Average Atomic Mass Average Atomic Mass IS on



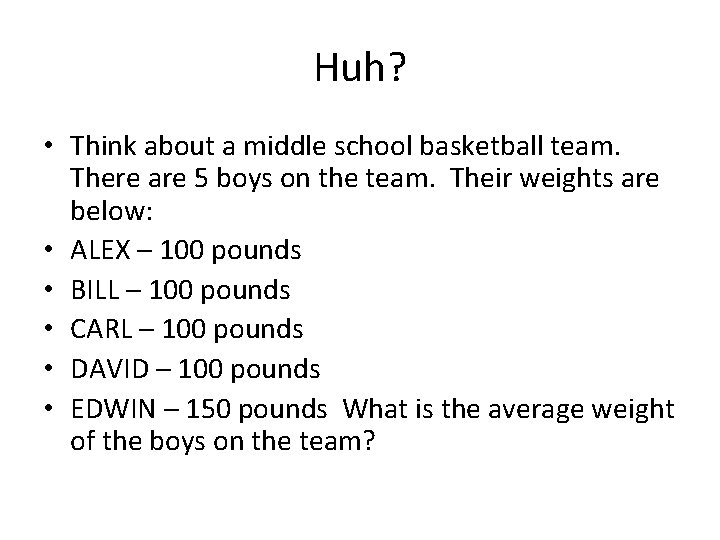







- Slides: 17

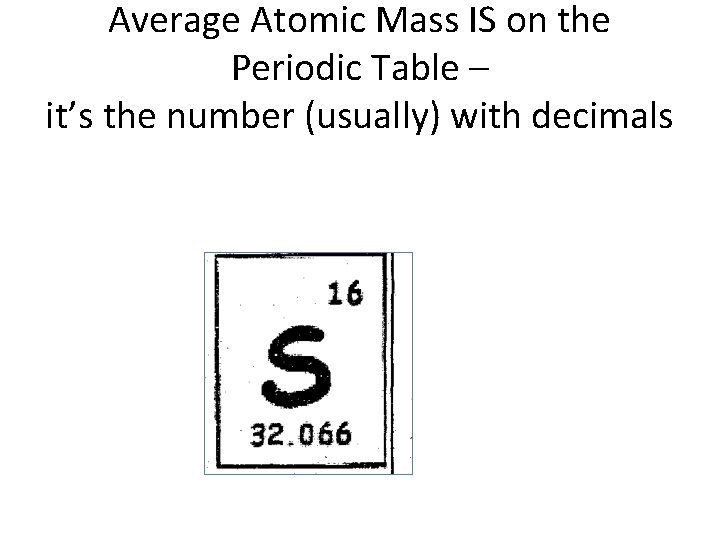
Average Atomic Mass

Average Atomic Mass IS on the Periodic Table – it’s the number (usually) with decimals

What are the units? • Amu – atomic mass unit • Approximately the same as the mass of one proton (or one neutron, since they’re the same size) • A weighted average of all the isotopes found in nature

What’s a WEIGHTED average? • It is an average that is weighted by the percent of each isotope present in nature.
Huh? • Think about a middle school basketball team. There are 5 boys on the team. Their weights are below: • ALEX – 100 pounds • BILL – 100 pounds • CARL – 100 pounds • DAVID – 100 pounds • EDWIN – 150 pounds What is the average weight of the boys on the team?

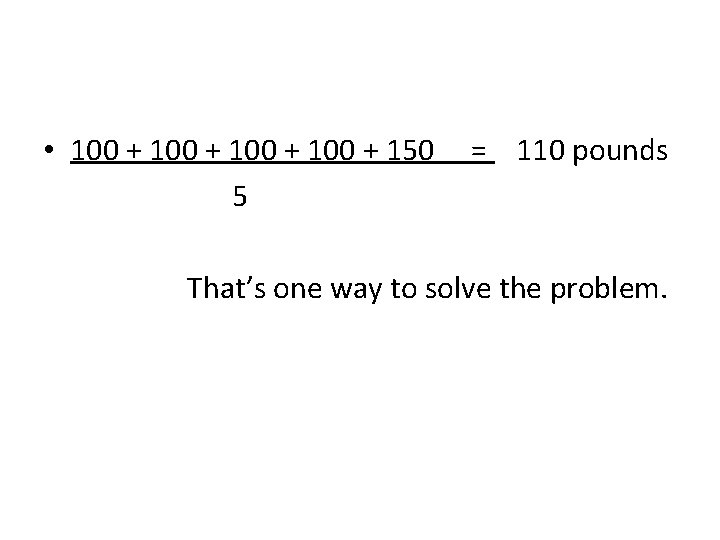
• 100 + 150 5 = 110 pounds That’s one way to solve the problem.

Another way to look at it… • 4 boys weigh 100 pounds. 4 = 80% 5 5 • 1 boy weighs 150 pounds 1 = 20% 5 5

So… 80% = 0. 80 20% = 0. 20 0. 80 X 100 pounds = 80 pounds + 0. 20 x 150 pounds = 30 pounds 110 pounds Same answer we got when we added the 4 @ 100 and 1 @150 and / 5

Remember: the various isotopes occur in different percentages • For Example: Rubidium has two common isotopes, Rb - 85 and Rb - 87. If the abundance of Rb-85 is 72. 2% and the abundance of Rb -87 is 27. 8%, what is the average atomic mass of rubidium that would be found on the Periodic Table?

Solution 0. 722 x 85 = 61. 37 + 0. 278 x 87 = 24. 19 85. 56 amu

Try this one • Chlorine – 35 = 75% • Chlorine – 37 = 25% • What is the average atomic mass of Chlorine that would be found on the Periodic Table?

Solution 35 X 0. 75 = 26. 25 amu + 37 x 0. 25 = 9. 25 amu 35. 50 amu Notice: 75% is Chlorine -35 so the weighted average is closer to 35 amu than 37.

Inferring the average based on % • Two isotopes of an element exist: X – 39 = 89% and X – 40 = 11% Which would the weighted average be closer to, 39 or 40?

Inferring the most abundant isotope… • It is possible to look at an average and take a good inference at which isotope is the most abundant in nature… • Argon – weighted average on PT is 39. 948 • Carbon – weighted average on PT is 12. 011 • Helium – weighted average is 4. 002

More inferences • Which isotope of Argon is most common if average is 39. 948, Argon -38 or Argon – 40? • Which isotope of Carbon is most abundant (average is 12. 011), Carbon- 12 or Carbon – 13? • Which isotope of Helium is most abundant (average is 4. 002), Helium- 3 or Helium – 4?

Look at the Periodic Table • What is the most abundant isotope of Rhodium, atomic number 45? • What is the most abundant isotope of Yttrium, atomic number 39? • What is the most abundant isotope of Sodium, atomic number 11?

Quick Review • What is the unit of atomic mass? • Oxygen has two numbers on the periodic table, 8 and 15. 994. Which one is the atomic mass? Which one is the atomic number? • If two isotopes of iodine are I-126 and I-127. The atomic mass on the Periodic Table is 126. 9045. Which isotope is most abundant? _______