Normal FPG 100 mgdl or 2 h PG


























![Structure Insulin Glargine Asp substitution A-chain A 21[Gly] COOH NH 2 COOH S B-chain Structure Insulin Glargine Asp substitution A-chain A 21[Gly] COOH NH 2 COOH S B-chain](https://slidetodoc.com/presentation_image_h/9188a7e72817ea2e28e41f9206319385/image-56.jpg)














- Slides: 78




Normal Ø FPG < 100 mg/dl or Ø 2 -h PG (OGTT) < 140 mg/dl

New Diagnostic Criteria for Diabetes ØDiabetic Sx & casual PG > 200 mg/dl or Ø FPG > 126 mg/dl or Ø 2 -h PG (OGTT, 75 gm) > 200 mg/dl

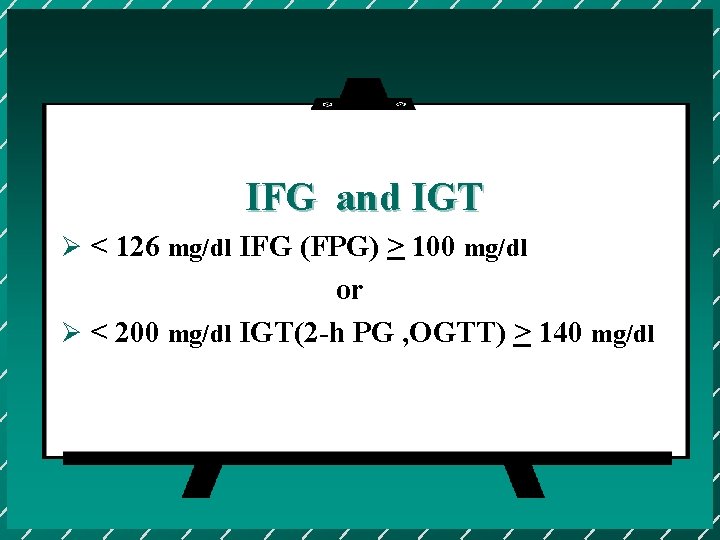
IFG and IGT Ø < 126 mg/dl IFG (FPG) > 100 mg/dl or Ø < 200 mg/dl IGT(2 -h PG , OGTT) > 140 mg/dl


分類方面常犯的錯誤 © Therapeutic Classification © Etiologic Classification

Classification of DM ( WHO, 1985 ) Diabetes mellitus (DM) Insulin-dependent diabetes mellitus ( IDDM ) Non-insulin-dependent diabetes mellitus ( NIDDM ) Malnutrition-related diabetes mellitus ( MRDM ) Other types pancreatic disease of hormonal etiology drug-induced or chemical-induced conditions abnormalities of insulin or its receptor genetic syndromes Gestational diabetes mellitus ( GDM ) Impaired glucose tolerance ( IGT )

New Classification of DM ( ADA, 1997 ) Type 1 diabetes Type 2 diabetes Other specific types genetic defects of b-cell function genetic defects of insulin action disease of the exocrine pancreas endocrinopathies drug- or chemical- induced infection uncommon forms of immune-mediated diabetes other genetic syndromes sometimes associated with diabetes Gestational diabetes mellitus ( GDM )

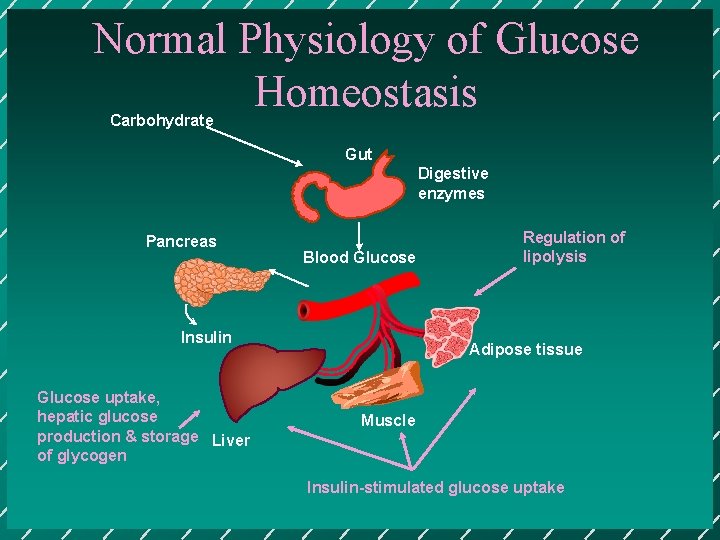
Normal Physiology of Glucose Homeostasis Carbohydrate Gut Digestive enzymes Pancreas Blood Glucose Insulin Glucose uptake, hepatic glucose production & storage Liver of glycogen Regulation of lipolysis Adipose tissue Muscle Insulin-stimulated glucose uptake

Types of Diabetes There are two main types of diabetes n. Type 1: Cellular-mediated autoimmune destruction of B-cells, usually leading to absolute insulin deficiency n. Type 2: Insulin resistance with relative insulin deficiency or insulin secretary defect with insulin resistance

Types of Diabetes Type 1 n 2~3 % of people with diabetes have type 1 n. Most frequently developed in children & adolescents, but now also increasingly found in adults n. Insulin is a life-sustaining medication n. Important management components diabetes education, a controlled diet & physical exercise

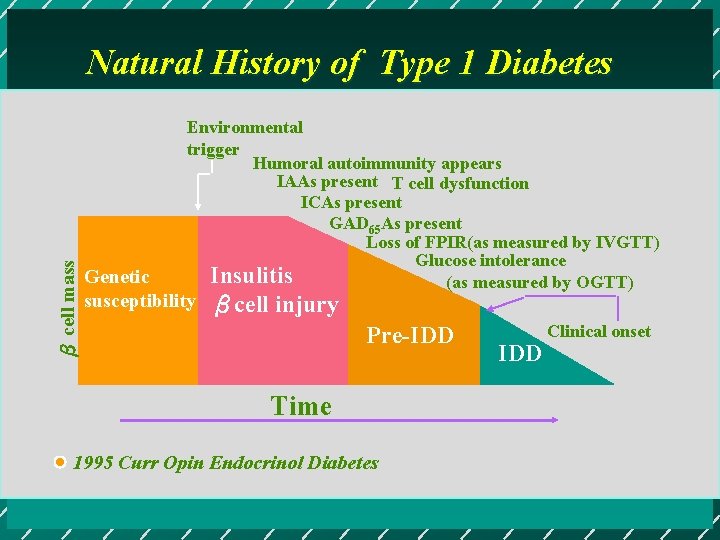
β cell mass Natural History of Type 1 Diabetes Environmental trigger Humoral autoimmunity appears IAAs present T cell dysfunction ICAs present GAD 65 As present Loss of FPIR(as measured by IVGTT) Glucose intolerance Insulitis (as measured by OGTT) Genetic susceptibility βcell injury Pre-IDD Time 1995 Curr Opin Endocrinol Diabetes IDD Clinical onset

Types of Diabetes > Type 2 n~ 97% of people with diabetes have type 2 n. Occurs mainly in adults but is becoming more common in young people n. To manage hyperglycaemia, oral medication may be required n. For metabolic control, insulin may be required n. Important management components diabetes education, a controlled diet & physical exercise

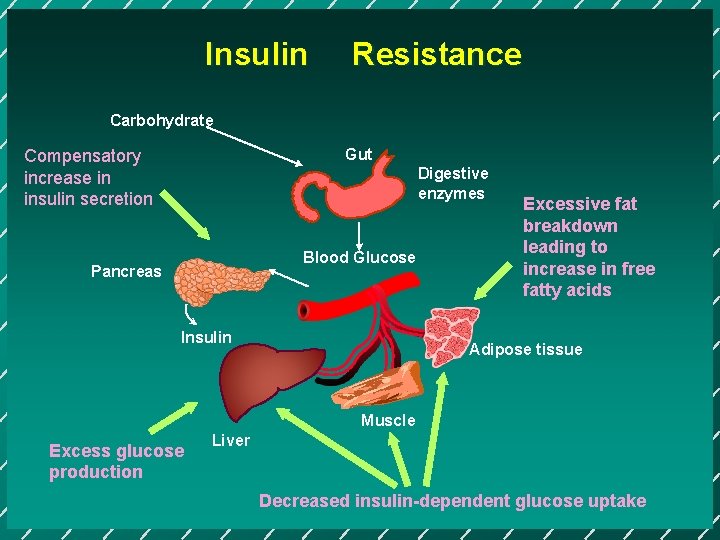
Insulin Resistance Carbohydrate Gut Compensatory increase in insulin secretion Digestive enzymes Blood Glucose Pancreas Insulin Excessive fat breakdown leading to increase in free fatty acids Adipose tissue Muscle Excess glucose production Liver Decreased insulin-dependent glucose uptake

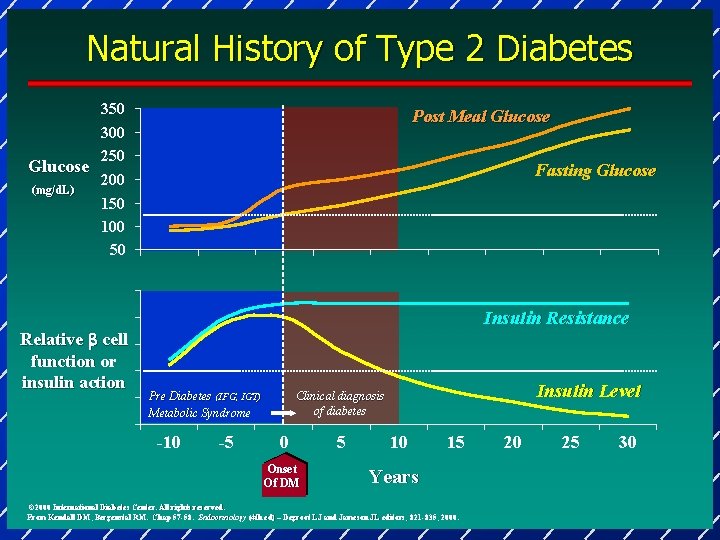
Natural History of Type 2 Diabetes Glucose (mg/d. L)) 350 300 250 Post Meal Glucose Fasting Glucose 200 150 100 50 Relative b cell function or insulin action Insulin Resistance -10 -5 Insulin Level Clinical diagnosis of diabetes Pre Diabetes (IFG, IGT) Metabolic Syndrome 0 Onset Of DM 5 10 15 Years © 2000 International Diabetes Center. All rights reserved. From Kendall DM, Bergenstal RM. Chap 57 -58. Endocrinology (4 th ed) – Degroot LJ and Jameson JL editors; 821 -835, 2000. 20 25 30



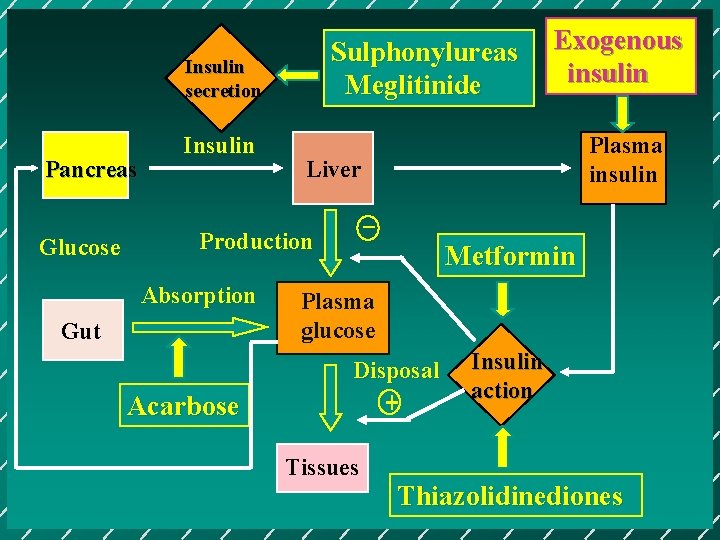
Oral hypoglycemic agents of DM Sulphonylureas Glinide - Novonorm ( Repaglinide ) - Starlix ( Nateglinide ) Biguanides α-glucosidase inhibitor - Acarbose Thiazolidinediones - Rosiglitazone Pioglitazone

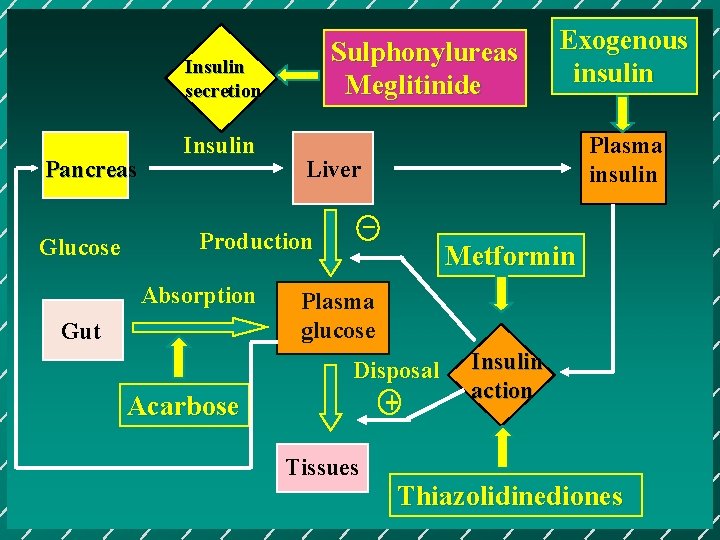
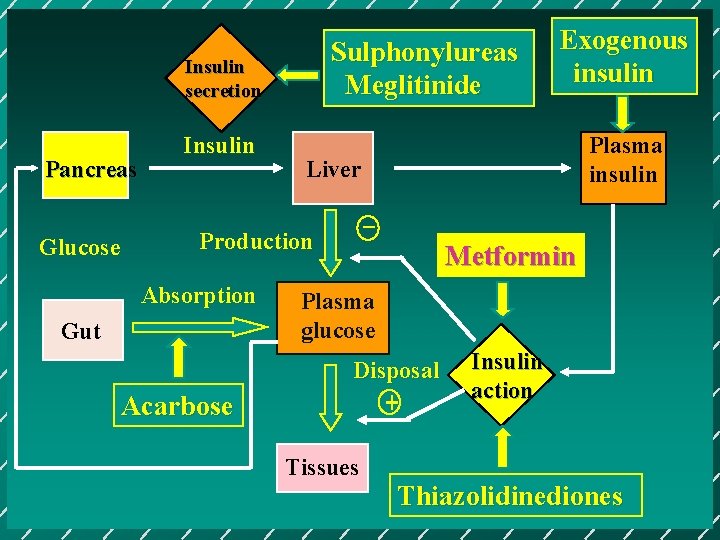
Sulphonylureas Meglitinide Insulin secretion Pancreas Glucose Insulin Gut Acarbose Plasma insulin Liver _ Production Absorption Exogenous insulin Metformin Plasma glucose Disposal + Tissues Insulin action Thiazolidinediones

Sulfonylureas First generation Second generation Tolbutamide Glibenclamide Chlorpropamide Acetohexamide Tolazamide Glipizide Gliclazide Glimepiride

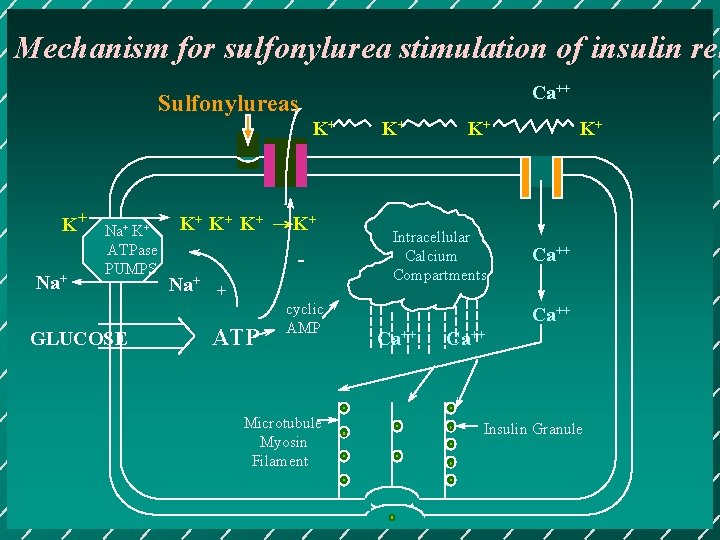
Mechanism for sulfonylurea stimulation of insulin rel Ca++ Sulfonylureas K+ K+ Na+ K+ ATPase PUMPS GLUCOSE K+ K+ K+ →K+ Na+ + ATP cyclic AMP Microtubule Myosin Filament K+ K+ Intracellular Calcium Compartments K+ Ca++ Insulin Granule

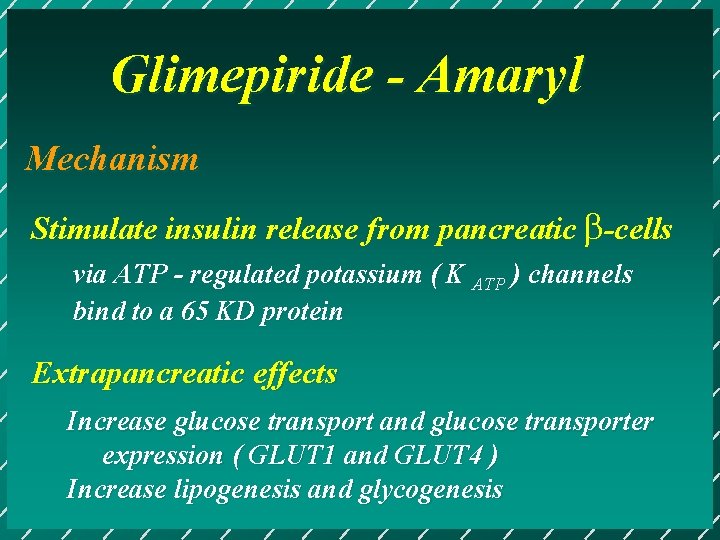
Glimepiride - Amaryl Mechanism Stimulate insulin release from pancreatic b-cells via ATP - regulated potassium ( K ATP ) channels bind to a 65 KD protein Extrapancreatic effects Increase glucose transport and glucose transporter expression ( GLUT 1 and GLUT 4 ) Increase lipogenesis and glycogenesis

DIAMICRON MR tablets

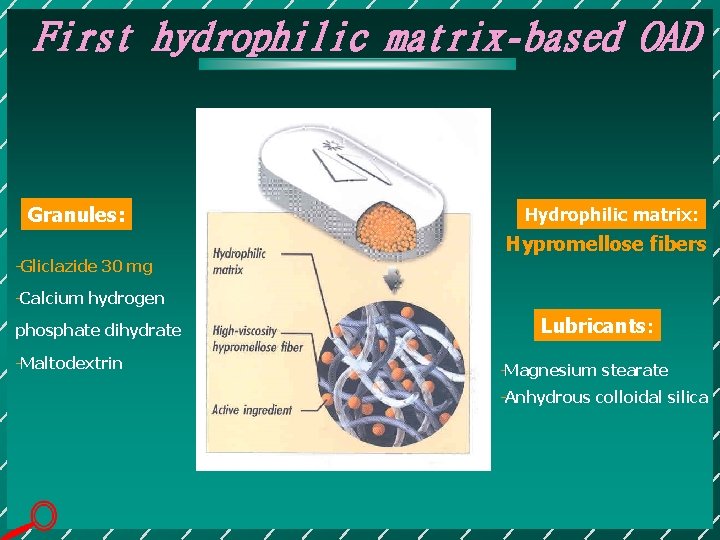
First hydrophilic matrix-based OAD Granules: Hydrophilic matrix: Hypromellose fibers Gliclazide 30 mg Calcium hydrogen phosphate dihydrate Maltodextrin Lubricants: Magnesium stearate Anhydrous colloidal silica

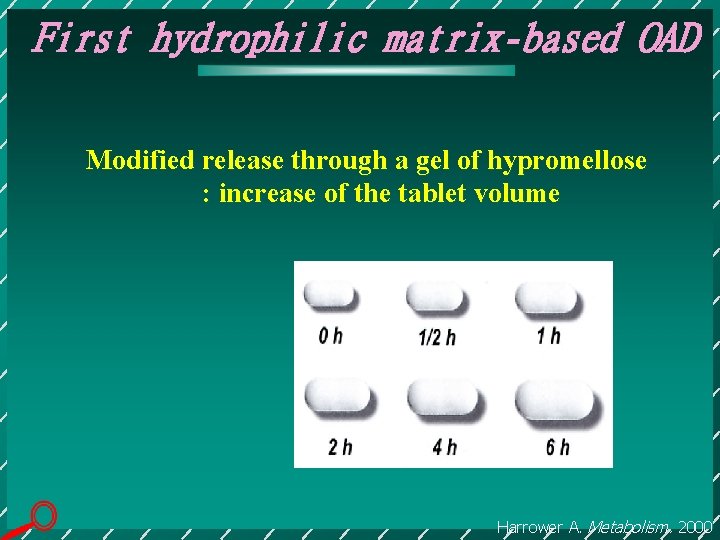
First hydrophilic matrix-based OAD Modified release through a gel of hypromellose : increase of the tablet volume Harrower A. Metabolism. 2000

The hydrophilic matrix Intestinal release of gliclazide - Immediate dissolution of a small fraction of active ingredient when in contact with water or GI fluid - Progressive infiltration of water induces the gelification of the hypromellose and progressive release of the granular - The hydrophilic matrix is excreted without degradation liquid

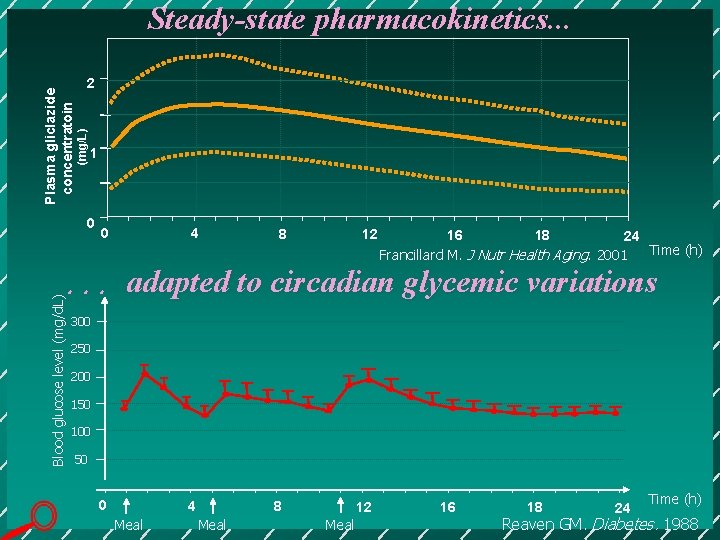
2 (mg/L) Plasma gliclazide concentratoin Steady-state pharmacokinetics. . . 1 0 0 4 8 12 16 18 24 Time (h) Francillard M. J Nutr Health Aging. 2001 Blood glucose level (mg/d. L) . . . adapted to circadian glycemic variations 300 250 200 150 100 50 0 4 Meal 8 Meal 12 Meal 16 18 24 Time (h) Reaven GM. Diabetes. 1988

Repaglinide - NOVONORM Mechanism Stimulate insulin release Pharmacology Rapidly absorb and clear (tmax and t 1/2 are approximately 1 hour) One meal, one dose - no meal, no dose

Fast-on for control; Fast-off for safety!

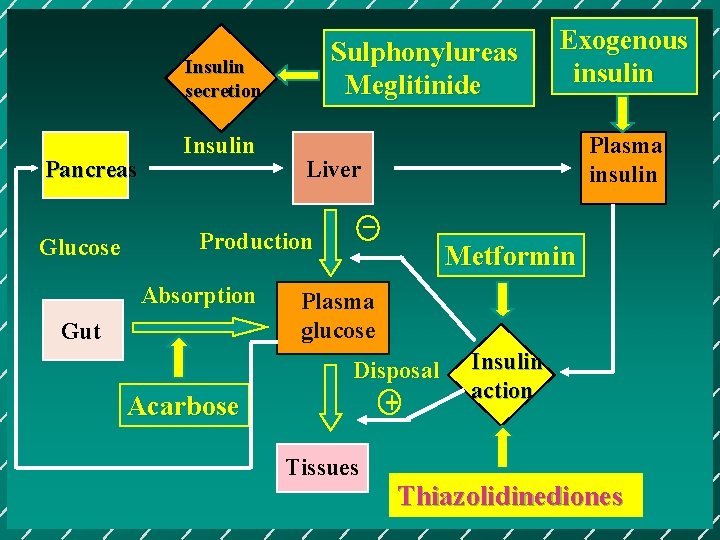
Sulphonylureas Meglitinide Insulin secretion Pancreas Glucose Insulin Gut Acarbose Plasma insulin Liver _ Production Absorption Exogenous insulin Metformin Plasma glucose Disposal + Tissues Insulin action Thiazolidinediones

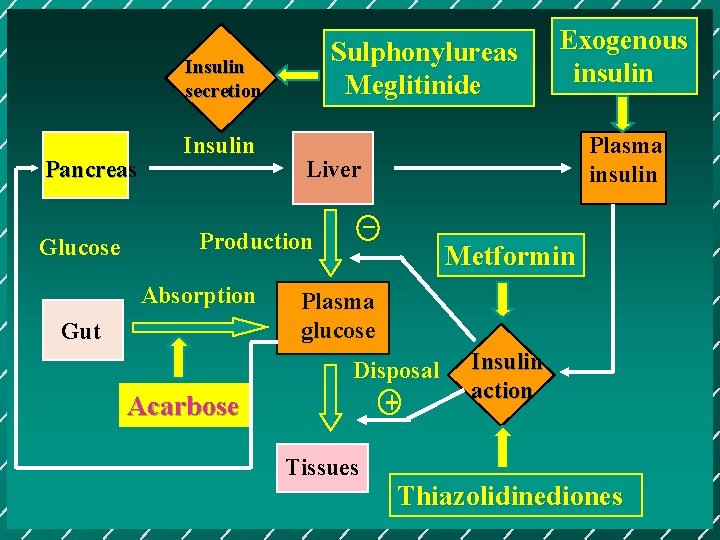
Sulphonylureas Meglitinide Insulin secretion Pancreas Glucose Insulin Gut Acarbose Plasma insulin Liver _ Production Absorption Exogenous insulin Metformin Plasma glucose Disposal + Tissues Insulin action Thiazolidinediones

Thiazolidinediones Troglitazone Rosiglitazone Pioglitazone

Rosiglitazone - Avandia Mechanism of action Decreasing insulin resistance Increasing insulin sensitivity Binding to peroxisome proliferator-activated receptor-gamma ( PPAR )

Piosiglitazone - ACTOS Mechanism of action Decreasing insulin resistance Increasing insulin sensitivity Binding to peroxisome proliferator-activated receptor-gamma ( PPAR ) & PPAR a.

Sulphonylureas Meglitinide Insulin secretion Pancreas Glucose Insulin Gut Acarbose Plasma insulin Liver _ Production Absorption Exogenous insulin Metformin Plasma glucose Disposal + Tissues Insulin action Thiazolidinediones

Acarbose Mechanism a - glucosidase inhibition slow digestion of sucrose, maltose, and complex carbohydrate blunt postprandial glucose rise Side Effect flatulence, abdominal discomfort and diarrhea

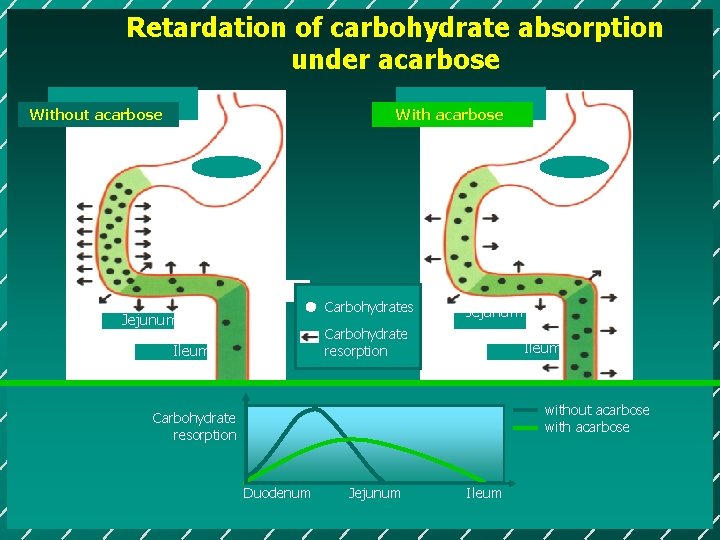
Retardation of carbohydrate absorption under acarbose Without acarbose With acarbose Carbohydrates Jejunum Carbohydrate resorption Ileum without acarbose with acarbose Carbohydrate resorption Duodenum Jejunum Ileum

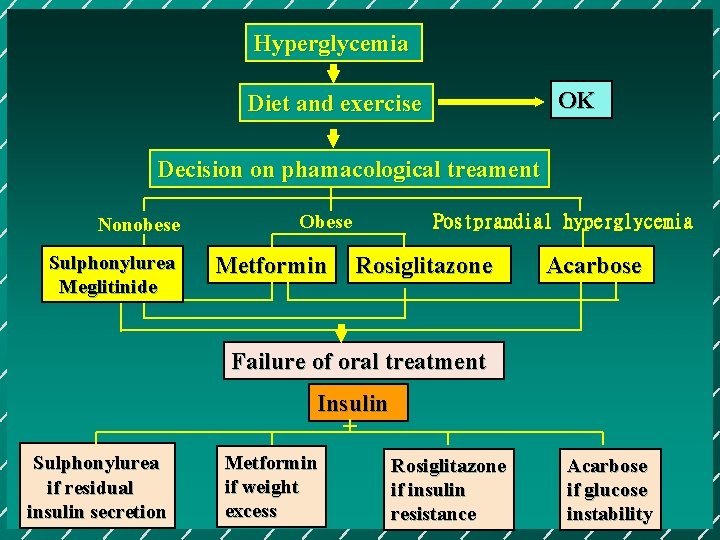
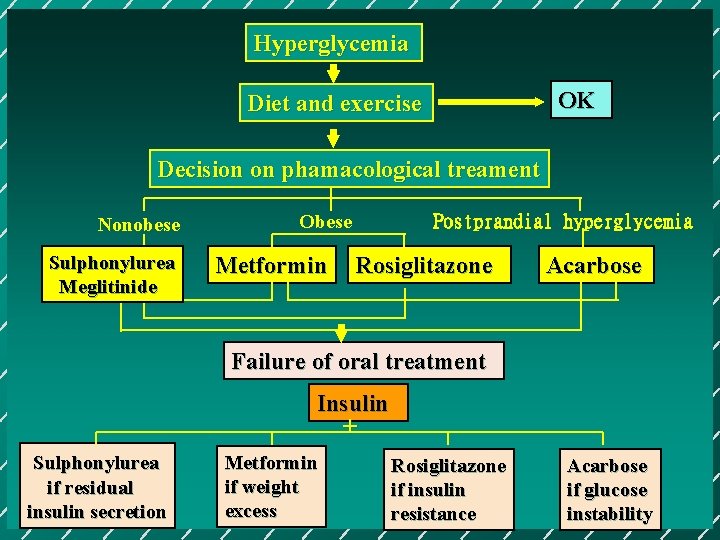
Hyperglycemia OK Diet and exercise Decision on phamacological treament Nonobese Sulphonylurea Meglitinide Obese Metformin Postprandial hyperglycemia Rosiglitazone Acarbose Failure of oral treatment Insulin + Sulphonylurea if residual insulin secretion Metformin if weight excess Rosiglitazone if insulin resistance Acarbose if glucose instability


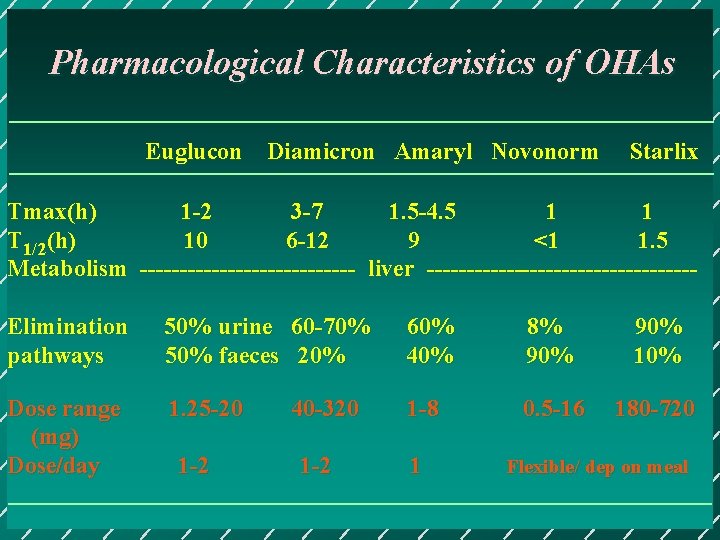
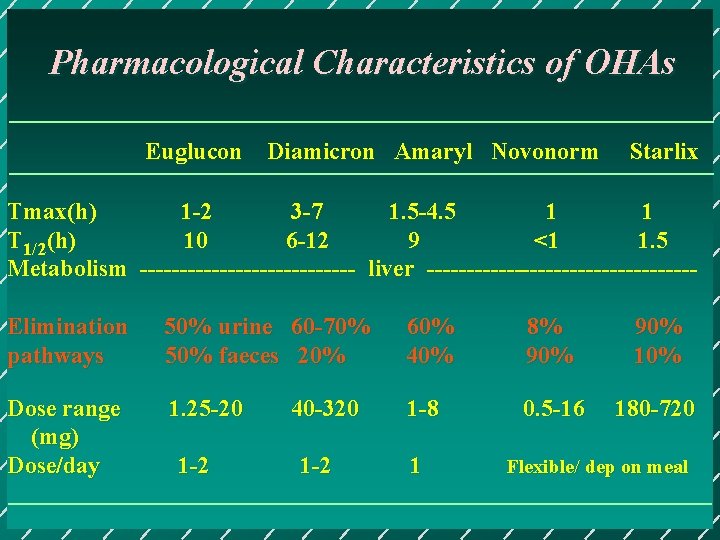
Pharmacological Characteristics of OHAs Euglucon Diamicron Amaryl Novonorm Starlix Tmax(h) 1 -2 3 -7 1. 5 -4. 5 1 1 T 1/2(h) 10 6 -12 9 <1 1. 5 Metabolism -------------- liver -----------------Elimination pathways 50% urine 60 -70% 50% faeces 20% 60% 40% 8% 90% 10% Dose range (mg) Dose/day 1. 25 -20 1 -8 0. 5 -16 180 -720 1 -2 40 -320 1 -2 1 Flexible/ dep on meal

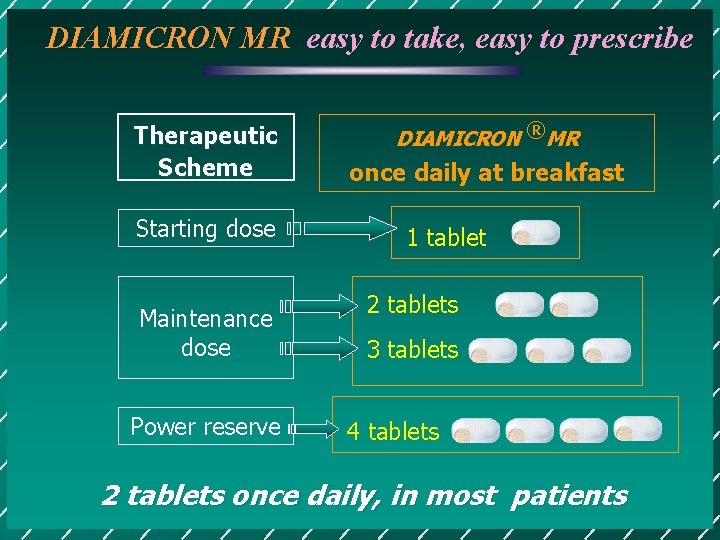
DIAMICRON MR easy to take, easy to prescribe Therapeutic Scheme Starting dose Maintenance dose Power reserve DIAMICRON ®MR once daily at breakfast 1 tablet 2 tablets 3 tablets 4 tablets 2 tablets once daily, in most patients

TZD Administration Avandia Once and twice daily, with or without meal ACTOS Once daily, with or without meal


Pharmacological Characteristics of OHAs Euglucon Diamicron Amaryl Novonorm Starlix Tmax(h) 1 -2 3 -7 1. 5 -4. 5 1 1 T 1/2(h) 10 6 -12 9 <1 1. 5 Metabolism -------------- liver -----------------Elimination pathways 50% urine 60 -70% 50% faeces 20% 60% 40% 8% 90% 10% Dose range (mg) Dose/day 1. 25 -20 1 -8 0. 5 -16 180 -720 1 -2 40 -320 1 -2 1 Flexible/ dep on meal

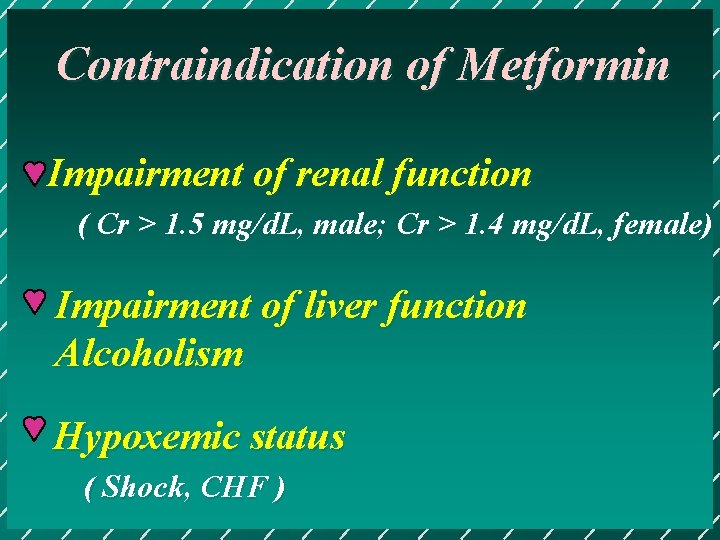
Contraindication of Metformin Impairment of renal function ( Cr > 1. 5 mg/d. L, male; Cr > 1. 4 mg/d. L, female) Impairment of liver function Alcoholism Hypoxemic status ( Shock, CHF )

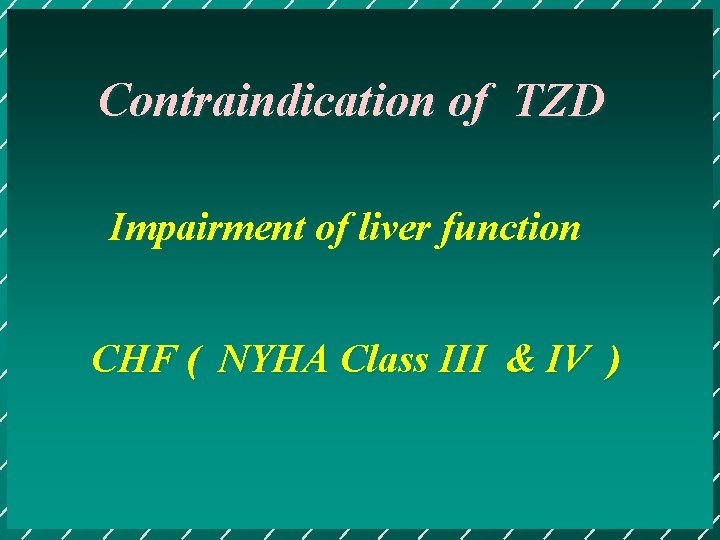
Contraindication of TZD Impairment of liver function CHF ( NYHA Class III & IV )

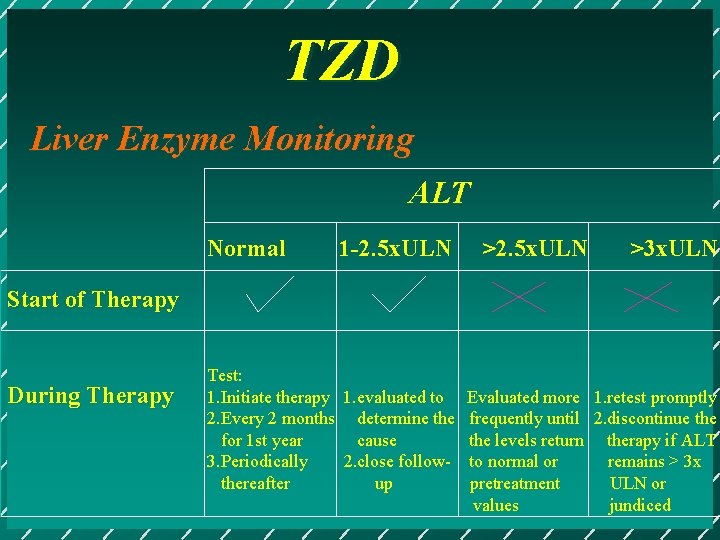
TZD Liver Enzyme Monitoring ALT Normal 1 -2. 5 x. ULN >3 x. ULN Start of Therapy During Therapy Test: 1. Initiate therapy 1. evaluated to Evaluated more 1. retest promptly 2. Every 2 months determine the frequently until 2. discontinue the for 1 st year cause the levels return therapy if ALT 3. Periodically 2. close follow- to normal or remains > 3 x thereafter up pretreatment ULN or values jundiced

Sulphonylureas Meglitinide Insulin secretion Pancreas Glucose Insulin Gut Acarbose Plasma insulin Liver _ Production Absorption Exogenous insulin Metformin Plasma glucose Disposal + Tissues Insulin action Thiazolidinediones

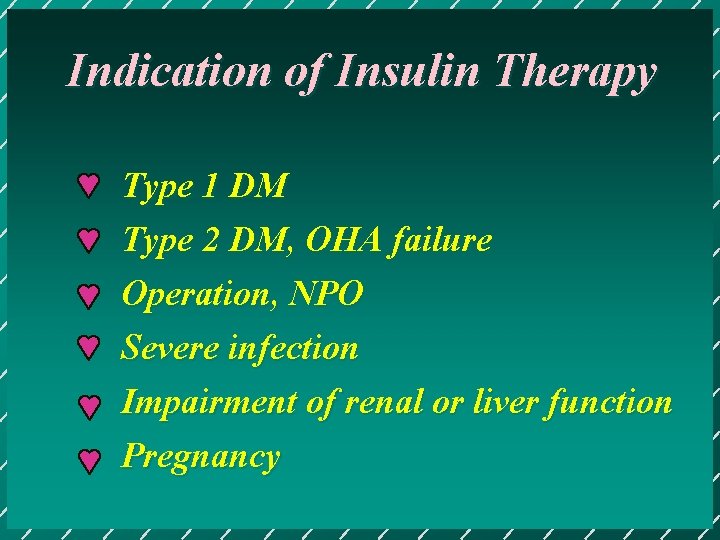
Indication of Insulin Therapy Type 1 DM Type 2 DM, OHA failure Operation, NPO Severe infection Impairment of renal or liver function Pregnancy

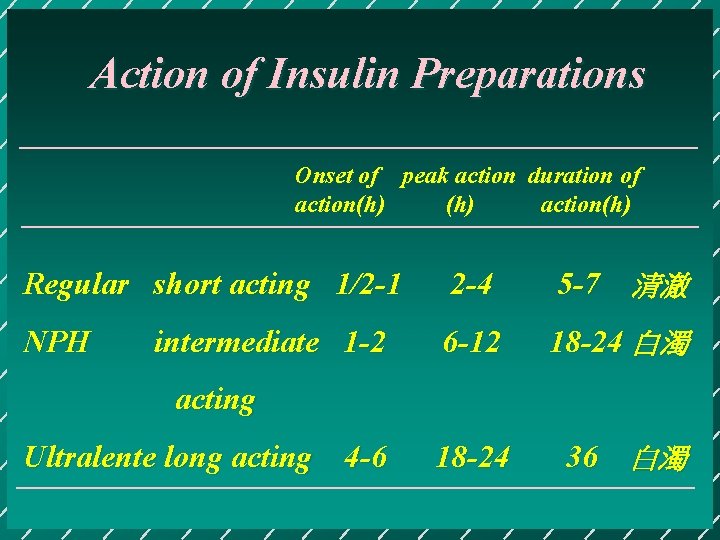
Action of Insulin Preparations Onset of peak action duration of action(h) Regular short acting 1/2 -1 2 -4 5 -7 清澈 NPH 6 -12 18 -24 白濁 18 -24 36 白濁 intermediate 1 -2 acting Ultralente long acting 4 -6

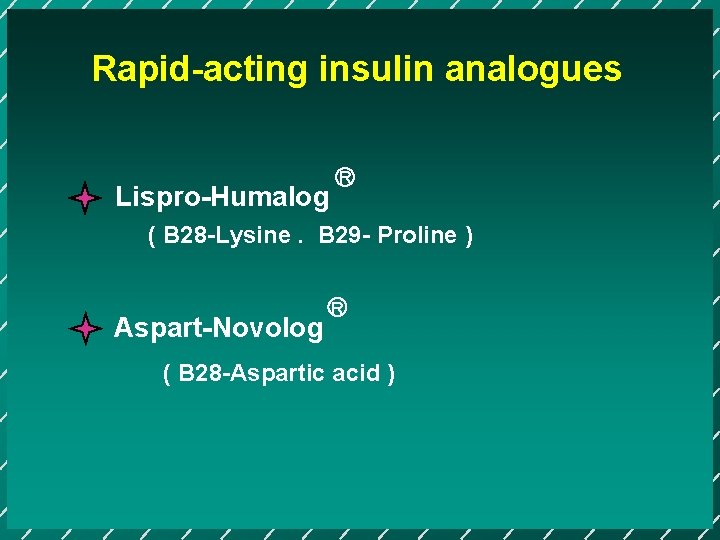
Rapid-acting insulin analogues Lispro-Humalog R ( B 28 -Lysine. B 29 - Proline ) Aspart-Novolog R ( B 28 -Aspartic acid )

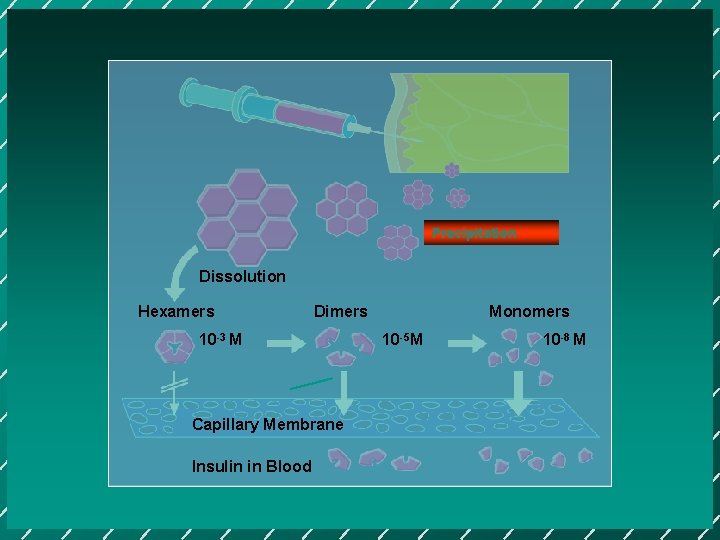
Precipitation Dissolution Hexamers Dimers 10 -3 M Capillary Membrane Insulin in Blood Monomers 10 -5 M 10 -8 M

The “Ideal” Long-Acting Insulin · Insulin Glargine (Lantus) – The first available long-acting insulin analogue – 24 -hour peakless profile – Once daily Subcutaneous administration Safe – – • no toxins, mitogenic or immunogenic effects – – Low intrasubject and intersubject variability Good glycaemic control
![Structure Insulin Glargine Asp substitution Achain A 21Gly COOH NH 2 COOH S Bchain Structure Insulin Glargine Asp substitution A-chain A 21[Gly] COOH NH 2 COOH S B-chain](https://slidetodoc.com/presentation_image_h/9188a7e72817ea2e28e41f9206319385/image-56.jpg)
Structure Insulin Glargine Asp substitution A-chain A 21[Gly] COOH NH 2 COOH S B-chain S S NH 2 S S S B 31[Arg] B 32[Arg] Arg extension These changes cause a shift in the isoelectric point from 5. 4 in native insulin to 7. 0 in insulin glargine Vajo & Duckworth, 2000. Pharmacological Reviews, 52: 1 -9; Heinemann L et al. Diabetes Care 2000; 23: 644– 649 ; Hilgenfeld R et al. Diabetologia 1992; 35[Suppl 1]: A 193

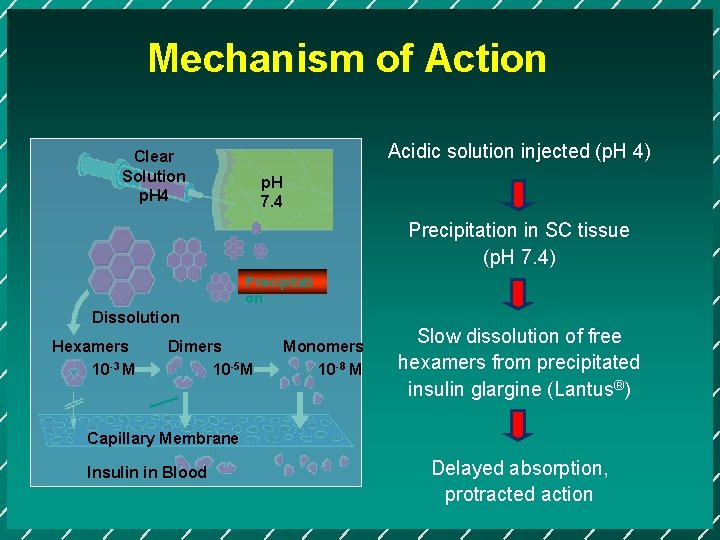
Mechanism of Action Acidic solution injected (p. H 4) Clear Solution p. H 4 p. H 7. 4 Precipitation in SC tissue (p. H 7. 4) Precipitati on Dissolution Hexamers 10 -3 M Dimers 10 -5 M Monomers 10 -8 M Slow dissolution of free hexamers from precipitated insulin glargine (Lantus®) Capillary Membrane Insulin in Blood Delayed absorption, protracted action

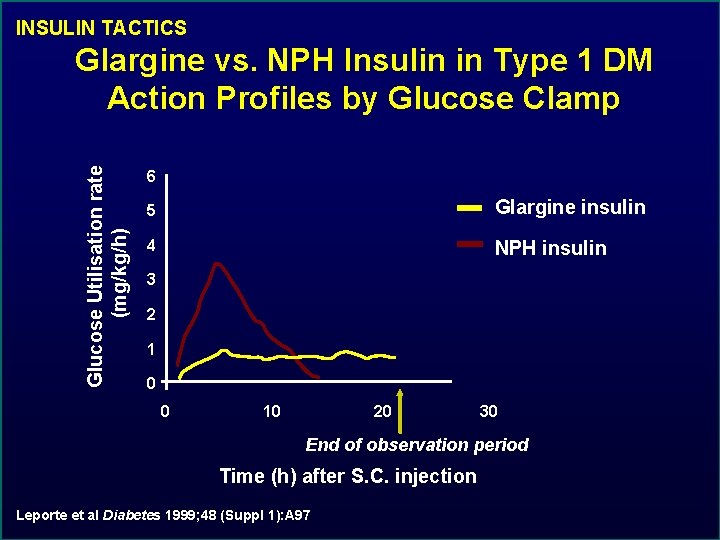
INSULIN TACTICS Glucose Utilisation rate (mg/kg/h) Glargine vs. NPH Insulin in Type 1 DM Action Profiles by Glucose Clamp 6 5 Glargine insulin 4 NPH insulin 3 2 1 0 0 10 20 30 End of observation period Time (h) after S. C. injection Leporte et al Diabetes 1999; 48 (Suppl 1): A 97

Hyperglycemia OK Diet and exercise Decision on phamacological treament Nonobese Sulphonylurea Meglitinide Obese Metformin Postprandial hyperglycemia Rosiglitazone Acarbose Failure of oral treatment Insulin + Sulphonylurea if residual insulin secretion Metformin if weight excess Rosiglitazone if insulin resistance Acarbose if glucose instability

Hyperglycemic Crisis DKA HHS


DKA Diabetic Ketoacidosis hyperglycemia hyperketomeia in the presence of acidosis

Diagnostic Criteria for DKA Glucose > 250 mg/d. L PH < 7. 30 Low HCO 3 - (<15 m. Eq/l) High anion gap Positive serum ketones

HHS (HHNK) Hyperosmolar Hyperglycemia State/ Hyperosmolar Hyperglycemic Nonketotic Coma 高血糖高滲透壓症候群/高血糖高滲透壓非酮體性昏迷 Severe hyperglycemia, hyperosmolarity, dehydration, no acidosis Occurs in middle aged & elderly patients Lack of ketosis Insulin levels high enough to prevent lipolysis & ketogenesis, not high enough to prevent hyperglycemia

Calculation of serum osmolality effective serum osmolality 2 (Na) + GLUCOSE 18 N = 285 + 5 m. Osm/L HHS > 320


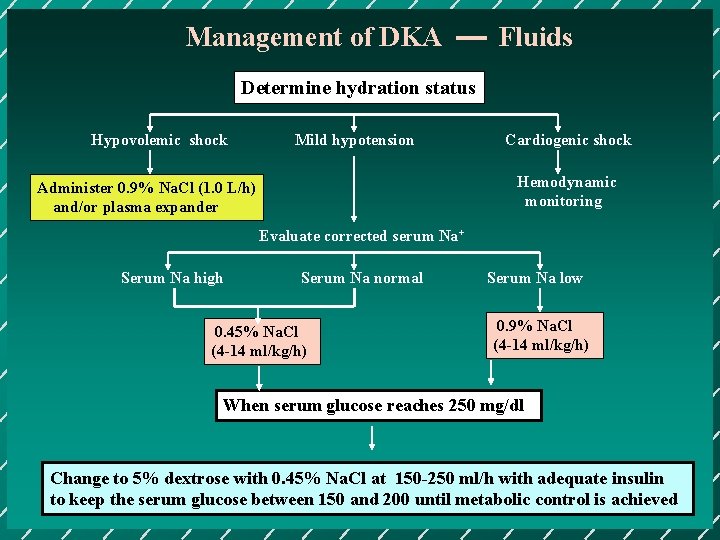
Management of DKA Fluids Determine hydration status Hypovolemic shock Mild hypotension Cardiogenic shock Hemodynamic monitoring Administer 0. 9% Na. Cl (1. 0 L/h) and/or plasma expander Evaluate corrected serum Na+ Serum Na high Serum Na normal 0. 45% Na. Cl (4 -14 ml/kg/h) Serum Na low 0. 9% Na. Cl (4 -14 ml/kg/h) When serum glucose reaches 250 mg/dl Change to 5% dextrose with 0. 45% Na. Cl at 150 -250 ml/h with adequate insulin to keep the serum glucose between 150 and 200 until metabolic control is achieved

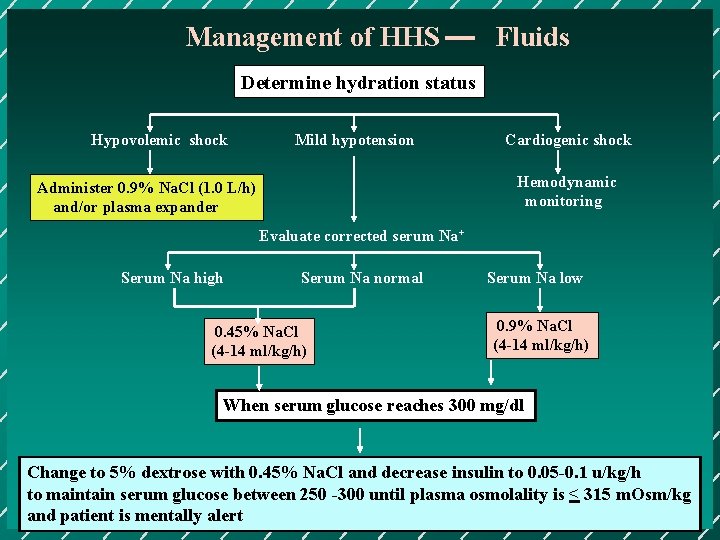
Management of HHS Fluids Determine hydration status Hypovolemic shock Mild hypotension Cardiogenic shock Hemodynamic monitoring Administer 0. 9% Na. Cl (1. 0 L/h) and/or plasma expander Evaluate corrected serum Na+ Serum Na high Serum Na normal 0. 45% Na. Cl (4 -14 ml/kg/h) Serum Na low 0. 9% Na. Cl (4 -14 ml/kg/h) When serum glucose reaches 300 mg/dl Change to 5% dextrose with 0. 45% Na. Cl and decrease insulin to 0. 05 -0. 1 u/kg/h to maintain serum glucose between 250 -300 until plasma osmolality is < 315 m. Osm/kg and patient is mentally alert


DKA Insulin Therapy 0. 2 u/kg regular, intravenous push 0. 1 u/kg/hr continous infusion Double each 1 -2 h if < 10% drop in glucose or no improvement in the anion gap or PH Reduce with glucose < 250 mg/d. L, if progressive improvement in anion gap and PH Add glucose to IV when serum glucose < 250

Insulin Therapy Low-dose insulin therapy avoid the hazards of rapid & erratic serum glucose fluctuation avoid episode of late hypoglycemia & hypokalemia Add glucose to IV when serum glucose < 250 mg/d. L is necessary for correction of tissue lipolysis & acidosis Plasma glucose level ordinary fall prior reversal of acidosis insulin should not be stopped when this occurs glucose should be infused to allow continuation of insulin therapy After discontinuing the insulin infusion give the first SC dose of insulin about 30 mins prior to discontinuing the infusion - total daily insulin dose of 2/3 u/kg

HHS Treatment - Insulin Immediate Subsequent 1. Regular insulin 0. 05 -0. 10 1. Decrease infusion rate to u/kg/hr IV. Monitor 1 -3 u/hr switch to SC glucose hourly insulin 2. Monitor glucose and electrolytes q 4 h


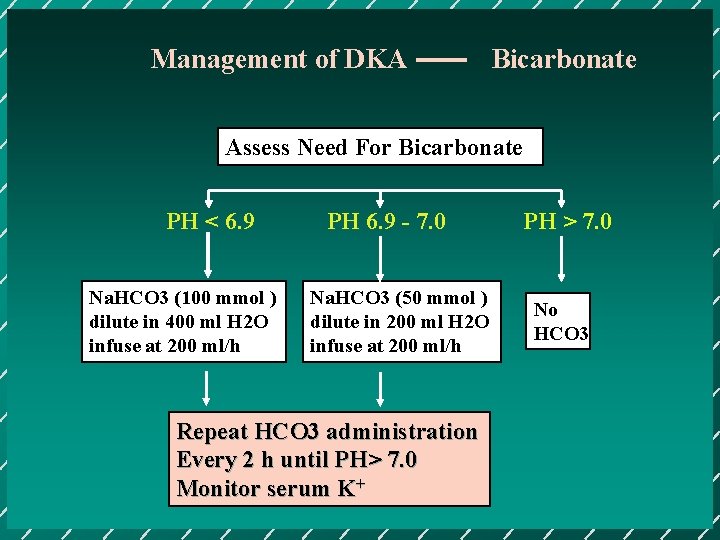
Management of DKA Bicarbonate Assess Need For Bicarbonate PH < 6. 9 Na. HCO 3 (100 mmol ) dilute in 400 ml H 2 O infuse at 200 ml/h PH 6. 9 - 7. 0 Na. HCO 3 (50 mmol ) dilute in 200 ml H 2 O infuse at 200 ml/h Repeat HCO 3 administration Every 2 h until PH> 7. 0 Monitor serum K+ PH > 7. 0 No HCO 3


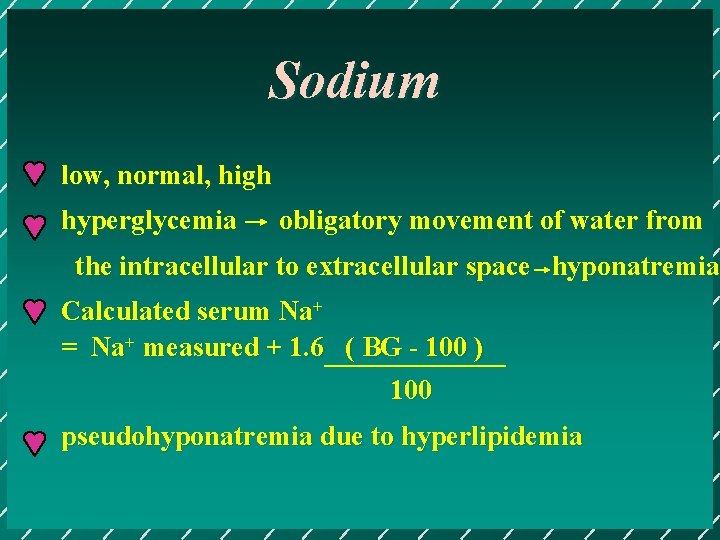
Sodium low, normal, high hyperglycemia obligatory movement of water from the intracellular to extracellular space hyponatremia Calculated serum Na+ = Na+ measured + 1. 6 ( BG - 100 ) 100 pseudohyponatremia due to hyperlipidemia

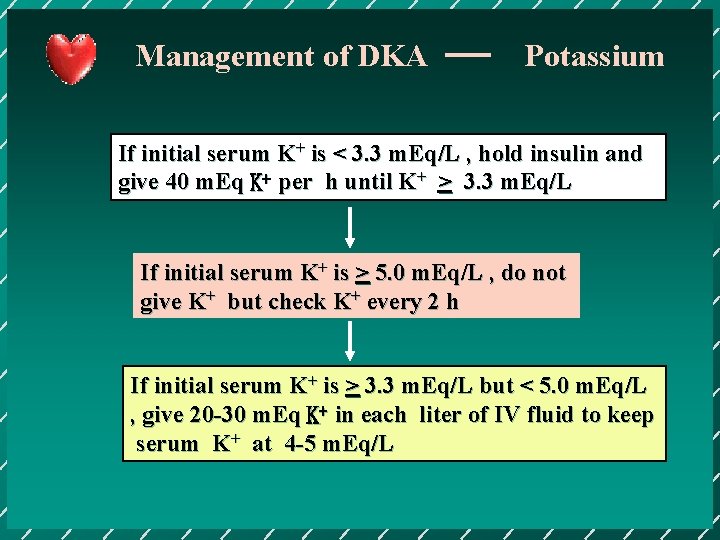
Management of DKA Potassium If initial serum K+ is < 3. 3 m. Eq/L , hold insulin and give 40 m. Eq K+ per h until K+ > 3. 3 m. Eq/L If initial serum K+ is > 5. 0 m. Eq/L , do not give K+ but check K+ every 2 h If initial serum K+ is > 3. 3 m. Eq/L but < 5. 0 m. Eq/L , give 20 -30 m. Eq K+ in each liter of IV fluid to keep serum K+ at 4 -5 m. Eq/L

THE END