Nuclear Imaging in Nephrology Wong Koh Wei References









- Slides: 74

Nuclear Imaging in Nephrology Wong Koh Wei

References: • • • Oxford Textbook of Nephrology Diagnostic Tests in Nephrology. Edited by John Bradley, Ken Smith Clinical Applications of Renal Scintigraphy. Andrew Taylor et al. AJR 1995; 164: 3141 Radionuclide Investigations of the Urinary Tract in the Era of Multimodality Imaging. Ariane Boubaker et al. The Journal of Nuclear Medicine. Vol. 47 (11), November 2006 Bristish Nuclear Medicine Society Guidelines Isotopic Scan for Diagnosis of Renal Disease. Yusuke Inoue et al. Saudi Journal of Kidney Diseases and Transplantation. 15(3) 2004; 257 -64

Introduction • Uses pharmaceuticals – labels with radionuclides (radiophamaceuticals) - radiotracers • Administers these to patients – radiation emitted is detected • Formation of an image using a gamma camera or positron emission tomography – Radionuclide imaging / nuclear scintigraphy • Shows the physiological function of the system being investigated

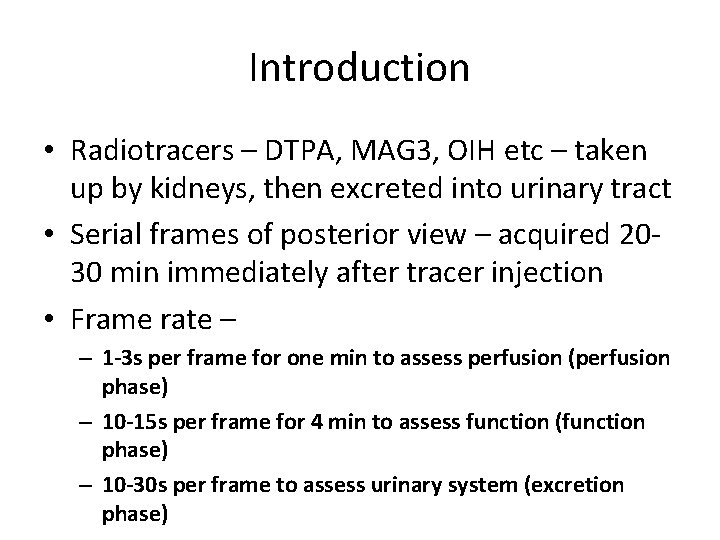
Introduction • Radiotracers – DTPA, MAG 3, OIH etc – taken up by kidneys, then excreted into urinary tract • Serial frames of posterior view – acquired 2030 min immediately after tracer injection • Frame rate – – 1 -3 s per frame for one min to assess perfusion (perfusion phase) – 10 -15 s per frame for 4 min to assess function (function phase) – 10 -30 s per frame to assess urinary system (excretion phase)

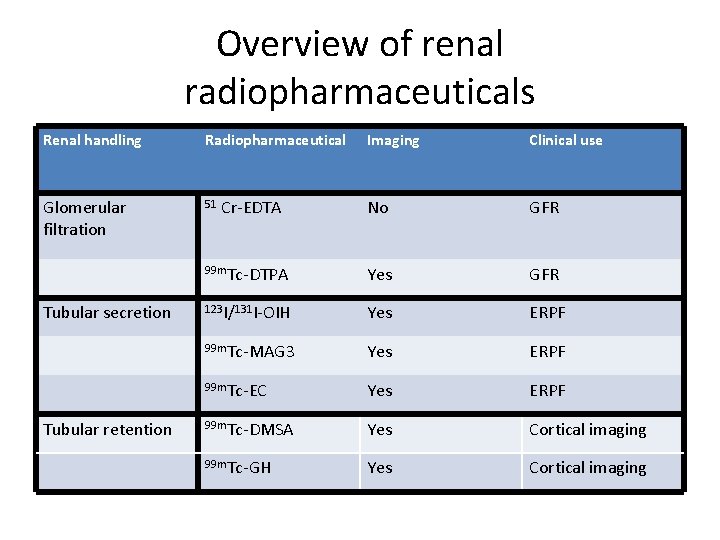
Overview of renal radiopharmaceuticals Renal handling Radiopharmaceutical Glomerular filtration 51 Tubular secretion Tubular retention Imaging Clinical use No GFR 99 m. Tc-DTPA Yes GFR 123 I/131 I-OIH Yes ERPF 99 m. Tc-MAG 3 Yes ERPF 99 m. Tc-EC Yes ERPF 99 m. Tc-DMSA Yes Cortical imaging 99 m. Tc-GH Yes Cortical imaging Cr-EDTA

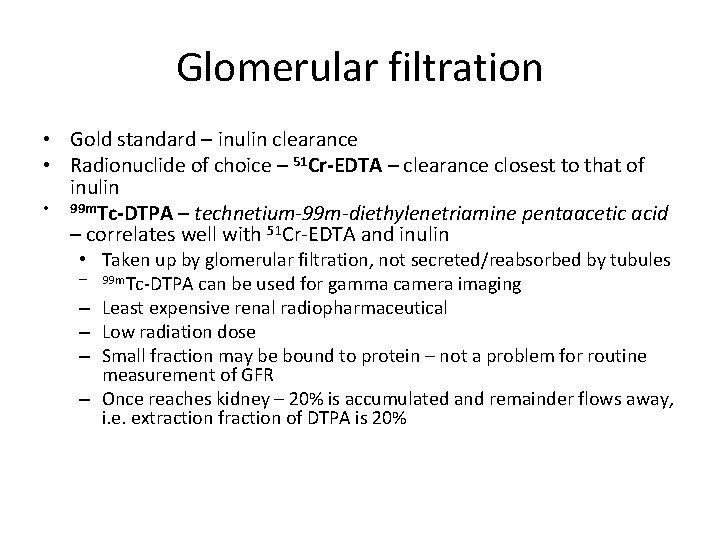
Glomerular filtration • Gold standard – inulin clearance • Radionuclide of choice – 51 Cr-EDTA – clearance closest to that of inulin • 99 m. Tc-DTPA – technetium-99 m-diethylenetriamine pentaacetic acid – correlates well with 51 Cr-EDTA and inulin • Taken up by glomerular filtration, not secreted/reabsorbed by tubules – 99 m. Tc-DTPA can be used for gamma camera imaging – Least expensive renal radiopharmaceutical – Low radiation dose – Small fraction may be bound to protein – not a problem for routine measurement of GFR – Once reaches kidney – 20% is accumulated and remainder flows away, i. e. extraction fraction of DTPA is 20%

Tubular secretion (1) • p-Aminohippuric acid (PAH) – gold standard for measurement of tubular cell function and its clearance – effective renal plasma flow (ERPF) • 123 I-OIH and 131 I-OIH – cleared by tubular secretion (small fraction by glomerular filtration) – Clearance rate – approx 500 -600 ml/min – Extraction of 131 I-OIH depends on renal plasma flow and extraction from plasma (proximal tubules) – 123 I-OIH – better imaging qualities, more expensi

Tubular secretion (2) • 99 m. Tc-MAG 3 – highly protein-bound, cleared mainly by proximal tubules – High extraction fraction (50%) – better scintigraphic images • 99 m. Tc-I, I and d, d-ethylenedicysteine (EC) – better than 99 m. Tc-MAG 3

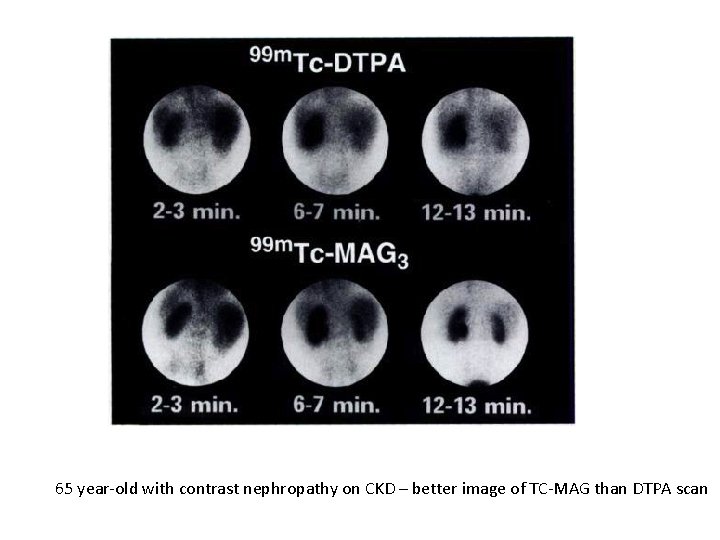
65 year-old with contrast nephropathy on CKD – better image of TC-MAG than DTPA scan

Tubular retention • 99 m. Tc-dimercaptosuccinic acid (DMSA) – Excellent cortical imaging agent – Concentrates largely in renal cortex (lesser in liver) – Strongly bound to proteins – At 2 h post-injection : 50% retained in kidneys, no visualization of urinary collecting system • 99 m. Tc-glucoheptonate (GH) • Filtered by glomerulus and bound by tubules • Highly protein-bound – glomerular filtration partial

DMSA • IV injection – bound to proximal tubules • Indications: – Assessment of kidneys in acute phase of UTI (acute pyelonephritis) – Assessment of kidneys in late phase of UTI – detection of scar – Assessment of horseshoe, solitary or ectopic kidney – Localisation of poor or very poorly functioning kidney – Assessment of renal function in the presence of an abdominal mass BNMS guidelines

DMSA • Pitfalls – Acute and chronic pyelonephritis cannot be distinguished on the cortical scan. If a defect is present 6 months after the last UTI then this is a scar – A recent UTI may cause temporary reduced uptake / focal defect and a followup DMSA scan should be undertaken – The diagnosis of renal scars is difficult in the infant under 3 -6 months of age because of renal immaturity. If appropriate the scan should be delayed • Controversies – To obtain the highest resolution, some centres recommend the use of a pin hole collimato, however many institutions obtain high resolution images with clear definition between cortex and medulla without the use of pin-hole collimation – Currently there is no evidence to support the routine use of SPECT in children to delineate focal defects BNMS guidelines

Analytic methods • Various methods • Single-sample methods and camera-based methods suitable for busy clinical practice • Single-sample – single venous blood sampling after tracer injection, and plasma radioactivity is measured – Plasma activity and injection dose substituted in a predefined, empiric equation = renal clearance

Analytic methods • Camera-based methods – renal uptake early after tracer injection reflects renal function – Calculate renal function from imaging data without blood sampling – A region of interest (ROI) is drawn for each kidney to estimate activity, then do a background subtraction, then attenuation correction for depth of each kidney, then normalized to the injection dose – finally substituted to an empiric equation = renal clearance – Less accurate than single-sample method – Can assess right and left kidney function separately – split renal function – Can do combined single-sample and camera-based methods Isotopic Scan for Diagnosis of Renal Disease. Yusuke Inoue et al. Saudi Journal of Kidney Diseases and Transplantation. 15(3) 2004; 257 -64

Analytic methods • Renogram curves – overview of the time course of renal radioactivity – Generated by setting an ROI for each kidney – Radioactivity in a kidney derives from renal parenchyma, upper tract and overlapping extrarenal tissues – Do background subtraction - renal parenchyma and upper tract – In normal subjects – a renogram demonstrates rapid increase during perfusion and function phases, then rapid decline during excretion phases – Hypofunction flattens the slopes during the function and excretion phases – Obstruction causes delayed excretion – Cannot discriminate between retention in renal parenchyma and that in urinary tract – visual assessment of scintigraphy images needed…

Clinical uses: • • Renal clearance measurements Obstruction Infection Renal artery stenosis Renal transplant Tumour/mass Trauma

Clinical uses: • • • Renal clearance measurements Obstruction Infection Renal artery stenosis Renal transplant

Renal clearance measurements • GFR and estimation of split renal function • Renal Plasma Flow

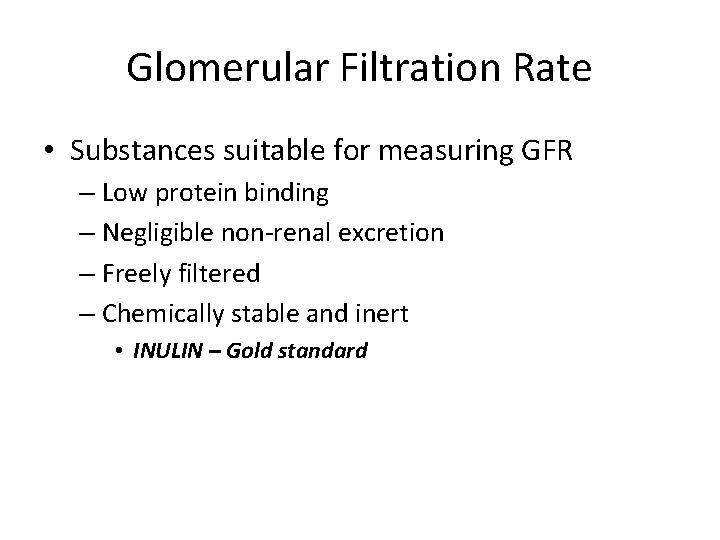
Glomerular Filtration Rate • Substances suitable for measuring GFR – Low protein binding – Negligible non-renal excretion – Freely filtered – Chemically stable and inert • INULIN – Gold standard

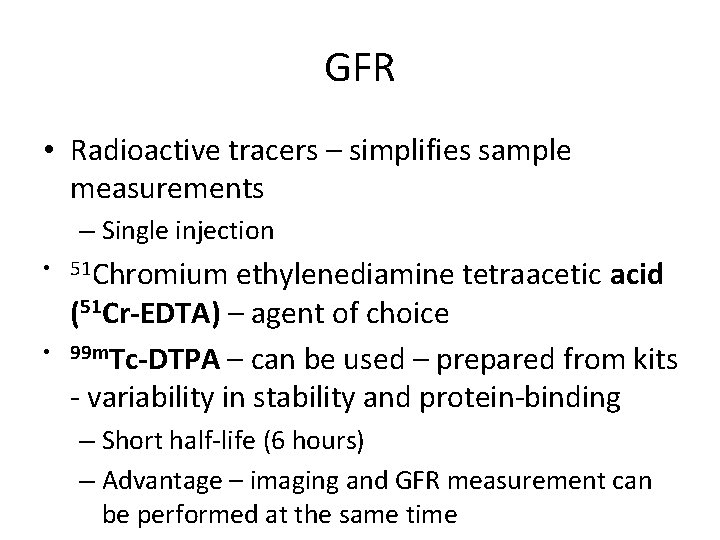
GFR • Radioactive tracers – simplifies sample measurements – Single injection • 51 Chromium • ethylenediamine tetraacetic acid (51 Cr-EDTA) – agent of choice 99 m. Tc-DTPA – can be used – prepared from kits - variability in stability and protein-binding – Short half-life (6 hours) – Advantage – imaging and GFR measurement can be performed at the same time

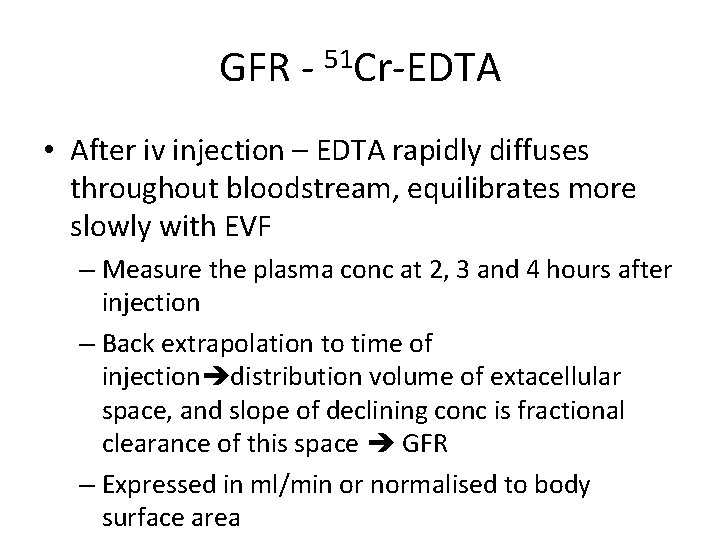
GFR - 51 Cr-EDTA • After iv injection – EDTA rapidly diffuses throughout bloodstream, equilibrates more slowly with EVF – Measure the plasma conc at 2, 3 and 4 hours after injection – Back extrapolation to time of injection distribution volume of extacellular space, and slope of declining conc is fractional clearance of this space GFR – Expressed in ml/min or normalised to body surface area

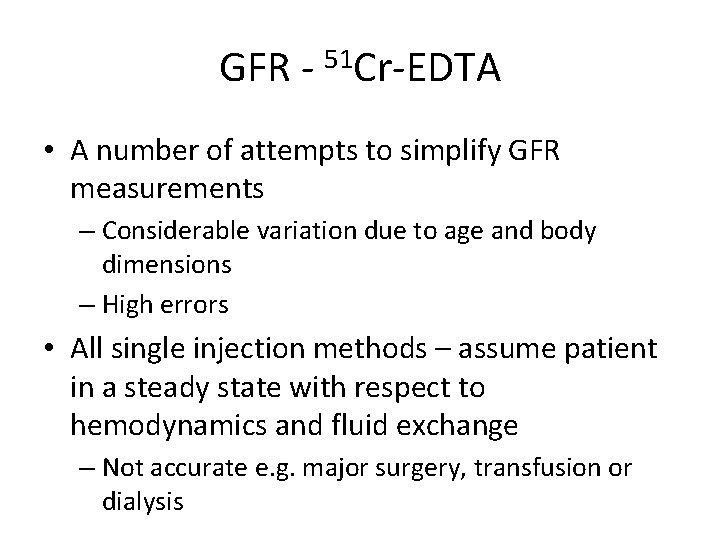
GFR - 51 Cr-EDTA • A number of attempts to simplify GFR measurements – Considerable variation due to age and body dimensions – High errors • All single injection methods – assume patient in a steady state with respect to hemodynamics and fluid exchange – Not accurate e. g. major surgery, transfusion or dialysis

EDTA • GFR - plasma clearance curve - which required multiple blood samples to be taken over a period of several hours • Slope-intercept method – after numerous simplification number of sample : 1 (recommended by the Radionuclides in Nephrology Committee on Renal Clearance (Blaufox et al, 1996) ) • It is recommended that the plasma clearance of EDTA from venous samples be taken as the standard measure of GFR • DTPA does have some technical advantages over EDTA but normal ranges are not so well established • Small systematic differences have been observed between GFR measurements obtained from EDTA and DTPA (Rehling et al, 1984, Fleming et al, 1991, Biggi et al, 1995)

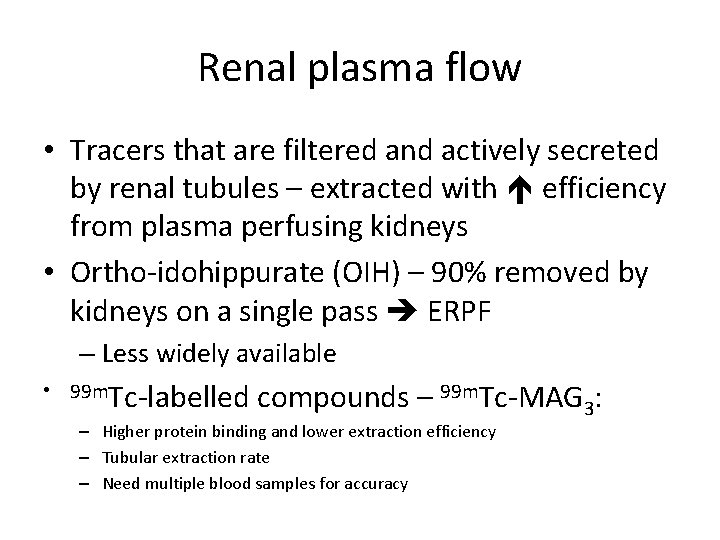
Renal plasma flow • Tracers that are filtered and actively secreted by renal tubules – extracted with efficiency from plasma perfusing kidneys • Ortho-idohippurate (OIH) – 90% removed by kidneys on a single pass ERPF – Less widely available • 99 m. Tc-labelled compounds – 99 m. Tc-MAG 3: – Higher protein binding and lower extraction efficiency – Tubular extraction rate – Need multiple blood samples for accuracy

Clinical uses: • • • Renal clearance measurements Obstruction Infection Renal artery stenosis Renal transplant

Obstruction/Urinary tract dilatation • Diuretic renography – diagnostic work-up of upper tract dilatation, and follow-up of patients with hydronephrosis • Method of choice – to differentiate a dilated unobstructed urinary system from a true stenosis – Can also assess urine flow and renal function

In children: • Sedation should be avoided – may interfere with bladder voiding • Increased radiation exposure to bladder mucosa – concern • Receiving the child with parents in a dedicated, calm environment • If needed – local guidelines for sedation – Short inhalation of equimolar mixture of nitrous oxide and oxygen J Nucl Med 2006; 47: 1819– 1836

Diuretic renography • 99 m. Tc-mercaptoacetyltriglycine(MAG 3) and 123 I-orthoiodohippurate (OIH) are preferred – higher renal extraction ratio and rapid plasma clearance, especially in infants and young children and in patients with impaired renal function • Use of frusemide 1 mg/kg (max 20 mg in children, 40 mg adults » Recommended by Society of Nuclear Medicine » European Nuclear Medicine Association

Diuretic renography • Timing of frusemide administration – 20 min or more after tracer injection – max distension of renal pelvis or ureter can be visually assessed (F+20) – 15 min before tracer injection – diuretic response of kidney is maximal (F-20) • Diuretic response is evaluated by visual and quantitative interpretation of the dynamic acquisition • Postmicturition images are mandatory because a full bladder may delay urinary flow even in an unobstructed system

Diuretic renography • The role of bladder catheterization – debated not recommended in clinical routine practice – Older children and adults - renography is performed after bladder emptying – Non–toilet-trained children, spontaneous micturition is usually observed during the acquisition • Adequate functioning of the affected kidney (GFR > 15 m. L/min) and adequate hydration are major determinants of the response to frusemide

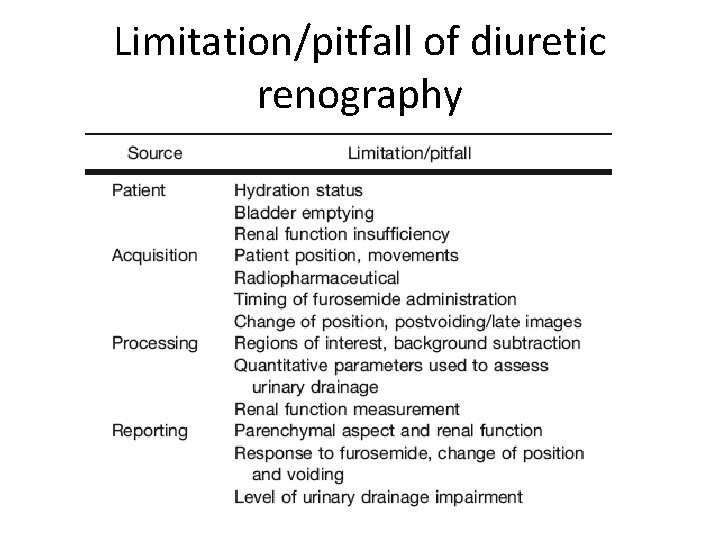
Limitation/pitfall of diuretic renography

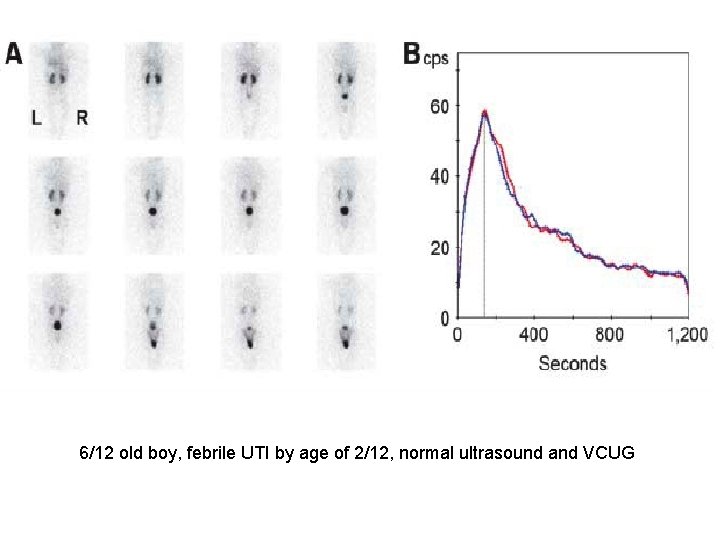
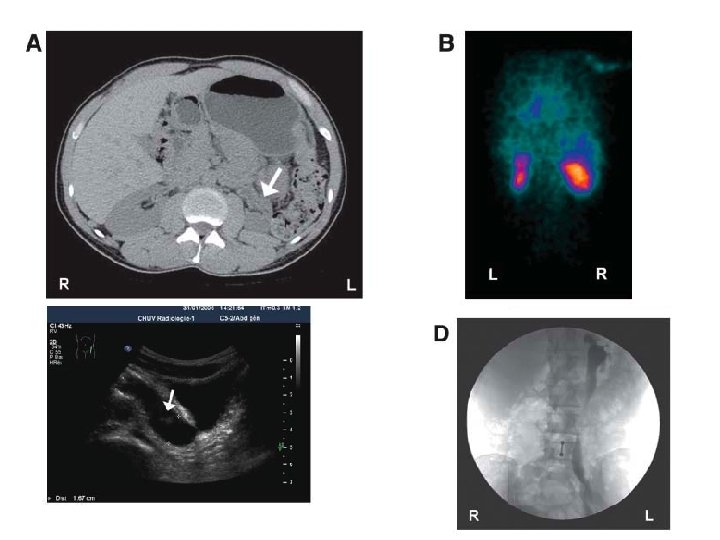
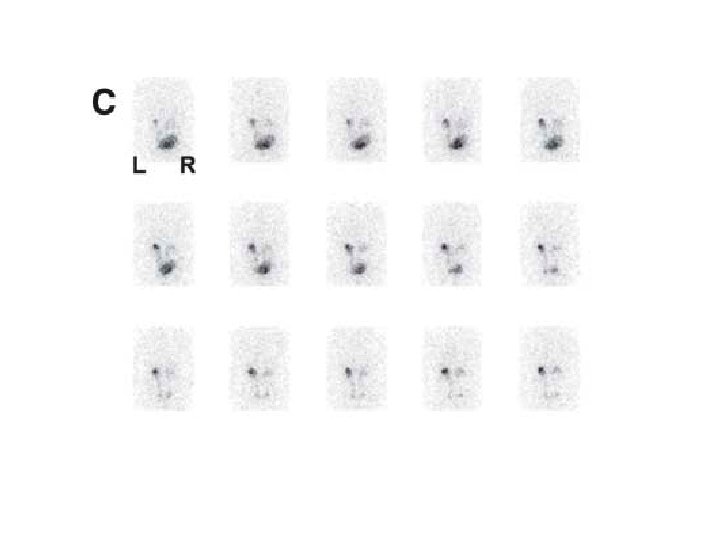
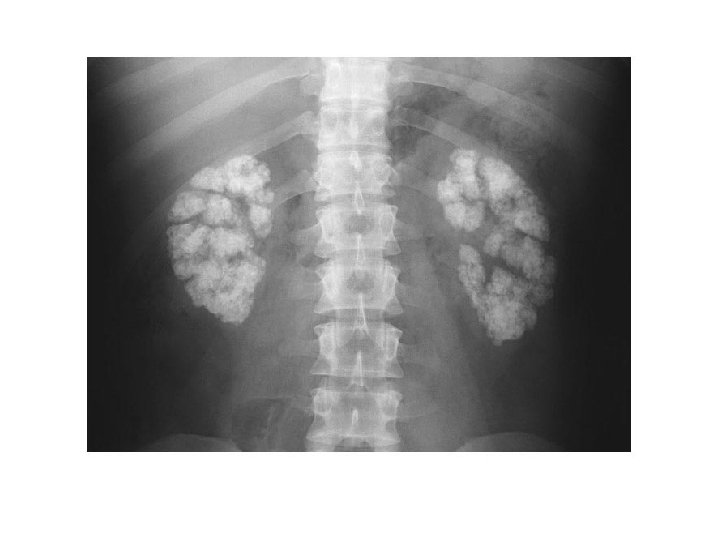
6/12 old boy, febrile UTI by age of 2/12, normal ultrasound and VCUG

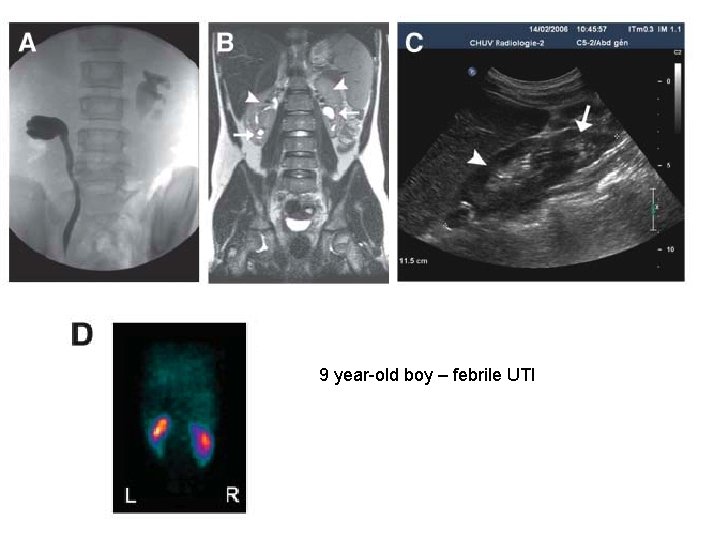
9 year-old boy – febrile UTI

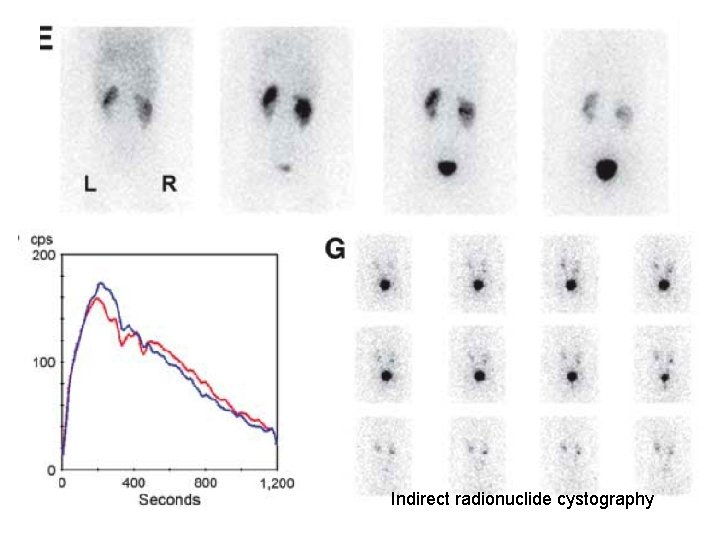
Indirect radionuclide cystography

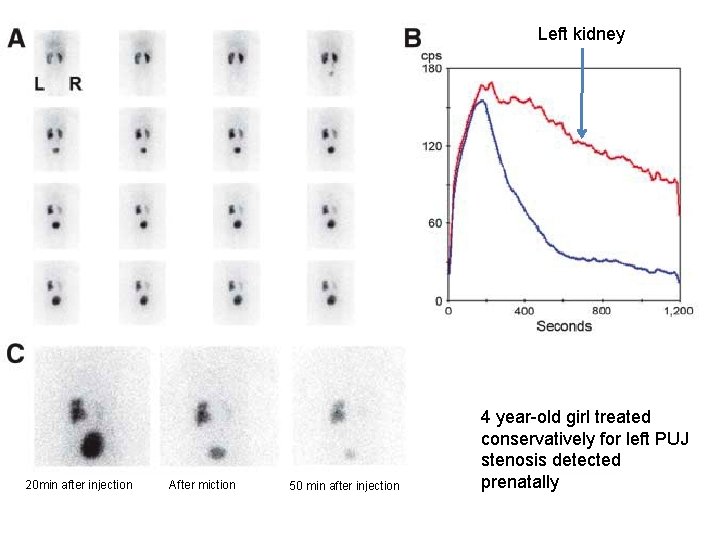
Left kidney 20 min after injection After miction 50 min after injection 4 year-old girl treated conservatively for left PUJ stenosis detected prenatally


Clinical uses: • • • Renal clearance measurements Obstruction Infection Renal artery stenosis Renal transplant

UTI • Frequent in children • Affect girls 2 x > boys • 80% first infections diagnosed during first 2 years of life • 5% in infants and young children – fever of unexplained origin • Diagnosis – urine culture • Risk of renal damage – delay of diagnosis/treatment, number of UTIs

UTI • The Subcommittee on Urinary Tract Infection of the American Academy of Pediatric Committee on Quality Improvement recommended imaging (mainly sonography, VCUG, or radionuclide cystography) of the urinary tract in children younger than 2 y old but considered the role of cortical renal scintigraphy still to be unclear despite its recognized high sensitivity

Cortical scintigraphy • Scintigraphy with 99 m. Tc-dimercaptosuccinic acid (DMSA) – simple and non-invasive – Static imaging 2 -4 hours after iv injection – Delayed or post-frusemide images in hydronephrosis • The sensitivity of 99 m. Tc-DMSA for the detection of parenchymal defects due to infection - from 80% to 100% – does not allow differentiation of acute pyelonephritis from renal scars

Cortical scintigraphy • Abnormal findings on cortical scintigraphy are found in 52%– 78% of children during acute pyelonephritis, and the risk that a renal scar will develop can reach 60% • The role of cortical scintigraphy is still largely debated in acute pyelonephritis but is widely accepted in the detection of renal scars • 99 m. Tc-DMSA scintigraphy is the reference method for detecting renal sequelae after UTI, is more sensitive than sonography, and should be performed no sooner than 6 mo after the last documented UTI • Also used to detect scars in VUR

Radionuclide cystography • Direct radionuclide cystograhy – A radiologic-VCUG alternative - lower radiation burden – As invasive as VCUG – bladder catheterization – More sensitive – acquisition is continuous in both filling and voiding phase • Indirect radionuclide cystography – Performed after conventional renography with 99 m. Tc-MAG 3 or 123 I-OIH – high excretion rate – No need catheterization – Less sensitive and specific than VCUG/direct cystography



Clinical uses: • • • Renal clearance measurements Obstruction Infection Renal artery stenosis Renal transplant

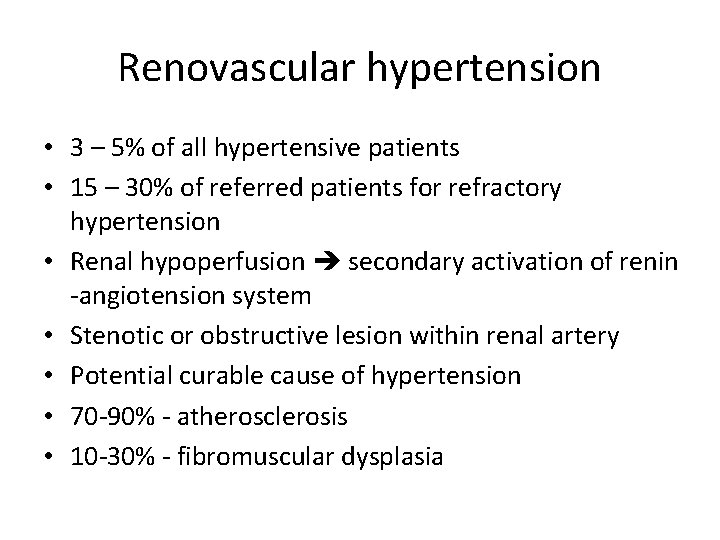
Renovascular hypertension • 3 – 5% of all hypertensive patients • 15 – 30% of referred patients for refractory hypertension • Renal hypoperfusion secondary activation of renin -angiotension system • Stenotic or obstructive lesion within renal artery • Potential curable cause of hypertension • 70 -90% - atherosclerosis • 10 -30% - fibromuscular dysplasia

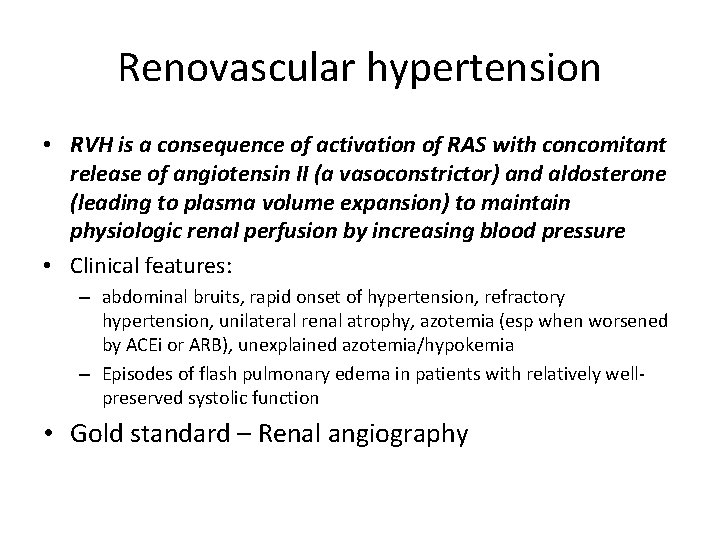
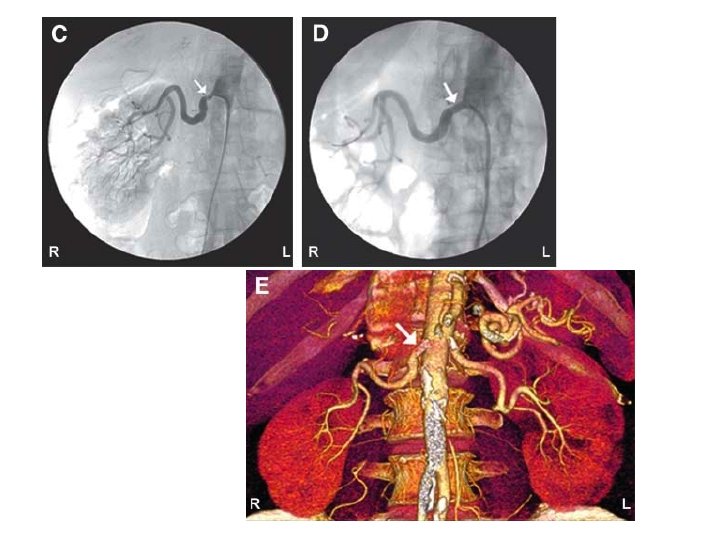
Renovascular hypertension • RVH is a consequence of activation of RAS with concomitant release of angiotensin II (a vasoconstrictor) and aldosterone (leading to plasma volume expansion) to maintain physiologic renal perfusion by increasing blood pressure • Clinical features: – abdominal bruits, rapid onset of hypertension, refractory hypertension, unilateral renal atrophy, azotemia (esp when worsened by ACEi or ARB), unexplained azotemia/hypokemia – Episodes of flash pulmonary edema in patients with relatively wellpreserved systolic function • Gold standard – Renal angiography


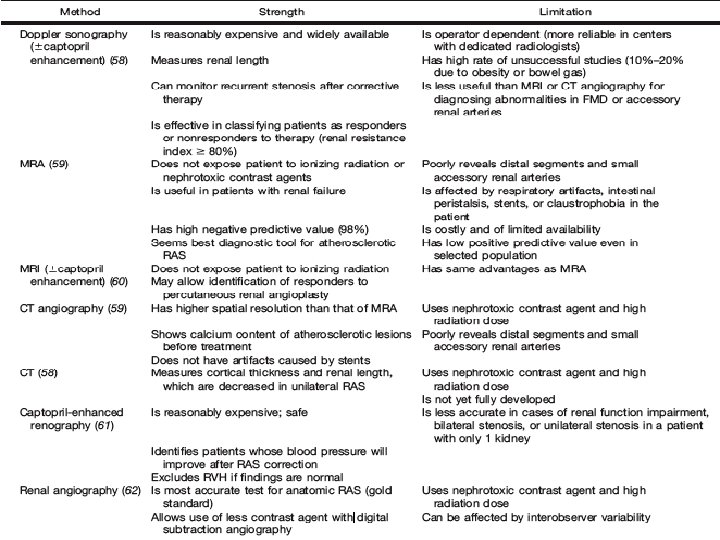
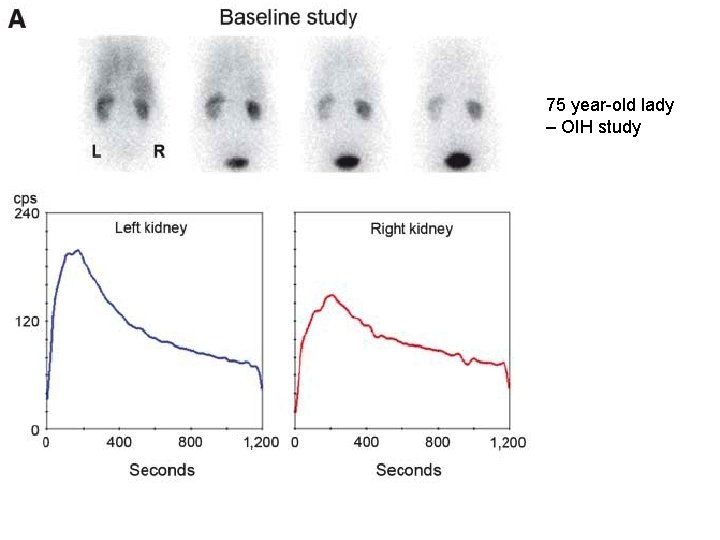
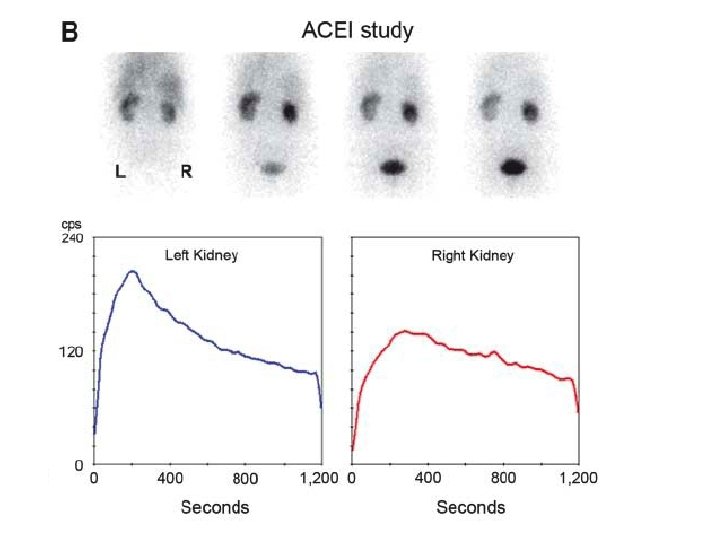
Captopril-enhanced renography • To determine which patients can expect normalization of BP or improvement of BP control after revascularization • ACEi reduce the conversion of angiotensin II - diminishing the vasoconstriction of the postglomerular efferent arteriole and decreasing the GFR, which can be detected by scintigraphy • Both glomerulus-filtered (99 m. Tc-DTPA) and tubulesecreted (99 m. Tc-MAG 3 or 123 I-OIH) - currently used • ACEi – captopril (25 -50 mg) orally 60 min before renography; or iv enalaprilat (40 mg/kg) over 325 min >15 min before renography

Captopril-enhanced renography • Known pitfalls erroneous results are – food ingestion within 4 h before receiving captopril, – infiltration, – dehydration, hypotension, – or a full bladder impairing drainage • ACEi enhanced renography now is rarely used as the primary imaging tool • Most reliable in predicting recovery in patients with FMD

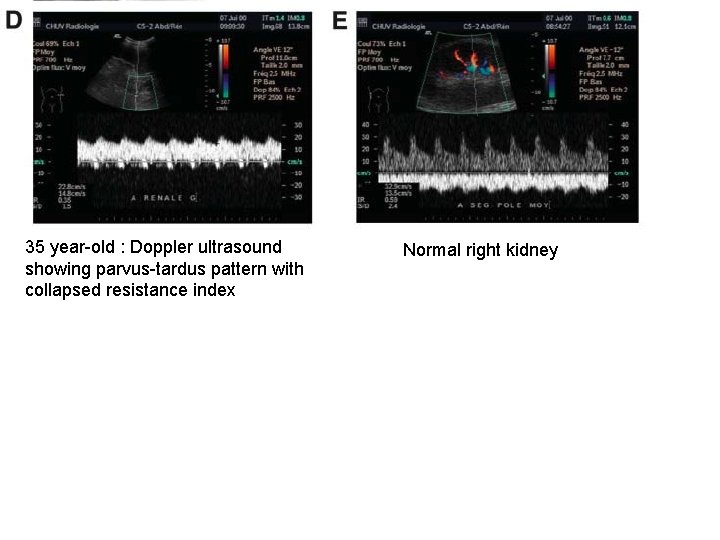
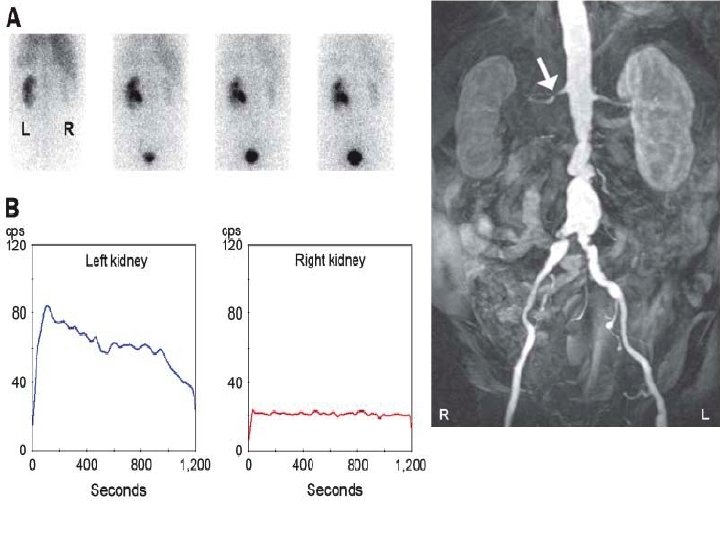
35 year-old : Doppler ultrasound showing parvus-tardus pattern with collapsed resistance index Normal right kidney

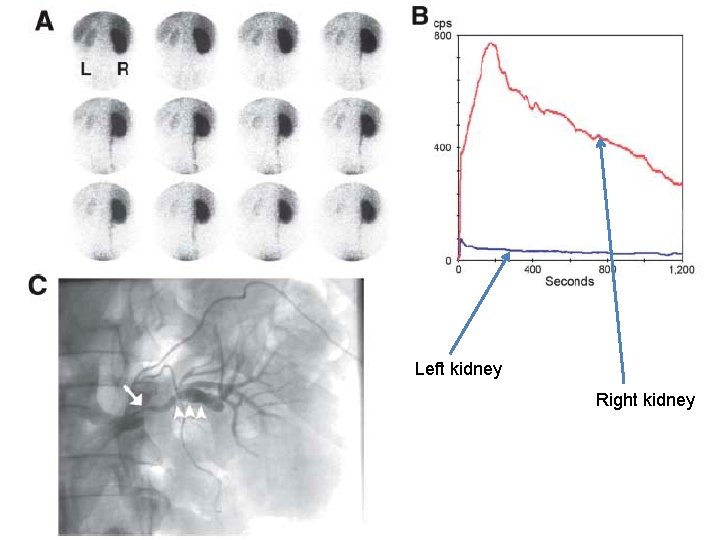
Left kidney Right kidney


75 year-old lady – OIH study



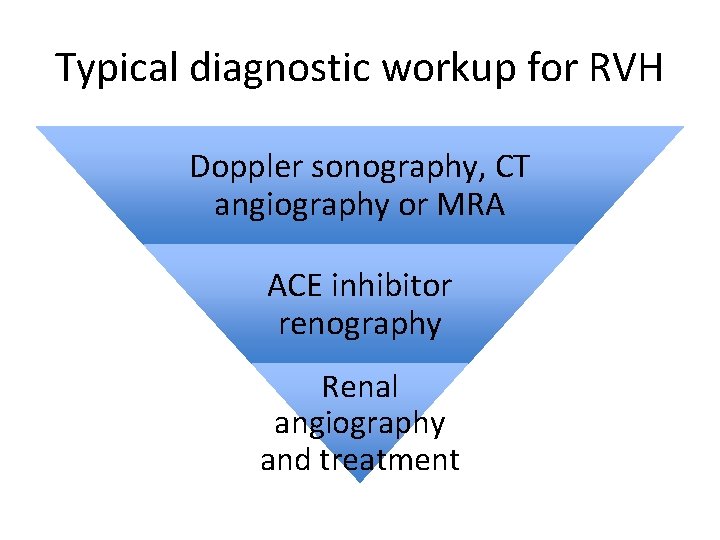
Typical diagnostic workup for RVH Doppler sonography, CT angiography or MRA ACE inhibitor renography Renal angiography and treatment

Clinical uses: • • • Renal clearance measurements Obstruction Infection Renal artery stenosis Renal transplant

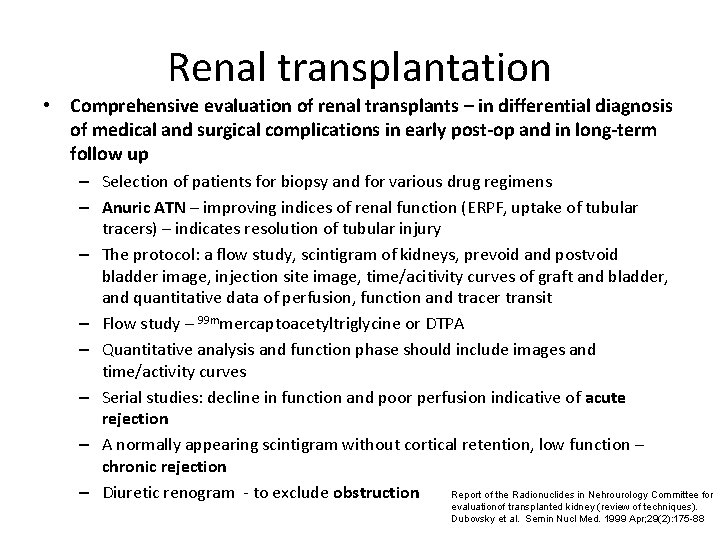
Renal transplantation • Comprehensive evaluation of renal transplants – in differential diagnosis of medical and surgical complications in early post-op and in long-term follow up – Selection of patients for biopsy and for various drug regimens – Anuric ATN – improving indices of renal function (ERPF, uptake of tubular tracers) – indicates resolution of tubular injury – The protocol: a flow study, scintigram of kidneys, prevoid and postvoid bladder image, injection site image, time/acitivity curves of graft and bladder, and quantitative data of perfusion, function and tracer transit – Flow study – 99 mmercaptoacetyltriglycine or DTPA – Quantitative analysis and function phase should include images and time/activity curves – Serial studies: decline in function and poor perfusion indicative of acute rejection – A normally appearing scintigram without cortical retention, low function – chronic rejection Report of the Radionuclides in Nehrourology Committee for – Diuretic renogram - to exclude obstruction evaluationof transplanted kidney (review of techniques). Dubovsky et al. Semin Nucl Med. 1999 Apr; 29(2): 175 -88

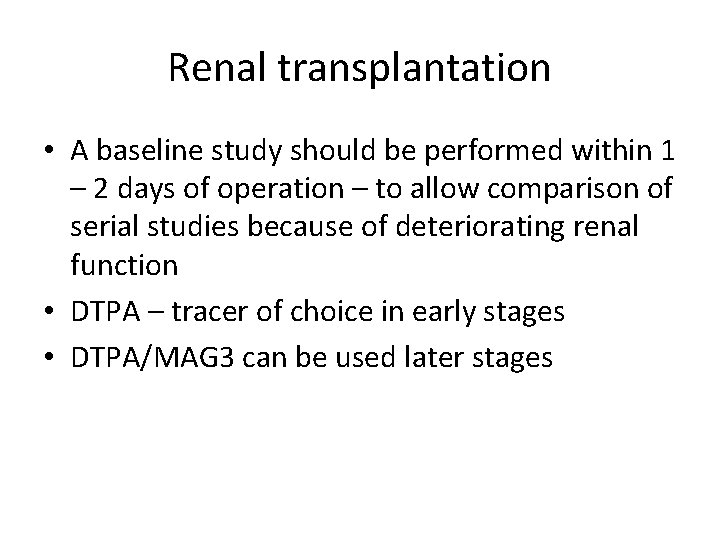
Renal transplantation • A baseline study should be performed within 1 – 2 days of operation – to allow comparison of serial studies because of deteriorating renal function • DTPA – tracer of choice in early stages • DTPA/MAG 3 can be used later stages

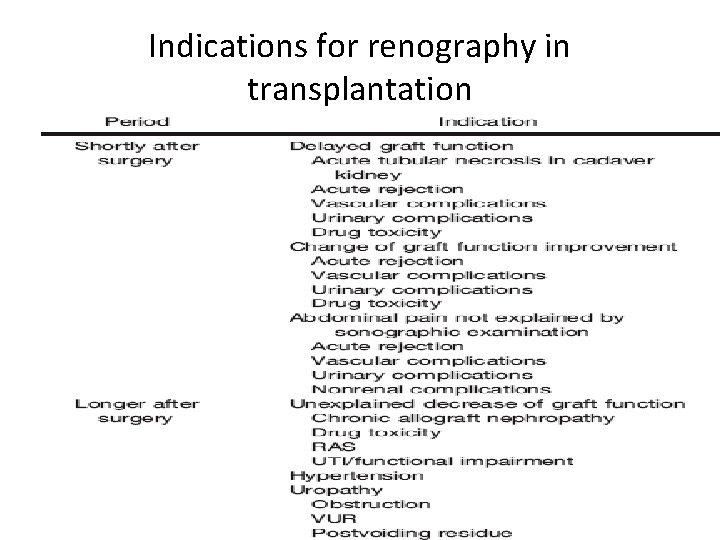
Indications for renography in transplantation

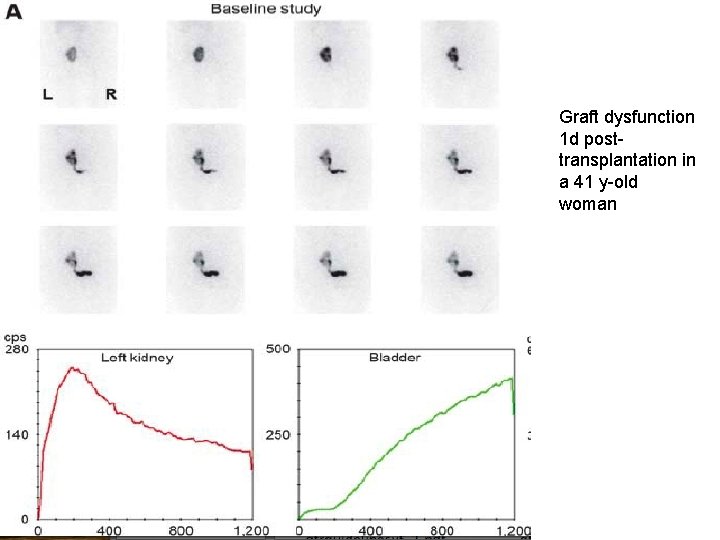
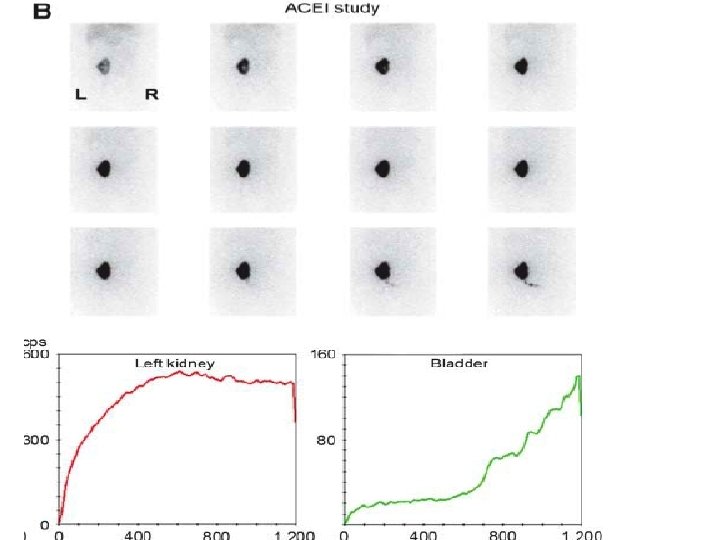
Renography in transplantation • Ultrasound – 1 st line evaluation in renal graft dysfunction • Renography – must be available on emergency basis – Non-visualization – irremediable loss of function – May not differentiate rejection from ischemia (RAS) – ACEi renography – help determine whether arterial hypertension is dependent on RAS – Help in diagnosis of urinary complications – obstruction or leak

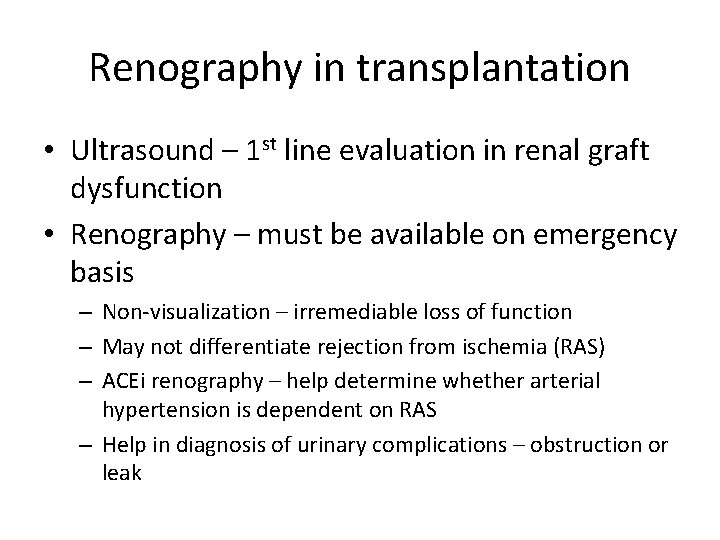
Graft dysfunction 1 d posttransplantation in a 41 y-old woman



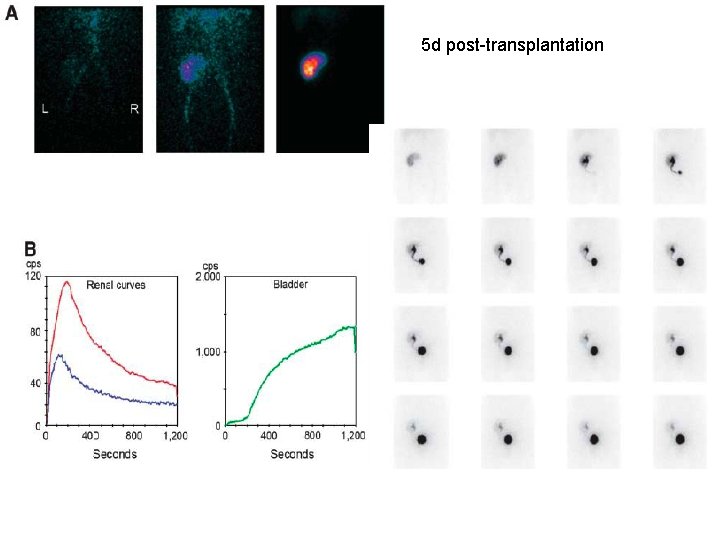
5 d post-transplantation

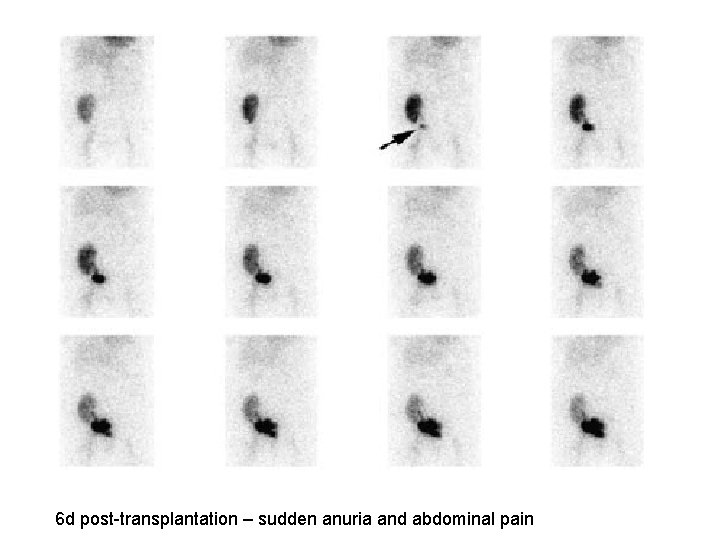
6 d post-transplantation – sudden anuria and abdominal pain

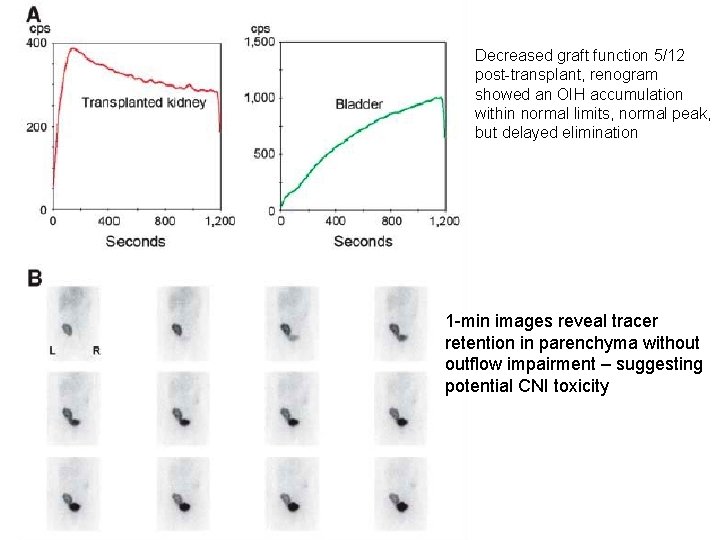
Decreased graft function 5/12 post-transplant, renogram showed an OIH accumulation within normal limits, normal peak, but delayed elimination 1 -min images reveal tracer retention in parenchyma without outflow impairment – suggesting potential CNI toxicity

Conclusion • Role of nuclear medicine – in investigation of renal parenchymal function and upper urinary tract abnormalities • Radiation burden low • Do not require sedation or specific patient preparation • Easy to perform • Knowledge of renal pathophysiology and recognition of limitation and technical pitfalls essential




