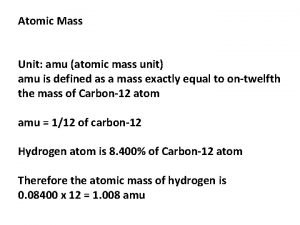Nuclear Chemistry College Chemistry Isotope Notation Atomic Mass































- Slides: 33

Nuclear Chemistry College Chemistry

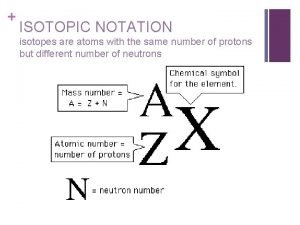
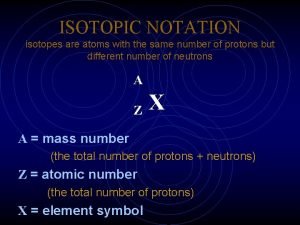
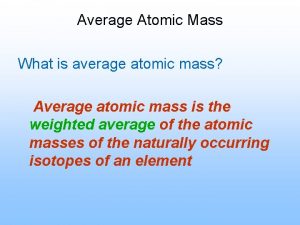
Isotope Notation Atomic Mass protons + neutrons Atomic Number the number of protons

Strong Nuclear Force l The strong nuclear force is usually strong enough to keep all of the + protons and neutral neutrons together in the nucleus. l When the strong nuclear force isn’t strong enough, the nucleus loses pieces (radioactivity) or breaks apart into 2 or more different atoms (fission).

Nuclear Reactions l When the nucleus of an atom is unstable, it will spontaneously decay and the nucleus will emit particles. l A change like this in the nucleus of an atom is called a nuclear reaction.

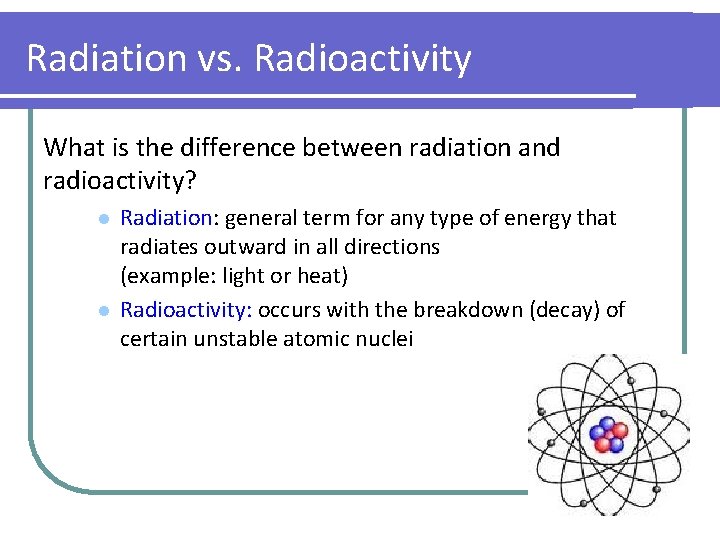
Radiation vs. Radioactivity What is the difference between radiation and radioactivity? l l Radiation: general term for any type of energy that radiates outward in all directions (example: light or heat) Radioactivity: occurs with the breakdown (decay) of certain unstable atomic nuclei

Alpha Decay l An alpha particle is a helium atom: l During alpha decay: l l Two protons are lost Two neutrons are lost

Alpha Decay Example Americium-241 is used in smoke detectors, where it emits an alpha particle that ionizes the oxygen and nitrogen in the air. Smoke interrupts the flow of ions and sets off an alarm. Write the alpha decay reaction for Am-241.

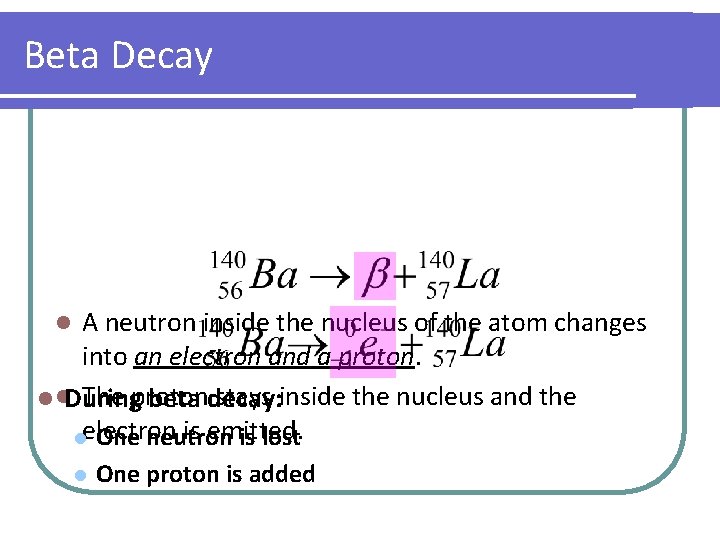
Beta Decay A neutron inside the nucleus of the atom changes into an electron and a proton. The proton stays inside the nucleus and the l l. During beta decay: is emitted. lelectron One neutron is lost l l One proton is added

Practice Problem l Complete the following reactions AND identify whether it is alpha or beta decay: a. b.

Nuclear Reactions Particle Mass Charge Alpha (helium) 4 N/A Beta (Electron) 0 -1 Proton 1 1 Neutron 1 0 Positron 0 1 Notation

Gamma Rays Gamma ( ) rays: high energy electromagnetic radiation (higher than X rays!) and the most dangerous type of radioactive emission. The following decay of cobalt-60 is used in cancer treatment: Note: gamma rays are accompanied by beta emission

Nuclear Reaction Example Beryllium-9 plus an alpha particle produces another element and a neutron. Write the nuclear reaction.

Positron Emission Positron emission: A positron is a particle with the same size and mass as an electron, but with a positive charge.

Electron Capture Electron capture: An electron is captured to turn a proton into a neutron Animation: http: //www 2. wwnorton. com/college/chemistry/gilbert/tutorials/ch 2. htm

Half Life: the time it takes for half a sample of unstable radioisotopes to decay l Elements take very different amounts of time to decay to half of the original sample: l Parent Isotope Half-Life Daughter Isotope Carbon-14 5730 yrs. Nitrogen-14 Potassium-40 1. 26 billion yrs. Argon-40 Thorium-230 75, 000 yrs. Radium-226 Uranium-235 700, 000 million yrs. Lead-207 Uranium-238 4. 5 billion yrs. Lead-206

Half Life Example

Half-life Calculations Radioactive decay rate is proportional to the number of radioactive nuclei (N) in a sample. Thus: Rate = k. N (k is called the decay constant) Using a little calculus, you can turn this into: OR Where: N = # left after a time interval and N 0 = # nuclei at time = zero If you assume ½ sample remains we can rearrange the above equation to: OR FYI: 0. 693 = ln 2

Radioactive Dating Radiocarbon dating is useful only for dating formerly living things l While an organism is alive it takes in carbon -- most of it as carbon-12 but a small % is carbon-14 (a radioactive form of carbon). l Carbon-14 is formed in the atmosphere by the following process (helped along by cosmic rays) l

Radioactive Dating As soon as the organism dies, it stops taking in carbon. The amount of carbon 12 stays the same over time, but the amount of carbon 14 decreases as it decays. l Carbon-14 has a half life of 5715 years. It decays by the following process: l l Geologists and archeologists can use the ratio of carbon-12 to carbon-14 to determine how old an object is

Nuclear Power in MN Source: http: //www. xcelenergy. com

Nuclear Power in MN Source: http: //www. eia. gov/state/? sid=mn#tabs-1

Nuclear Power in MN Prairie Island Nuclear Generating Plant Source: www. xcelenergy. com Monticello Nuclear Generating Plant

Nuclear Power 2006 Xcel Energy-owned Generating Facilities Unit Type Number of 2005 Xcel Energy-owned Generating Facilities Generating Units Generating Capacity (MW) Coal 17 35 8, 207 Natural Gas 26 61 4, 913 Nuclear 2 3 1, 617 Hydro 28 83 508 Oil 9 24 460 Refuse-derived Fuel 3 6 67 Wind 1 37 27* *Xcel Energy purchased 1, 296 megawatts of wind power in 2006. Source: XCel Energy web site (http: //www. xcelenergy. com)

Nuclear Power l l Uranium Mines/Milling Sites in the USA Image from http: //www. nrc. gov/infofinder/materials/uranium/

http: //www. wise-uranium. org/umaps. html

Nuclear Power l Operating Nuclear Reactors in the USA l Image from http: //www. nrc. gov/info-finder/reactor/

Nuclear power is produced in reactors. These include various components principally, nuclear fuel, moderators, coolants, steam generators, turbines, condensers, cooling towers and of course a containment structure. Typical pressurized water reactor

Chapter 2. Nuclear Energy Production Nuclear plant fuel pool Nuclear plant containment building

New nuclear plants incorporate multiple safety layers.

Uranium provides the energy source for nuclear reactors. 1 ton of uranium has the equivalent energy of 20, 000 tons of coal! Typical fuel pellet Fuel assembly in a representative boiling water reactor (about 4. 3 meters [14 feet]) tall and each weighing about 317. 5 kilograms (700 pounds). NFI type 9 x 9 Fuel.

Commercial spent fuel storage Spent fuel pool storage at reactors Above-ground fuel storage containers Note: The blue glow is called Cerenkov radiation. It only happens when highly radioactive fuel rods are immersed in water. They cause the water molecules to get excited and emit blue light when they drop back to their normal state.

http: //en. wikipedia. org/wiki/File: Nuclear_waste_locations_USA. jpg

Permanent Storage Facility? The proposed Yucca Mountain Nuclear Waste Repository near Las Vegas, Nevada
 Is atomic mass and relative atomic mass the same
Is atomic mass and relative atomic mass the same Abundance calculation chemistry
Abundance calculation chemistry Differentiate between atomic number and mass number
Differentiate between atomic number and mass number Isotope symbol
Isotope symbol Isotopic notation
Isotopic notation Fe atomic number
Fe atomic number Isotope notation
Isotope notation Isotope notation practice
Isotope notation practice Mass and atomic number
Mass and atomic number Moles to particles
Moles to particles Atomic mass vs molar mass
Atomic mass vs molar mass Lesson 15 nuclear quest nuclear reactions
Lesson 15 nuclear quest nuclear reactions Fisión nuclear vs fision nuclear
Fisión nuclear vs fision nuclear Nuclear notation
Nuclear notation Nuclear symbol notation
Nuclear symbol notation Periodic trend
Periodic trend Atomic radius trends on periodic table
Atomic radius trends on periodic table Atomic number vs atomic radius
Atomic number vs atomic radius Given the atomic notation of 64/30 zn
Given the atomic notation of 64/30 zn Engineering notation prefixes
Engineering notation prefixes Infix notation to prefix notation
Infix notation to prefix notation Regex polish letters
Regex polish letters Reverse polish notation examples
Reverse polish notation examples Nuclear chemistry webquest answer key
Nuclear chemistry webquest answer key Nuclear chemistry
Nuclear chemistry Application of nuclear chemistry
Application of nuclear chemistry Application of nuclear chemistry
Application of nuclear chemistry Zeff trend
Zeff trend Chapter 24 nuclear chemistry answer key
Chapter 24 nuclear chemistry answer key Chapter 25 nuclear chemistry answer key
Chapter 25 nuclear chemistry answer key Anatomy of a wave
Anatomy of a wave Applications of nuclear chemistry
Applications of nuclear chemistry T half life formula
T half life formula Rectangle real life examples
Rectangle real life examples