Lesson 2 3 Water on Earth The Earth




















- Slides: 28
Lesson 2 & 3: Water on Earth

The Earth

Earth • 71% covered by oceans • Name the 4 oceans • Only 3% of the water is fresh • Majority is trapped in ice (glaciers & sea ice caps) • Dynamic with changing “spheres” • • Hydrosphere Biosphere Atmosphere Lithosphere

Earth • Contains both renewable and nonrenewable resources • Renewable resource – can replenish itself naturally over a relatively short period of time. • Nonrenewable resource – exhausted faster than they can be naturally replaced. • Are oceans a renewable or a nonrenewable resource?

Earth • Marine habitats are distributed based on water’s physical properties. • Water’s unique properties are due to the chemistry of its molecule.

Basic Chemistry

Review • Water’s unique properties are due to the chemistry of its molecule. • Atom – the basic particle of each unique element. • Neutron • Proton • Electron

Review • Bonding – the process that makes individual elements stable What determines how elements bond with other elements? • Covalent bonds – elements share their valence electrons • Diatomic molecules • Polar covalent bonds • Ionic bonds – elements give and take their valence electrons

Water Molecules • Polar covalent bonding between hydrogen and oxygen atoms • Hydrogen bonding between water molecules • May bond with 4 additional water molecules • Shape depends on state of matter • Liquids – creates a pyramid shape • Solids – creates a lattice shape • Most dense at 4 o. C • Hydrogen bonding gets stronger at lower temperatures causing water molecules to move further apart

Phases of Matter

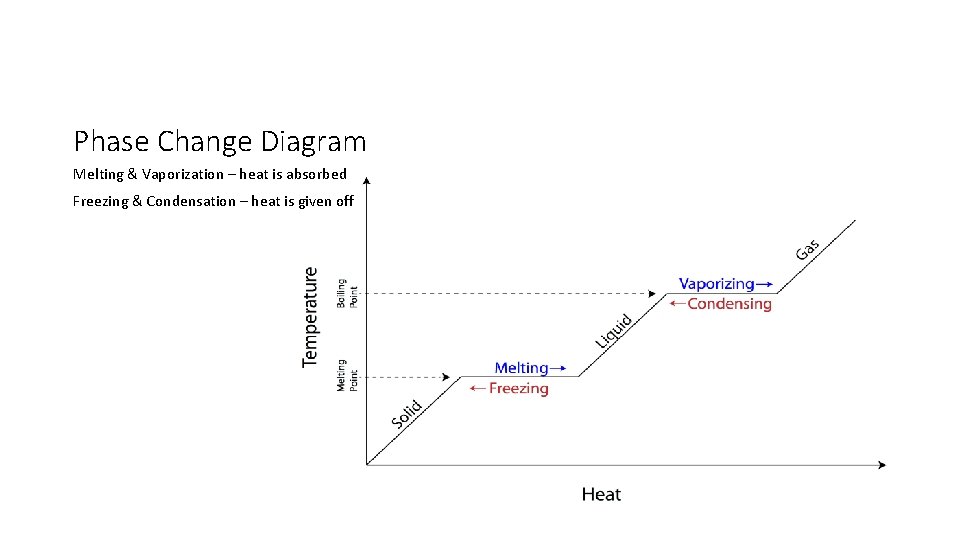
Phase Change Diagram Melting & Vaporization – heat is absorbed Freezing & Condensation – heat is given off

Phase Change Diagrams • Typical temperatures on the diagram refers to pure (distilled) water • Salt water • Boils at a higher temperature (b/c more difficult to evaporate) • Freezes at a lower temperature (b/c more difficult to form a crystalize structure) • High altitude • Boils and freezes at a lower temperature (b/c the air pressure is lower)

Density and Marine Organisms

Buoyancy • Definition: the upward force that keeps materials afloat in fluids • Equaled to the weight of the water being displaced • Objects float better in salt water than fresh water • Density (fresh water) = 1. 00 g/cm 3 • Density (salt water) = 1. 03 g/cm 3

Objects & Organisms in Ocean • Ships add weight when traveling in salt water • How? • Ballast water • Nonnative species (Zebra mussel) • Animals have been designed to allow them to float, sink, or maintain a neutral buoyancy

Sea Ice • Floating sea ice is important for many marine organisms • Habitat • Insulates water below • Resting spot

Solutions

Definitions • Solvent – a substance other items are dissolve in • Solutes – a substance that is dissolved • Solution – a uniform mixture of 2 or more substances • Solubility – the ability of a substance to be dissolved in a solvent • Affected by temperature of solvent

Water: The Universal Solvent • Why? • The polarity of the water molecules • The uneven distribution of shared electrons • Ionic compounds (salts) • Anions – negatively charged nonmetals attracted to the H portion of water molecule • Cation – positively charged metal attracted to the O portion of water molecule • Nonpolar molecules (oils and fats) • Contains symmetrically shared electrons

Salinity

Salinity • Definition – measurement of the dissolved salts in water • Distilled water – 0 parts per thousand • Fresh water – 1 part per thousand • Ocean water (average) – 35 parts per thousand (3. 5% salt makeup)

Why is the Sea Salty? • Land • Streams and rivers • 4 billion tons each years • Water evaporates leaving the salts behind • Inside the Earth • Undersea volcanoes and hydrothermal vents • Atmosphere • Particles carried by wind

Brackish Water • A salinity between salty and fresh • Seen in estuaries – areas where rivers meet the sea • “nurseries of the sea” • Resting places for migrating birds • Protect coastlines by preventing the movement of sediment (erosion) • Changing levels of salinity

Osmoregulation • Process used by individual cells to control the balance of water • Fig. 3. 17 and 3. 18 (page 50) – osmoregulation in marine and freshwater fish • Sea turtles – drink seawater and secrete excess salts in specialized glands in their eyes • Marine mammals – get water from the food they eat, produce urine with a greater salt concentration

Osmoregulation • What would happen to a freshwater fish that was placed into the ocean?

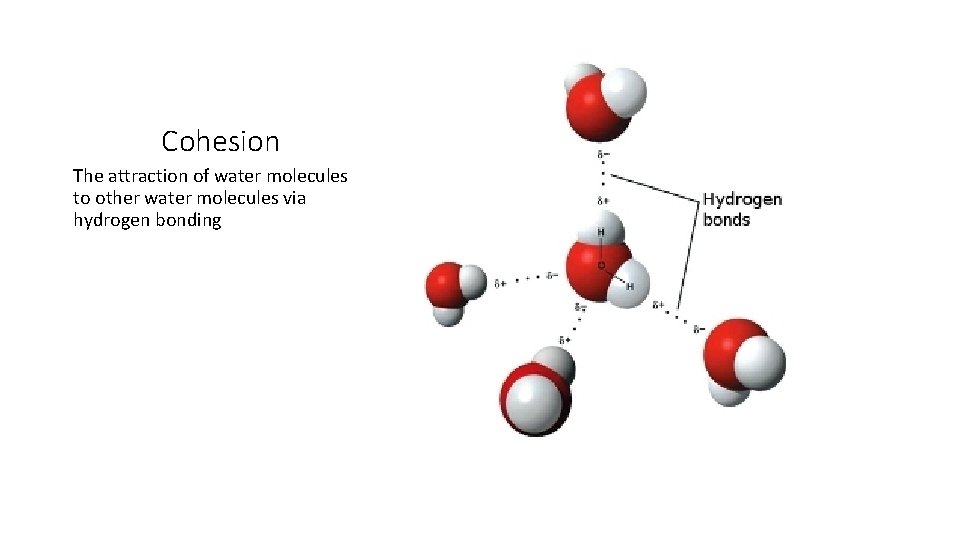
Cohesion

Cohesion The attraction of water molecules to other water molecules via hydrogen bonding

Cohesion • The cause of surface tension seen in water • The reason why water can flow through organisms (from animals with closed circulatory systems to filter feeding clams and sponges and plants carrying substances from roots to the leaves)