Macromolecules NOTES TO BE WRITTEN ARE IN RED

Macromolecules NOTES TO BE WRITTEN ARE IN RED.

Carbon Compounds �Biomolecule is an organic molecule produced by a living organism �Carbon can bond with many elements, including hydrogen, oxygen, phosphorus, sulfur, and nitrogen to form the molecules of life. �Carbon can share its electrons with other atoms to form up to four covalent bonds. �Carbon-carbon bonds can be single, double, or triple covalent bonds. �Chains of carbon atoms can even close up on themselves to form rings.
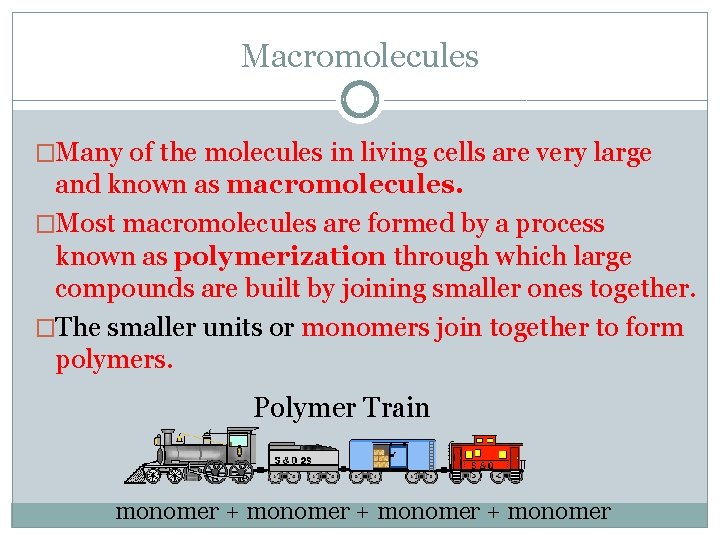
Macromolecules �Many of the molecules in living cells are very large and known as macromolecules. �Most macromolecules are formed by a process known as polymerization through which large compounds are built by joining smaller ones together. �The smaller units or monomers join together to form polymers. Polymer Train monomer + monomer
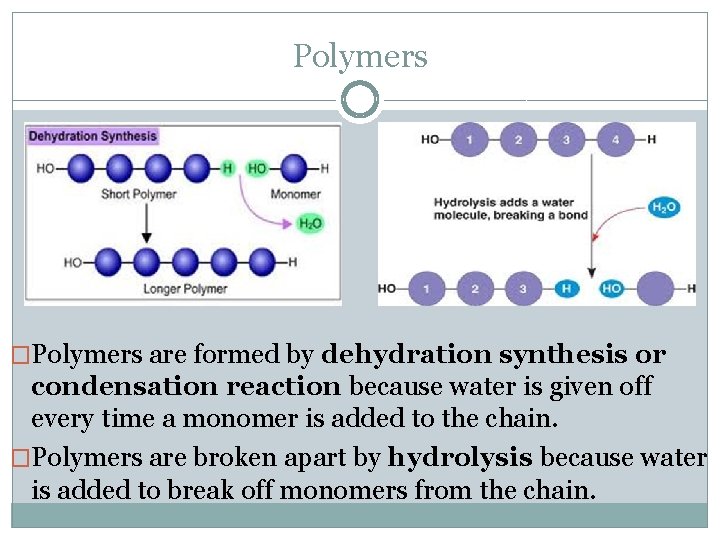
Polymers �Polymers are formed by dehydration synthesis or condensation reaction because water is given off every time a monomer is added to the chain. �Polymers are broken apart by hydrolysis because water is added to break off monomers from the chain.
Types of Macromolecules �Carbohydrates �Lipids �Proteins �Nucleic Acids

Macromolecules: Carbohydrates Type Structures Monomers Function Sugar, starches, and cellulose Carbon: Hydrogen: Oxygen Glucose Living things use carbohydrates as their main source of energy. Monosaccharide 1: 2: 1 ratio C 6 H 12 O 6 Disaccharides Polysaccharides
Types of Carbohydrates �Monosaccharide: one simple sugar. Examples are glucose (plant sugar), fructose(fruit sugar), galactose(milk sugar) �The formula for glucose is C 6 H 12 O 6. Plants produce glucose during photosynthesis �Disaccharide: two simple sugars joined by a saccharide bond. Examples are sucrose(table sugar made of glucose bonded to fructose), lactose(milk sugar), and maltose (malt beverage sugar) �http: //www. youtube. com/watch? v=Qckf. Yv. Il. Vu 4
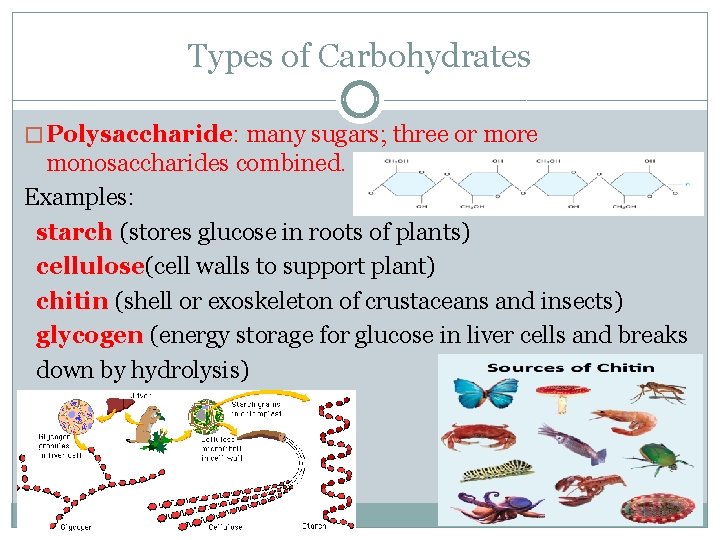
Types of Carbohydrates � Polysaccharide: many sugars; three or more monosaccharides combined. Examples: starch (stores glucose in roots of plants) cellulose(cell walls to support plant) chitin (shell or exoskeleton of crustaceans and insects) glycogen (energy storage for glucose in liver cells and breaks down by hydrolysis)
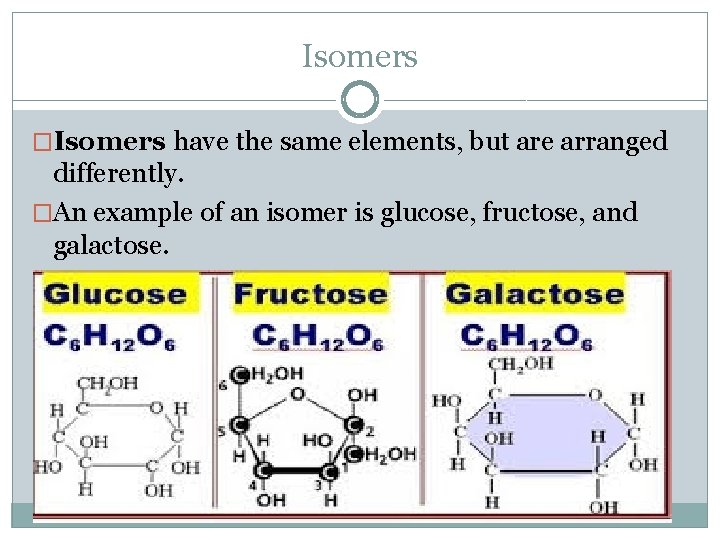
Isomers �Isomers have the same elements, but are arranged differently. �An example of an isomer is glucose, fructose, and galactose.
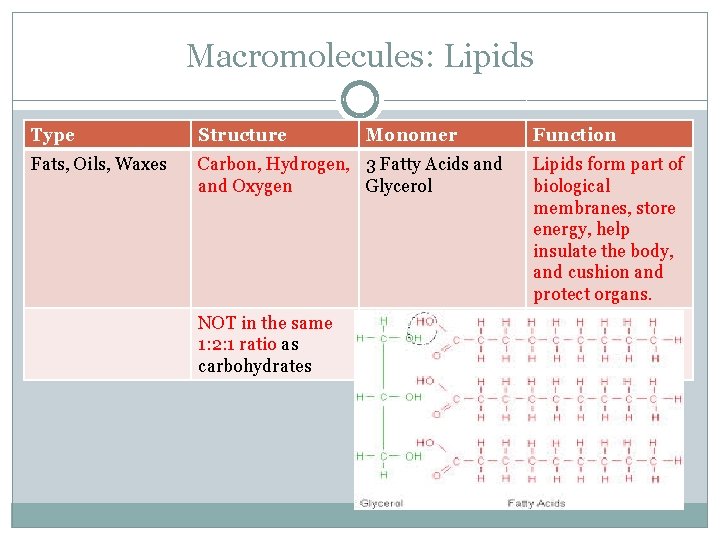
Macromolecules: Lipids Type Structure Fats, Oils, Waxes Carbon, Hydrogen, 3 Fatty Acids and Oxygen Glycerol NOT in the same 1: 2: 1 ratio as carbohydrates Monomer Function Lipids form part of biological membranes, store energy, help insulate the body, and cushion and protect organs.

Lipids �Lipids are made of glycerol and three fatty acids. �Lipids contain fewer oxygen than carbohydrates �Lipids are used for protection, cushion, structure, insulation, and long term energy storage. �Main types of lipids are fat, phospholipids, steroids, and wax. �http: //www. youtube. com/watch? v=3 x. F_LK 9 pn. L 0
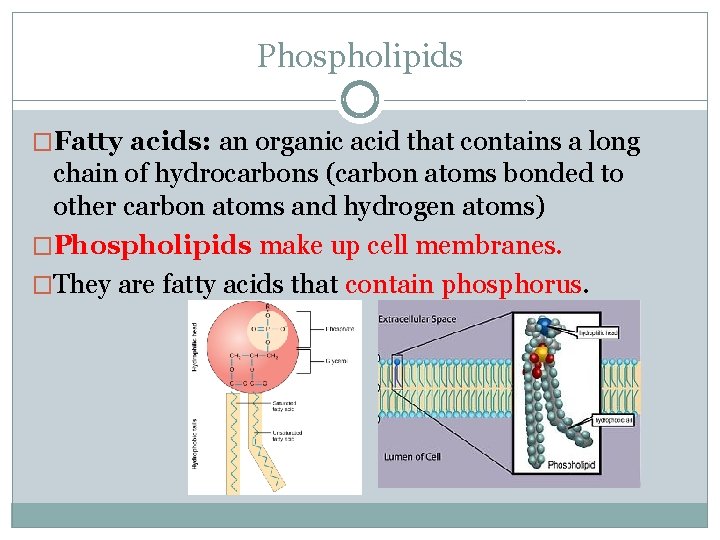
Phospholipids �Fatty acids: an organic acid that contains a long chain of hydrocarbons (carbon atoms bonded to other carbon atoms and hydrogen atoms) �Phospholipids make up cell membranes. �They are fatty acids that contain phosphorus.
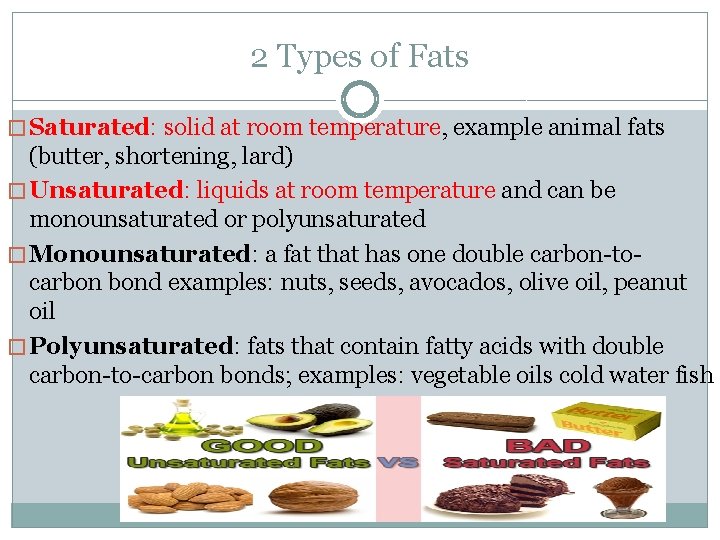
2 Types of Fats � Saturated: solid at room temperature, example animal fats (butter, shortening, lard) � Unsaturated: liquids at room temperature and can be monounsaturated or polyunsaturated � Monounsaturated: a fat that has one double carbon-tocarbon bond examples: nuts, seeds, avocados, olive oil, peanut oil � Polyunsaturated: fats that contain fatty acids with double carbon-to-carbon bonds; examples: vegetable oils cold water fish

Other Types of Lipids �Steroid: a type of lipid that can be present in cell membranes or can make up certain hormones which are chemical messengers or regulators. �Wax: a type of lipid that is used to waterproof leaves, skins, and feathers.
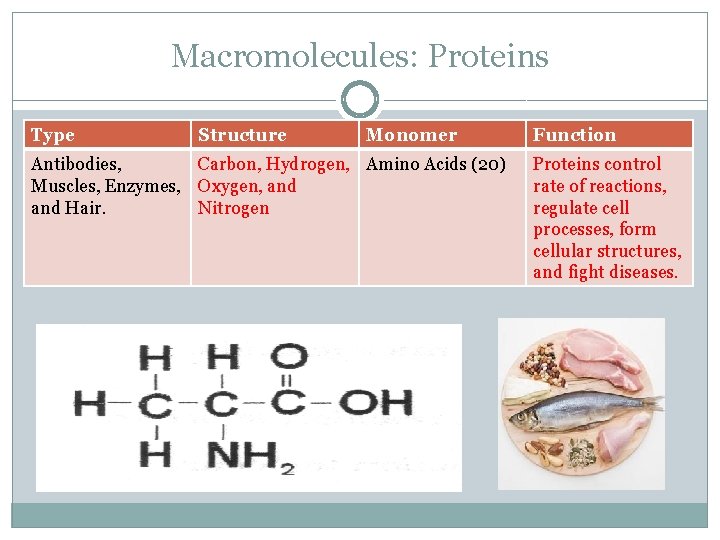
Macromolecules: Proteins Type Structure Monomer Antibodies, Carbon, Hydrogen, Amino Acids (20) Muscles, Enzymes, Oxygen, and Hair. Nitrogen Function Proteins control rate of reactions, regulate cell processes, form cellular structures, and fight diseases.
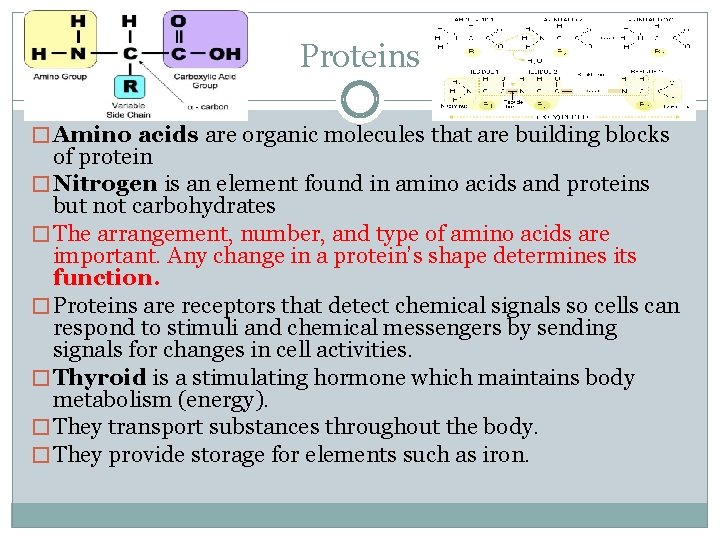
Proteins � Amino acids are organic molecules that are building blocks of protein � Nitrogen is an element found in amino acids and proteins but not carbohydrates � The arrangement, number, and type of amino acids are important. Any change in a protein’s shape determines its function. � Proteins are receptors that detect chemical signals so cells can respond to stimuli and chemical messengers by sending signals for changes in cell activities. � Thyroid is a stimulating hormone which maintains body metabolism (energy). � They transport substances throughout the body. � They provide storage for elements such as iron.

Proteins �Proteins are composed of carbon, hydrogen, oxygen, and nitrogen with sulfur and two amino acids �The building blocks of protein are amino acids held together by a peptide bond, a chain of amino acids. �Examples of proteins are antibodies (protect against disease), main structural component of muscles, skin, bone, hair, and enzymes. �http: //www. youtube. com/watch? v=w-ctk. PUUp. Uc

Macromolecules: Nucleic Acids Type Structure Monomer DNA RNA Carbon, Hydrogen, Nucleotides Oxygen, Nitrogen, Phosphorus Function Nucleic acids store and transmit genetic information.
Nucleic Acids Nucleic acids are used for controlling cellular activities and making protein (genes) Nucleic acids are made of: � a simple sugar: deoxyribose for DNA (what the “D” stands for) and ribose for RNA (what the “R” stands for_ � phosphate group � nitrogen base: adenine, cytosine, guanine occur in both DNA and RNA, and thymine occurs only in DNA and adenine, cytosine, guanine, and uracil only in RNA � DNA stores and transmits genetic information. The main function of RNA is protein synthesis, making proteins. � http: //www. youtube. com/watch? v=j. KMw. Lrb. Yy. J 0
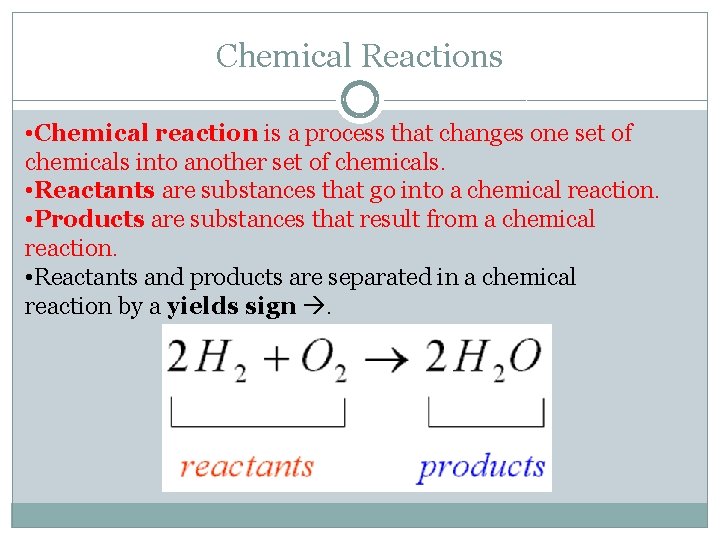
Chemical Reactions • Chemical reaction is a process that changes one set of chemicals into another set of chemicals. • Reactants are substances that go into a chemical reaction. • Products are substances that result from a chemical reaction. • Reactants and products are separated in a chemical reaction by a yields sign .
Enzymes � Activation energy is the term used to describe the energy needed to get a reaction started. A catalyst is a substance that accelerates the rate of a chemical reaction. � Enzymes are proteins that act as biological catalysts. Enzymes do not cause reactions to happen. They speed up chemical reactions that take place in cells. � Enzymes act by lowering activation energies, which has a dramatic effect on how quickly reactions are completed. Without enzymes the reactions of the cells would proceed very slowly. � Enzymes are never used up in the reaction. They can be used over and over again. � Enzymes get their names from the substances they act on and on the action of the enzyme and end in “ase. ” � Examples: Lipase – breaks down a lipid; protease breaks down protein; amylase breaks down carbohydrates; lactase breaks down lactose
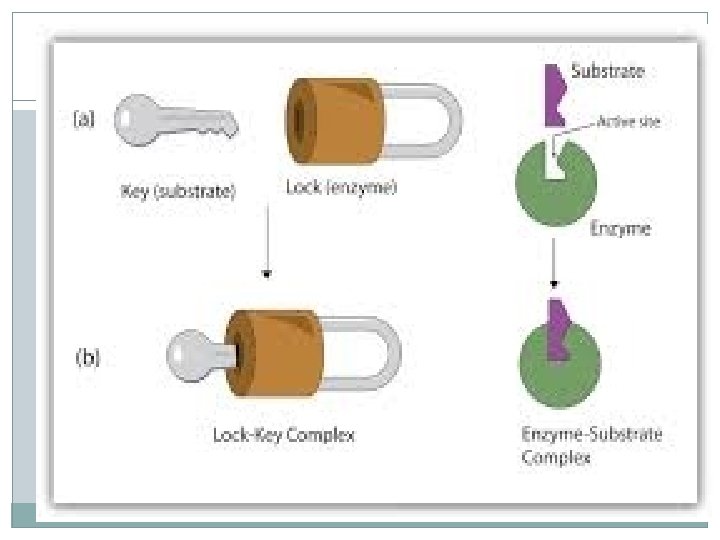
How Enzymes Work �Enzymes are very specific, generally catalyzing only one chemical reaction. �The reactants of the enzyme-catalyzed reactions are known as substrates. �The substrates bind to a site on the enzyme called the active site. �The active site and the substrate have complementary shapes. �The fit is so precise the active site and substrate are often compared to a lock and key.
Enzyme Regulation � Concentration- the number of particles either substrate or enzyme can cause a reaction to speed up until it reaches a saturation point in which all enzyme or substrate molecules are exchanged in reaction. � Temperature – Enzymes produced by the human cells generally work best at temperatures close to 37°C, the normal temperature of the human body. � p. H – Enzymes work best at certain p. H values. For example, the stomach enzyme pepsin, which begins protein digestion, works best under acidic conditions. � Salinity – refers to the concentration of salt in a solution or the number of ions in a solution. � Regulatory Molecules- The activities of most enzymes are regulated by molecules that carry certain chemical signals within cells, switching enzymes “on” or “off” as needed.
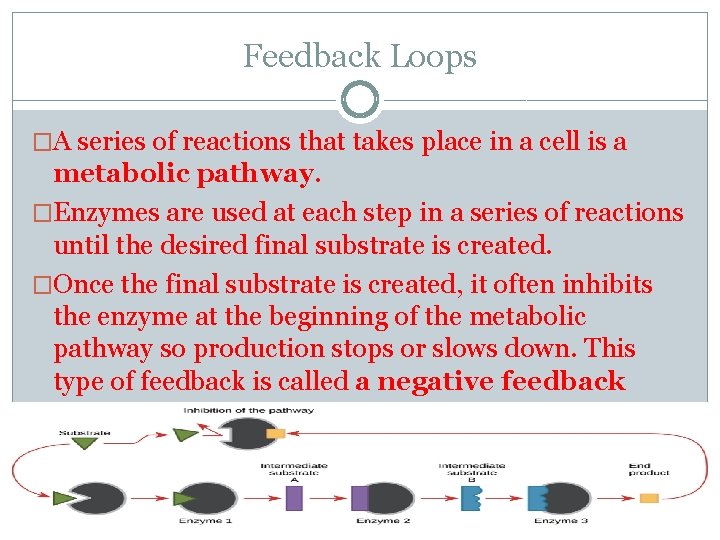
Feedback Loops �A series of reactions that takes place in a cell is a metabolic pathway. �Enzymes are used at each step in a series of reactions until the desired final substrate is created. �Once the final substrate is created, it often inhibits the enzyme at the beginning of the metabolic pathway so production stops or slows down. This type of feedback is called a negative feedback loop.
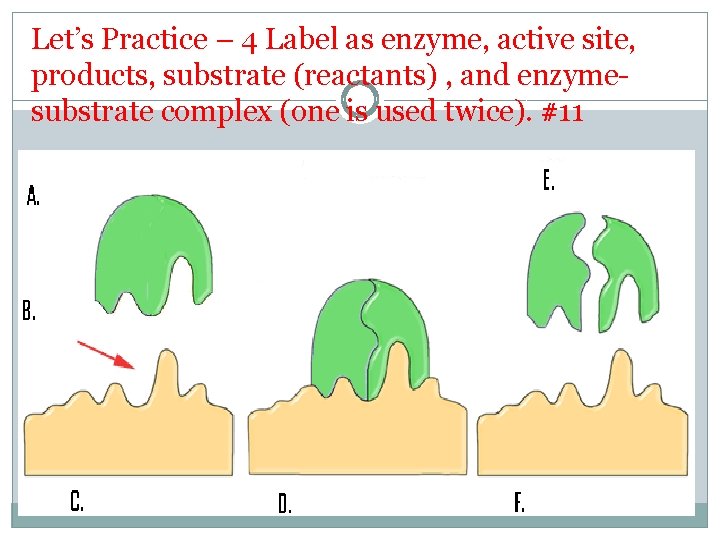
Let’s Practice – 4 Label as enzyme, active site, products, substrate (reactants) , and enzymesubstrate complex (one is used twice). #11

Answers #11
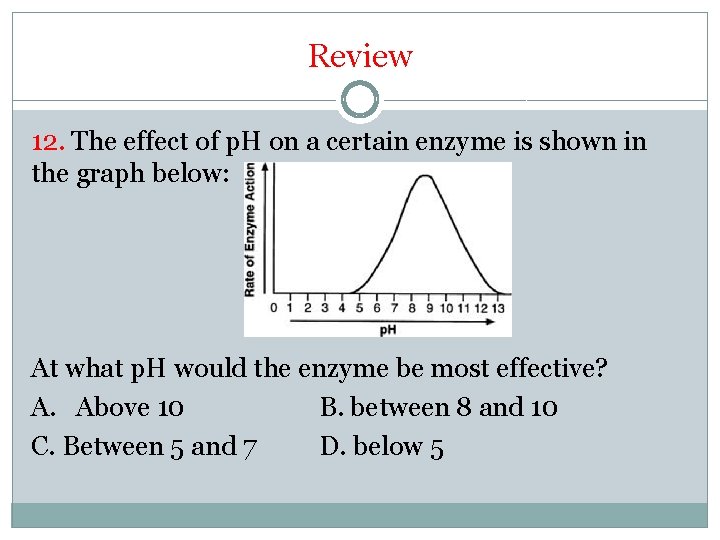
Review 12. The effect of p. H on a certain enzyme is shown in the graph below: At what p. H would the enzyme be most effective? A. Above 10 B. between 8 and 10 C. Between 5 and 7 D. below 5
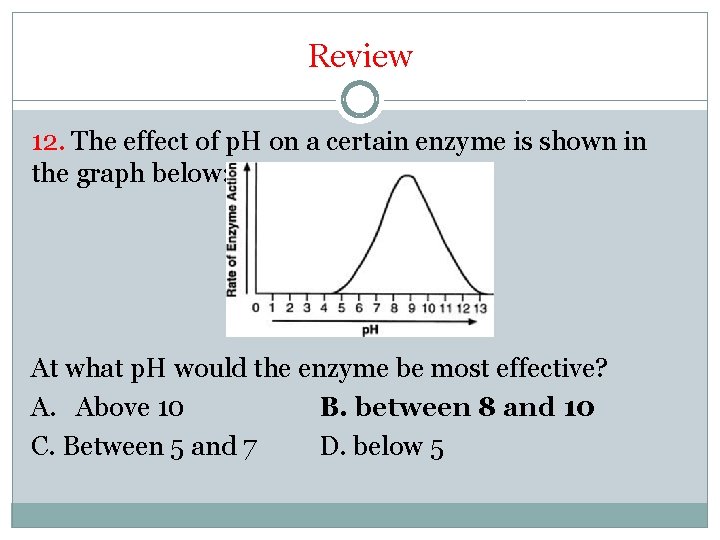
Review 12. The effect of p. H on a certain enzyme is shown in the graph below: At what p. H would the enzyme be most effective? A. Above 10 B. between 8 and 10 C. Between 5 and 7 D. below 5
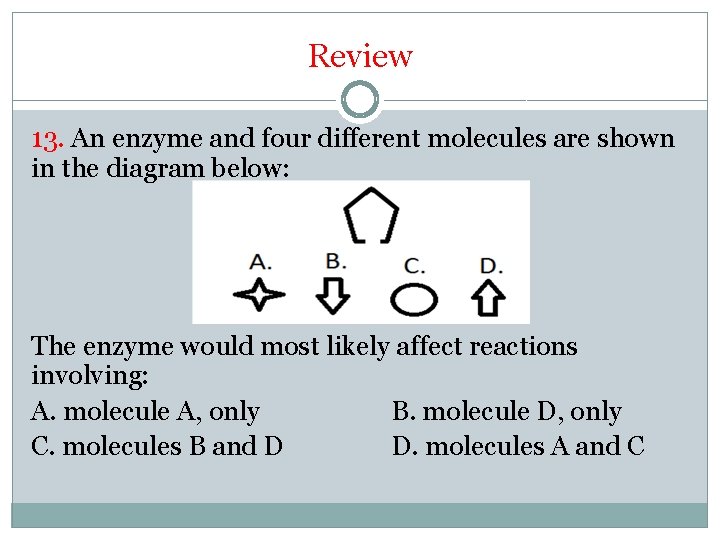
Review 13. An enzyme and four different molecules are shown in the diagram below: The enzyme would most likely affect reactions involving: A. molecule A, only B. molecule D, only C. molecules B and D D. molecules A and C
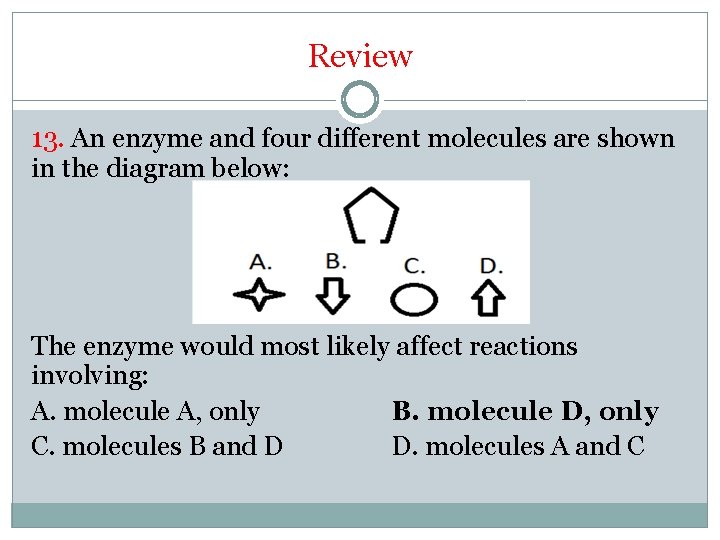
Review 13. An enzyme and four different molecules are shown in the diagram below: The enzyme would most likely affect reactions involving: A. molecule A, only B. molecule D, only C. molecules B and D D. molecules A and C

Review 14. Ice floats on a lake. This characteristic of water is responsible for A. Suffocation of aquatic organisms B. Mixing a lake’s thermal layers C. Preventing a lake from freezing solid D. Altering migration patterns of fish

Review 14. Ice floats on a lake. This characteristic of water is responsible for A. Suffocation of aquatic organisms B. Mixing a lake’s thermal layers C. Preventing a lake from freezing solid (High Heat Capacity is the amount of heat energy required to increase its temperature. ) D. Altering migration patterns of fish
Review 15. Proteins are formed from monomers (subunits) called: A. Nucleic acids B. Fatty acids C. Nucleotides D. Amino acids
Review 15. Proteins are formed from monomers (subunits) called: A. Nucleic acids (it’s a type of macromolecule) B. Fatty acids (monomers of lipids) C. Nucleotides (monomers for nucleic acids) D. Amino acids
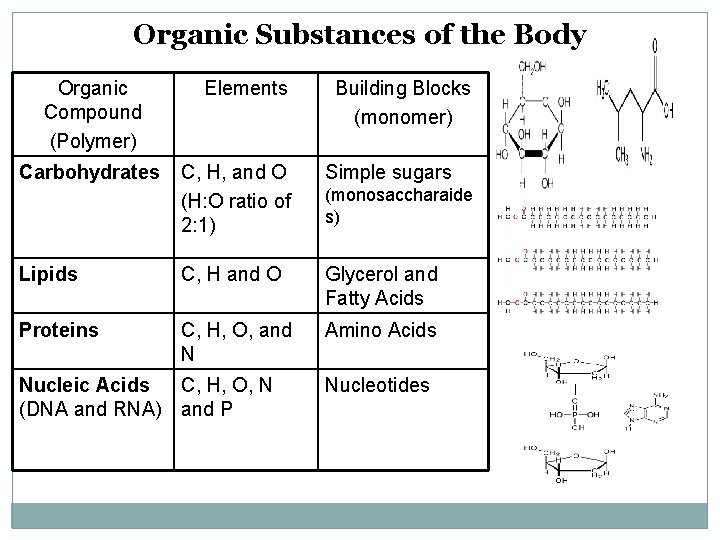
Organic Substances of the Body Organic Compound (Polymer) Carbohydrates Elements Building Blocks (monomer) C, H, and O (H: O ratio of 2: 1) Simple sugars Lipids C, H and O Glycerol and Fatty Acids Proteins C, H, O, and N Amino Acids Nucleic Acids C, H, O, N (DNA and RNA) and P (monosaccharaide s) Nucleotides
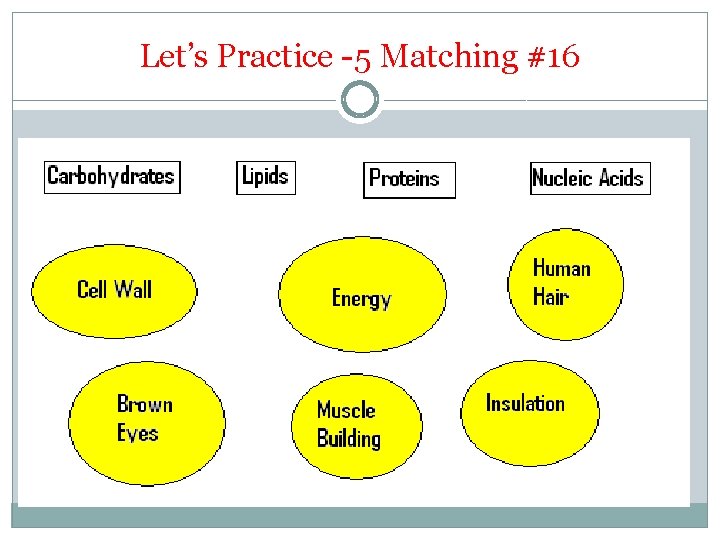
Let’s Practice -5 Matching #16
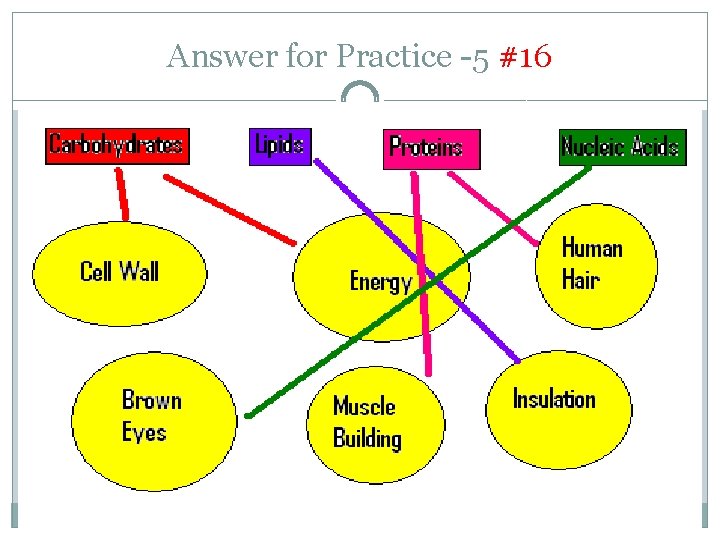
Answer for Practice -5 #16

Review 17. Which of the following macromolecules are a prominent part of animal tissues that function in insulation, helping animals to conserve heat? A. Carbohydrates B. Lipids C. Proteins D. Nucleic Acids

Review 17. Which of the following macromolecules are a prominent part of animal tissues that function in insulation, helping animals to conserve heat? A. Carbohydrates B. Lipids C. Proteins D. Nucleic Acids
- Slides: 40