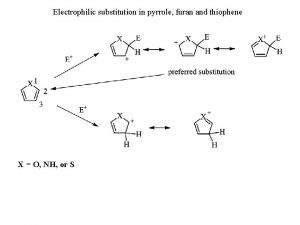
Feedback inhibitor Furan electrophilic substitution
Furan electrophilic substitution Identify nucleophile and electrophile
Identify nucleophile and electrophile Electrophilic carbonyl
Electrophilic carbonyl Senyawa pirol
Senyawa pirol Quinoline electrophilic substitution
Quinoline electrophilic substitution Halogenations
Halogenations