Please select a Team 1 2 3 4










![What is the molarity of [OH]- in a strong base solution with a p. What is the molarity of [OH]- in a strong base solution with a p.](https://slidetodoc.com/presentation_image/3d855cca11aa5ac0179a8744cf18a740/image-30.jpg)



- Slides: 53

Please select a Team. 1. 2. 3. 4. 5. Team 1 Team 2 Team 3 Team 4 Team 5

A solution that is mildly acidic would have a p. H of approximately 1. 2. 3. 4. 5. 2. 4. 6. 8. 12

A solution that is most strongly acidic would have a p. H of approximately 1. 2. 3. 4. 5. 2. 4. 6. 8. 12

A solution that is most strongly basic would have a p. H of approximately 1. 2. 3. 4. 5. 2. 4. 6. 8. 12

A solution that is weakly basic would have a p. H of approximately 1. 2. 3. 4. 5. 2. 4. 6. 8. 12

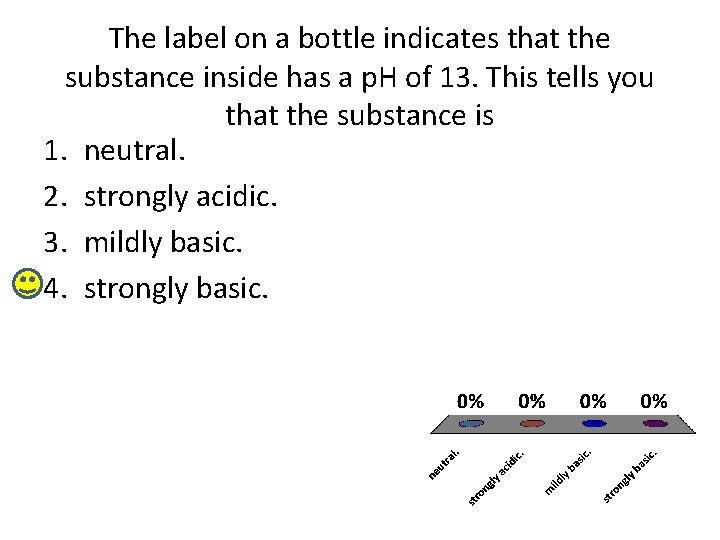
The label on a bottle indicates that the substance inside has a p. H of 13. This tells you that the substance is 1. neutral. 2. strongly acidic. 3. mildly basic. 4. strongly basic.

Team Scores 0 0 Team 1 Team 2 Team 3 Team 4 0 Team 5

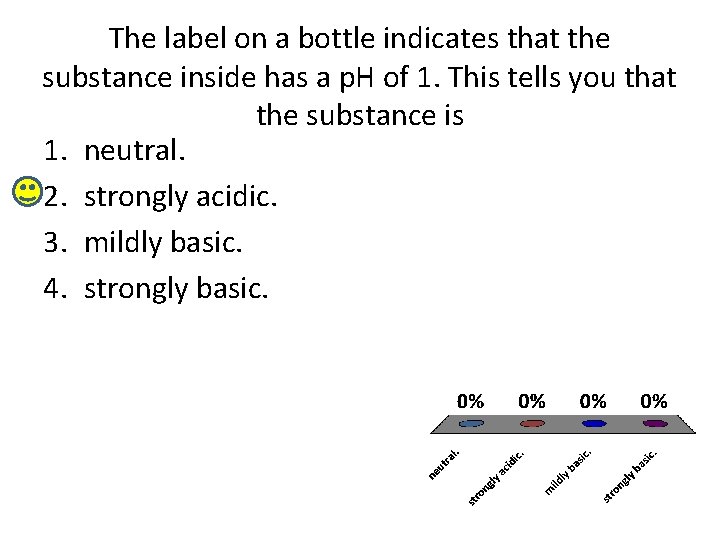
The label on a bottle indicates that the substance inside has a p. H of 1. This tells you that the substance is 1. neutral. 2. strongly acidic. 3. mildly basic. 4. strongly basic.

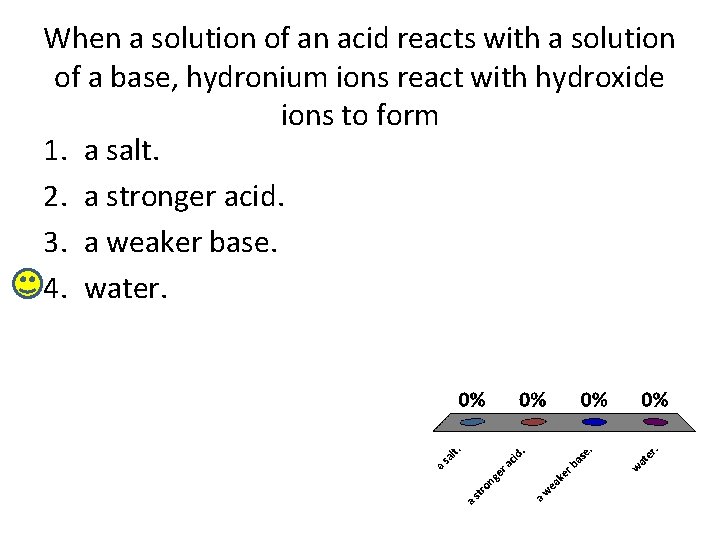
When a solution of an acid reacts with a solution of a base, hydronium ions react with hydroxide ions to form 1. a salt. 2. a stronger acid. 3. a weaker base. 4. water.

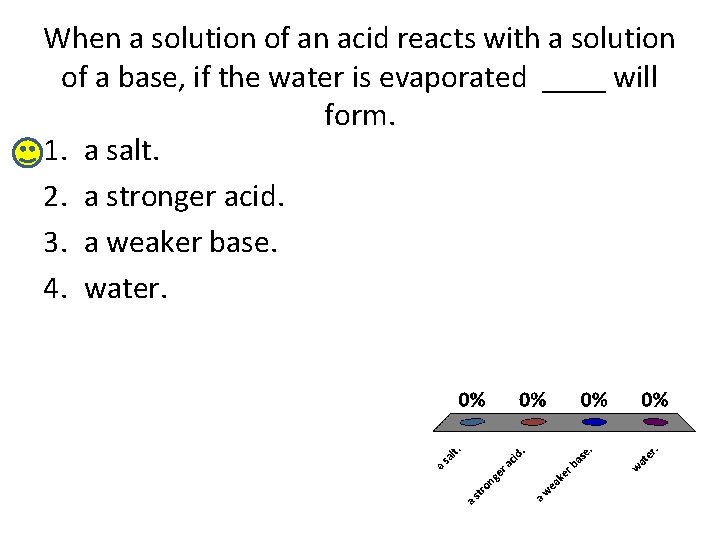
When a solution of an acid reacts with a solution of a base, if the water is evaporated ____ will form. 1. a salt. 2. a stronger acid. 3. a weaker base. 4. water.

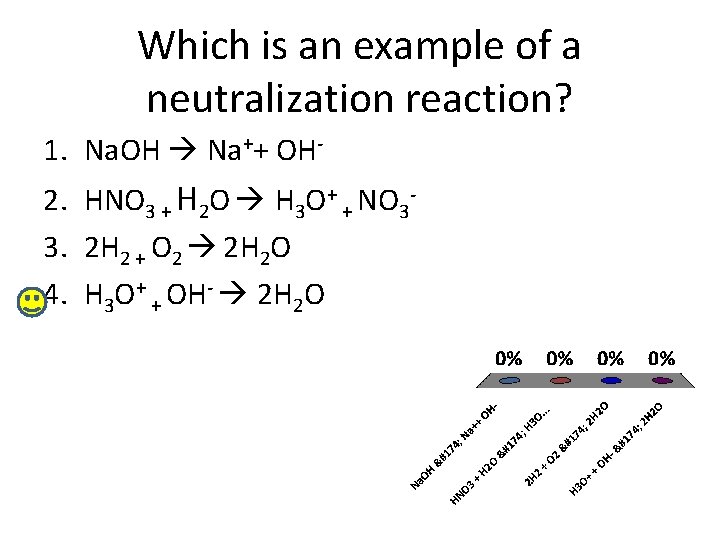
Which is an example of a neutralization reaction? 1. Na. OH Na++ OH 2. HNO 3 + H 2 O H 3 O+ + NO 33. 2 H 2 + O 2 2 H 2 O 4. H 3 O+ + OH- 2 H 2 O

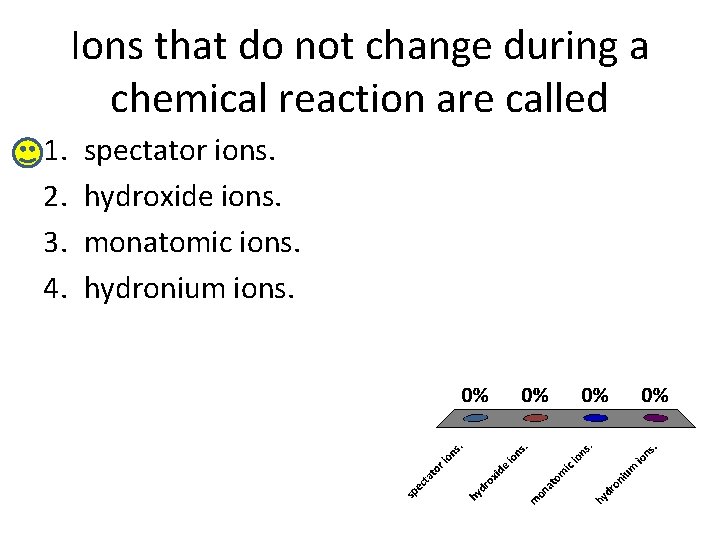
Ions that do not change during a chemical reaction are called 1. 2. 3. 4. spectator ions. hydroxide ions. monatomic ions. hydronium ions.

Team Scores 0 0 Team 1 Team 2 Team 3 Team 4 0 Team 5

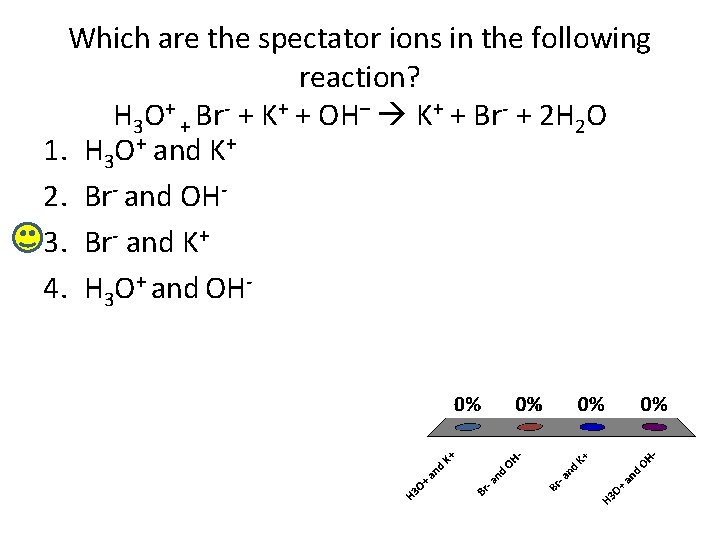
Which are the spectator ions in the following reaction? H 3 O+ + Br- + K+ + OH– K+ + Br- + 2 H 2 O 1. H 3 O+ and K+ 2. Br- and OH 3. Br- and K+ 4. H 3 O+ and OH-

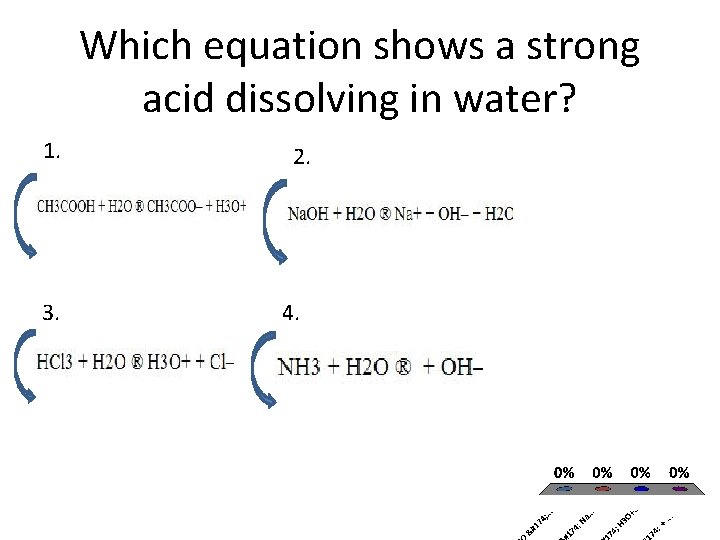
Which equation shows a strong acid dissolving in water? 1. 3. 2. 4.

A base forms a greater concentration of which ions in solution than an acid? 1. 2. 3. 4. oxygen hydroxide hydronium hydrogen

Which phrase is not true about strong acids? 1. 2. 3. 4. conduct electricity well ionize completely in water turn blue litmus paper red form OH- ions in solution

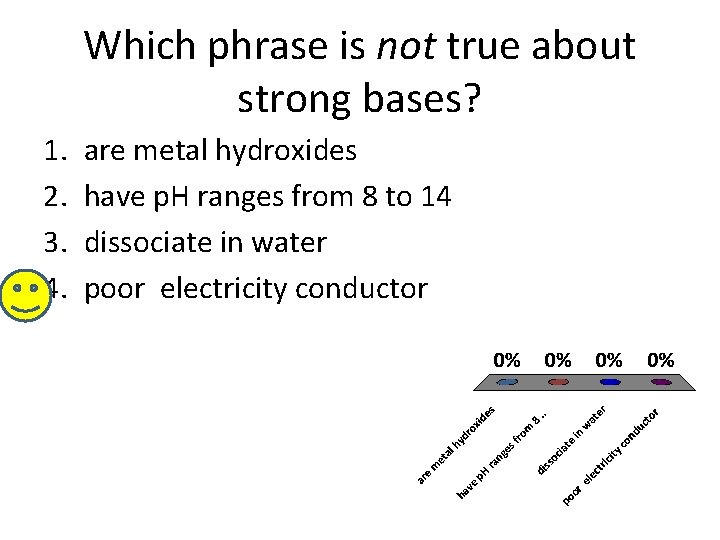
Which phrase is not true about strong bases? 1. 2. 3. 4. are metal hydroxides have p. H ranges from 8 to 14 dissociate in water poor electricity conductor

Team Scores 0 0 Team 1 Team 2 Team 3 Team 4 0 Team 5

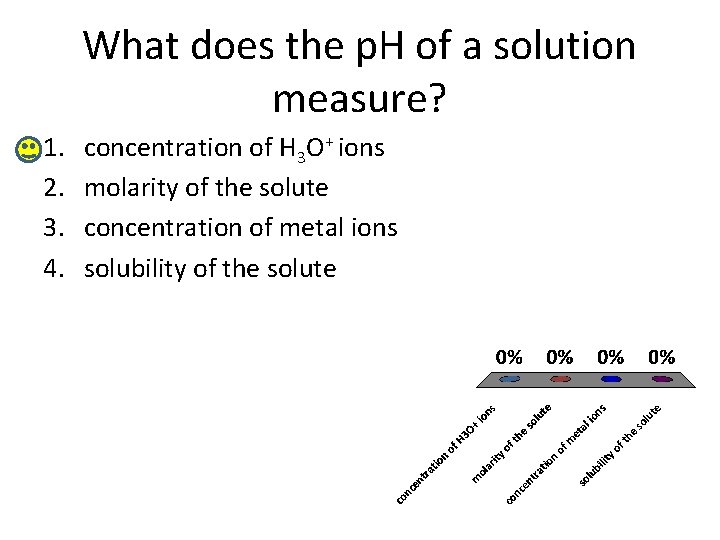
What does the p. H of a solution measure? 1. 2. 3. 4. concentration of H 3 O+ ions molarity of the solute concentration of metal ions solubility of the solute

Which solution is the most acidic? 1. 2. 3. 4. apple juice, p. H = 3 pure water, p. H = 7 hand soap solution, p. H = 10 drain cleaner solution, p. H = 14

Which solution is the least acidic? 1. apple juice, p. H = 3 2. pure water, p. H = 7 3. hand soap solution, p. H = 10 4. drain cleaner solution, p. H = 14

Which solution is the most basic? 1. apple juice, p. H = 3 2. pure water, p. H = 7 3. hand soap solution, p. H = 10 4. drain cleaner solution, p. H = 14

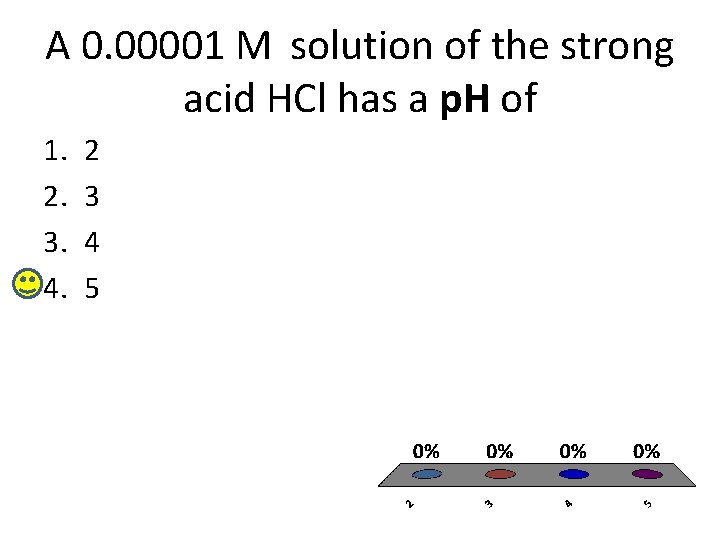
A 0. 00001 M solution of the strong acid HCl has a p. H of 1. 2. 3. 4. 2 3 4 5

Team Scores 0 0 Team 1 Team 2 Team 3 Team 4 0 Team 5

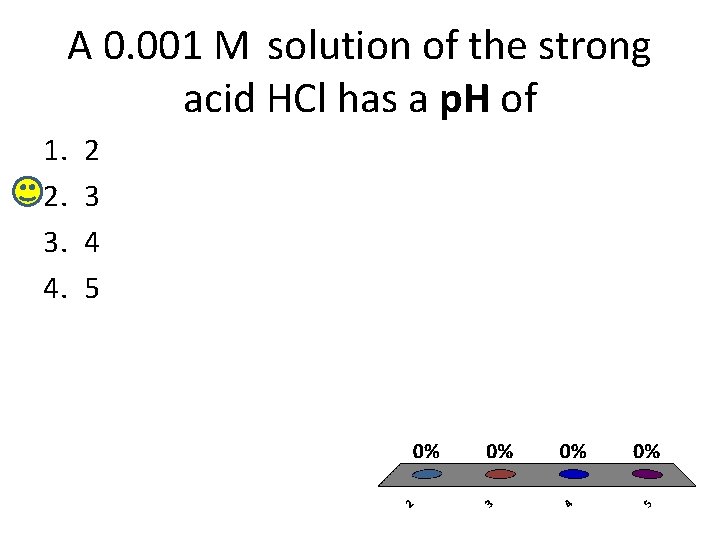
A 0. 001 M solution of the strong acid HCl has a p. H of 1. 2. 3. 4. 2 3 4 5

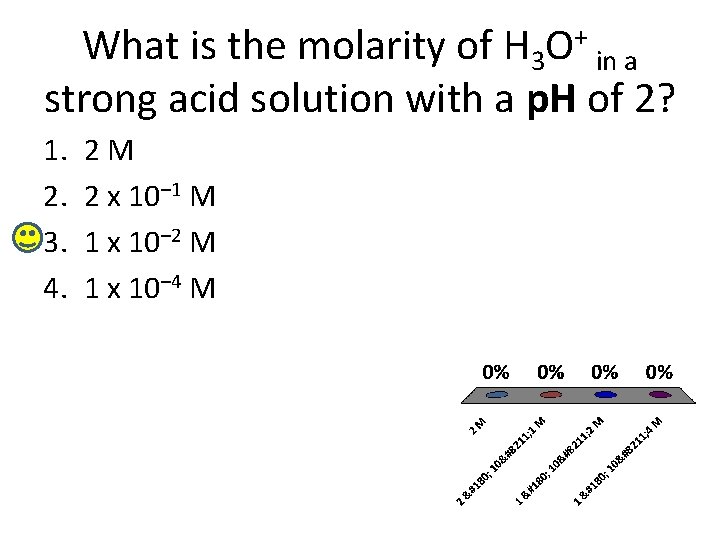
What is the molarity of H 3 O+ in a strong acid solution with a p. H of 2? 1. 2. 3. 4. 2 M 2 x 10– 1 M 1 x 10– 2 M 1 x 10– 4 M

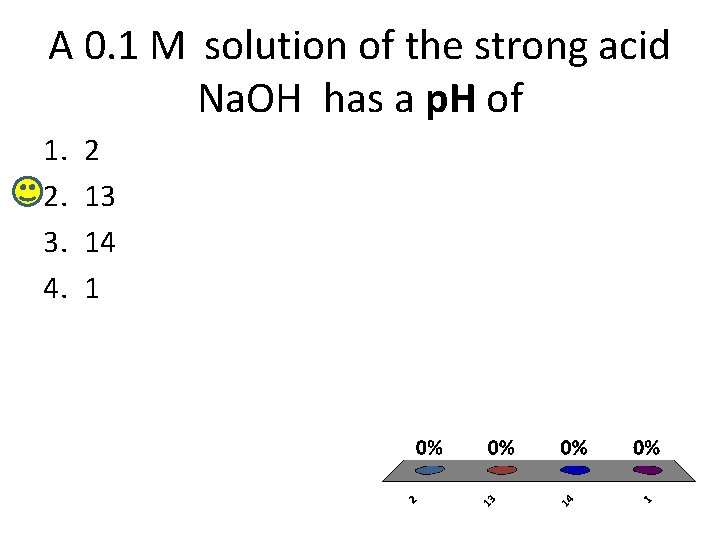
A 0. 1 M solution of the strong acid Na. OH has a p. H of 1. 2. 3. 4. 2 13 14 1

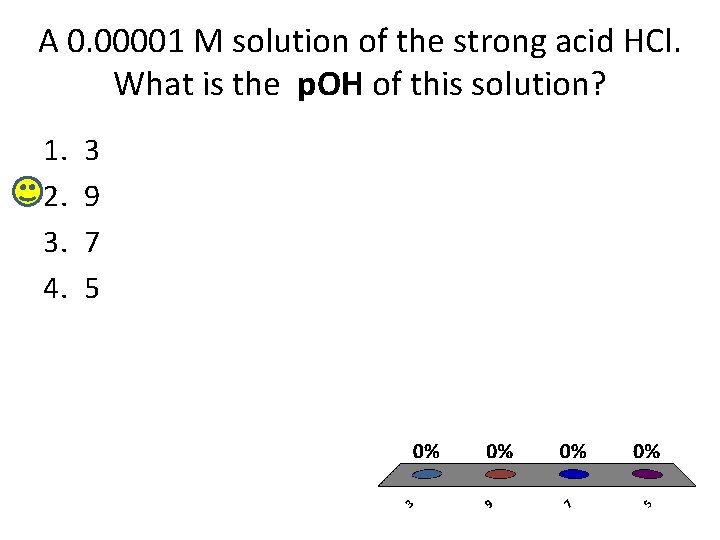
A 0. 00001 M solution of the strong acid HCl. What is the p. OH of this solution? 1. 2. 3. 4. 3 9 7 5
![What is the molarity of OH in a strong base solution with a p What is the molarity of [OH]- in a strong base solution with a p.](https://slidetodoc.com/presentation_image/3d855cca11aa5ac0179a8744cf18a740/image-30.jpg)
What is the molarity of [OH]- in a strong base solution with a p. OH of 2? 1. 2. 3. 4. 2 M 1 x 10– 12 M 1 x 10– 4 M

Team Scores 0 0 Team 1 Team 2 Team 3 Team 4 0 Team 5

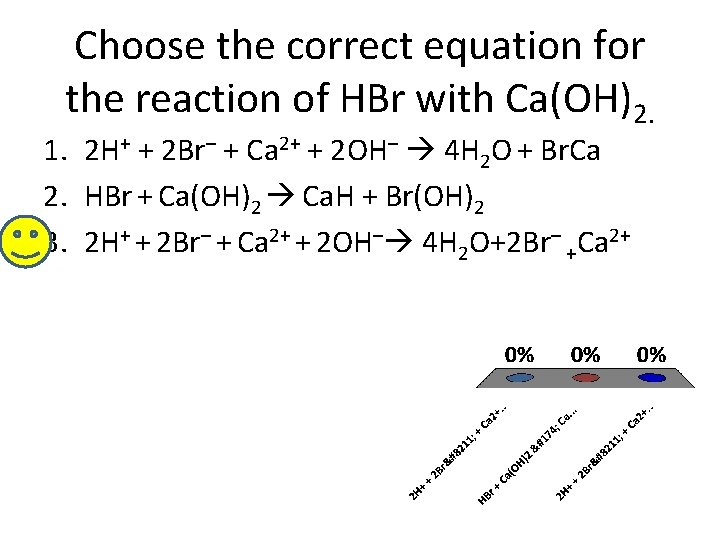
Choose the correct equation for the reaction of HBr with Ca(OH)2. 1. 2 H+ + 2 Br– + Ca 2+ + 2 OH– 4 H 2 O + Br. Ca 2. HBr + Ca(OH)2 Ca. H + Br(OH)2 3. 2 H+ + 2 Br– + Ca 2+ + 2 OH– 4 H 2 O+2 Br– +Ca 2+

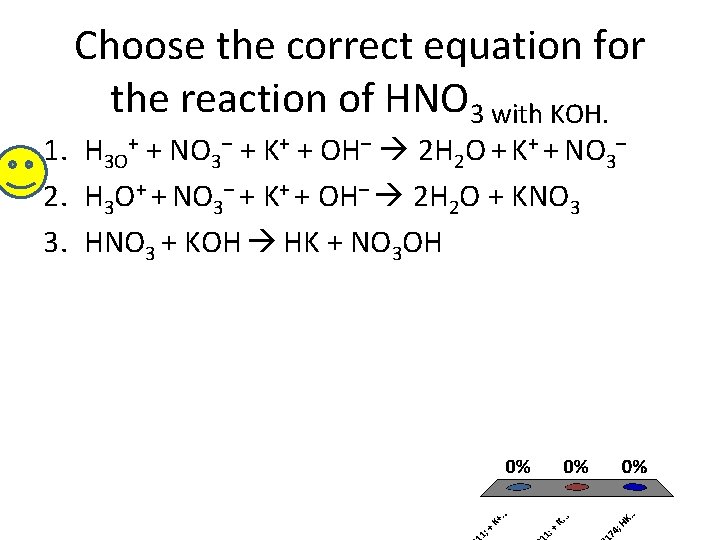
Choose the correct equation for the reaction of HNO 3 with KOH. 1. H 3 O+ + NO 3– + K+ + OH– 2 H 2 O + K+ + NO 3– 2. H 3 O+ + NO 3– + K+ + OH– 2 H 2 O + KNO 3 3. HNO 3 + KOH HK + NO 3 OH

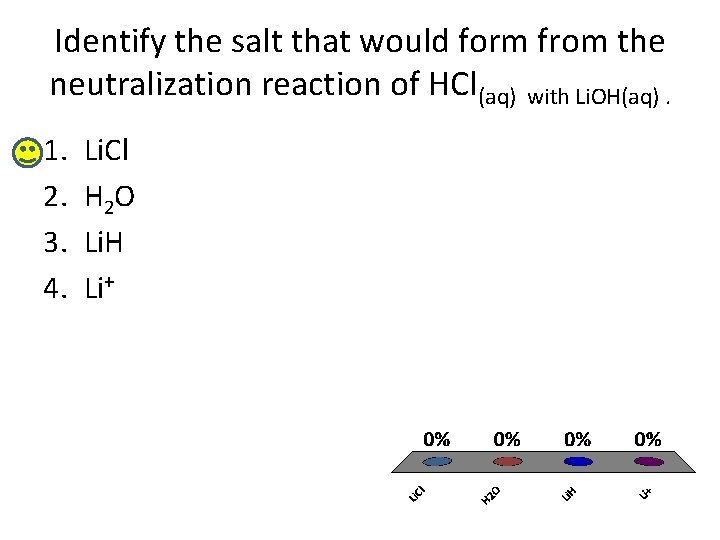
Identify the salt that would form from the neutralization reaction of HCl(aq) with Li. OH(aq). 1. 2. 3. 4. Li. Cl H 2 O Li. H Li+

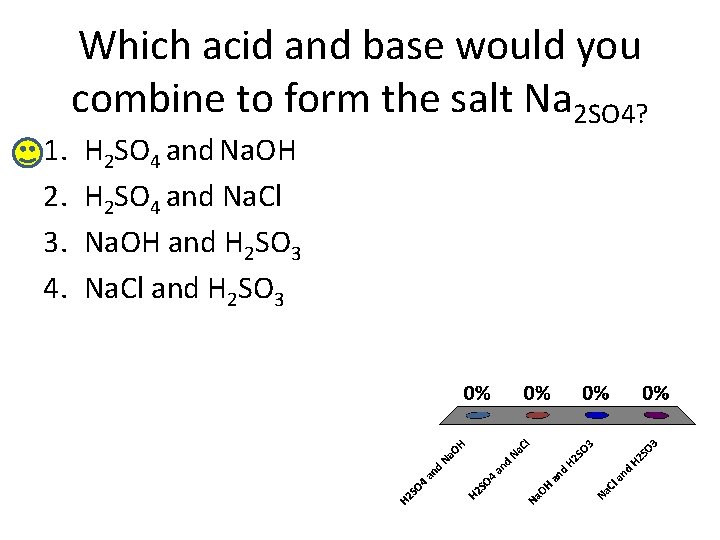
1. 2. 3. 4. Which acid and base would you combine to form the salt Na 2 SO 4? H 2 SO 4 and Na. OH H 2 SO 4 and Na. Cl Na. OH and H 2 SO 3 Na. Cl and H 2 SO 3

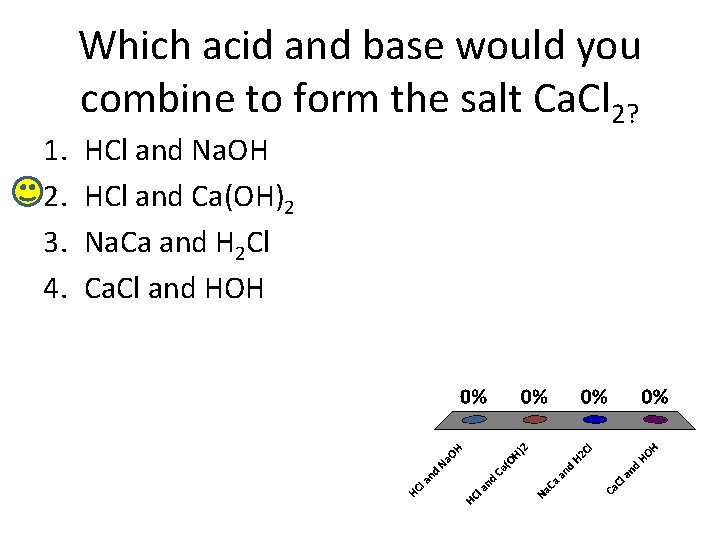
1. 2. 3. 4. Which acid and base would you combine to form the salt Ca. Cl 2? HCl and Na. OH HCl and Ca(OH)2 Na. Ca and H 2 Cl Ca. Cl and HOH

Team Scores 0 0 Team 1 Team 2 Team 3 Team 4 0 Team 5

At the equivalence point of a strong acid–strong base titration the p. H is 1. 2. 3. 4. 0 7 10 14

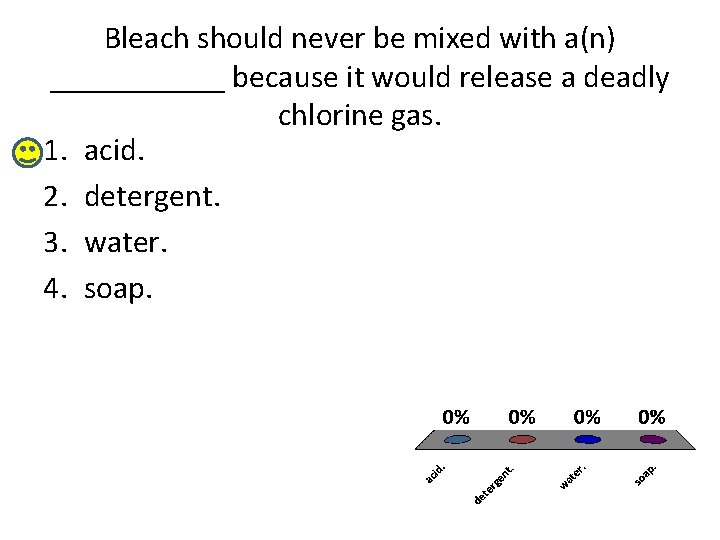
Bleach should never be mixed with a(n) ______ because it would release a deadly chlorine gas. 1. acid. 2. detergent. 3. water. 4. soap.

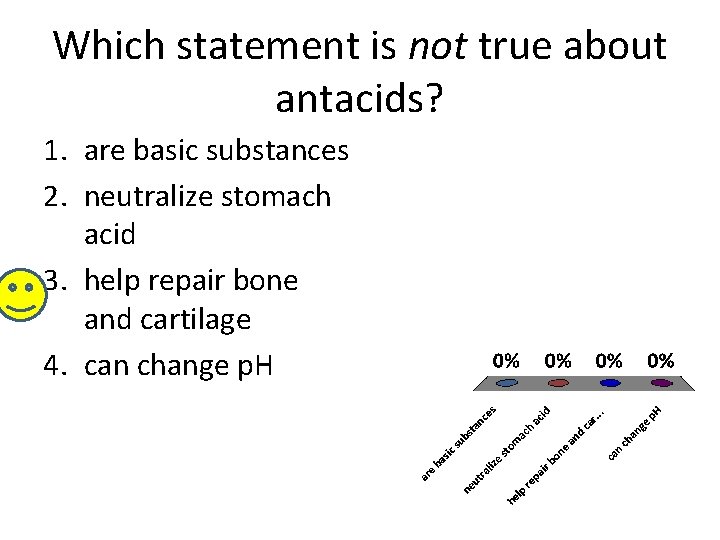
Which statement is not true about antacids? 1. are basic substances 2. neutralize stomach acid 3. help repair bone and cartilage 4. can change p. H

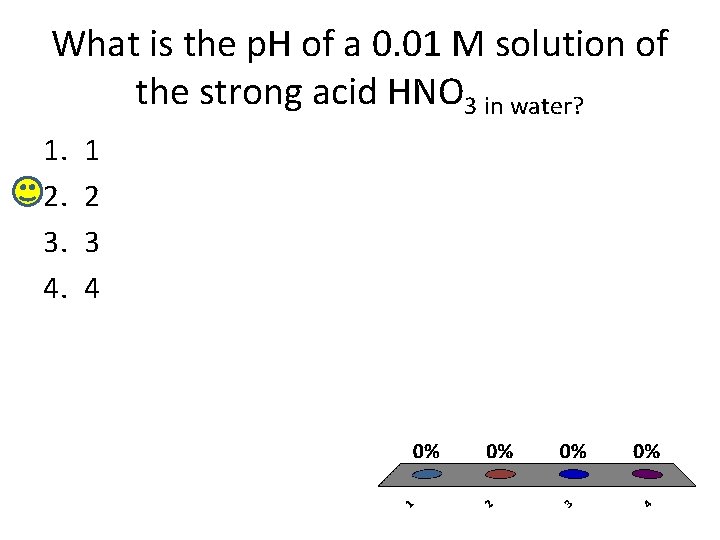
What is the p. H of a 0. 01 M solution of the strong acid HNO 3 in water? 1. 2. 3. 4. 1 2 3 4

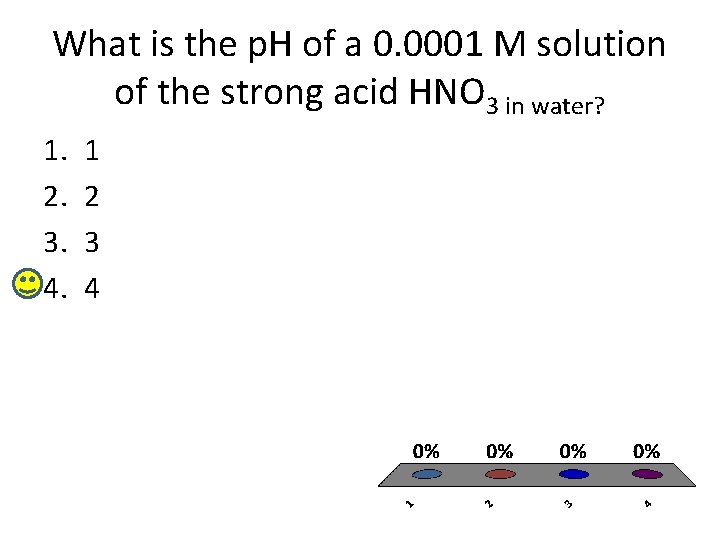
What is the p. H of a 0. 0001 M solution of the strong acid HNO 3 in water? 1. 2. 3. 4. 1 2 3 4

Team Scores 0 0 Team 1 Team 2 Team 3 Team 4 0 Team 5

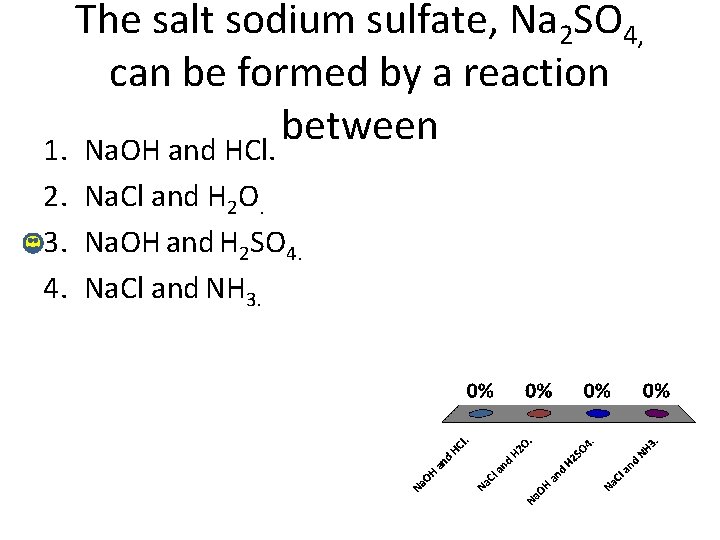
The salt sodium sulfate, Na 2 SO 4, can be formed by a reaction between 1. Na. OH and HCl. 2. Na. Cl and H 2 O. 3. Na. OH and H 2 SO 4. 4. Na. Cl and NH 3.

Solution A has a p. H of 6. Solution B has a p. H of 8. What is true about these solutions? 1. 2. 3. 4. Solution A has 10 times as many H+ ions as Solution B. Solution A has 1000 times as many H+ ions as Solution B. Solution A has 0. 10 times as many H+ ions as Solution B.

Solution A has a p. H of 2. Solution B has a p. H of 5. What is true about these solutions? 1. 2. 3. 4. Solution A has 10 times as many H+ ions as Solution B. Solution A has 1000 times as many H+ ions as Solution B. Solution A has 0. 10 times as many H+ ions as Solution B.

Acid rain is caused by: 1. cloud seeding. 2. silver iodide particles in clouds. 3. by-products of the combustion of fossil fuels. 4. carbon tetrachloride

If the hydroxide ion concentration of a solution is 1 x 10 -10, the hydronium ion concentration =________. Is this solution that is acidic, basic (alkaline), or neutral? 1. acidic 2. alkaline (basic) 3. neutral

Team Scores 0 0 Team 1 Team 2 Team 3 Team 4 0 Team 5

What is the best description for a solution with a hydroxide-ion concentration of 1 10 -4 M? 1. acidic 2. basic 3. neutral

A 0. 1 M solution of an acid that ionizes only slightly in solution would be termed ____. 1. concentrated and weak 2. strong and dilute 3. dilute and weak 4. concentrated and strong

The reaction HCl + KOH KCl + H 2 O 1. synthesis reaction 2. ionization reaction 3. neutralization reaction 4. decomposition reaction is a

FINAL Team Scores 0 0 Team 1 Team 2 Team 3 Team 4 0 Team 5