LECTURE 7 INTRODUCTION TO CHROMATOGRAPHIC SEPARATIONS Chromatography Is





















- Slides: 65

LECTURE 7 INTRODUCTION TO CHROMATOGRAPHIC SEPARATIONS

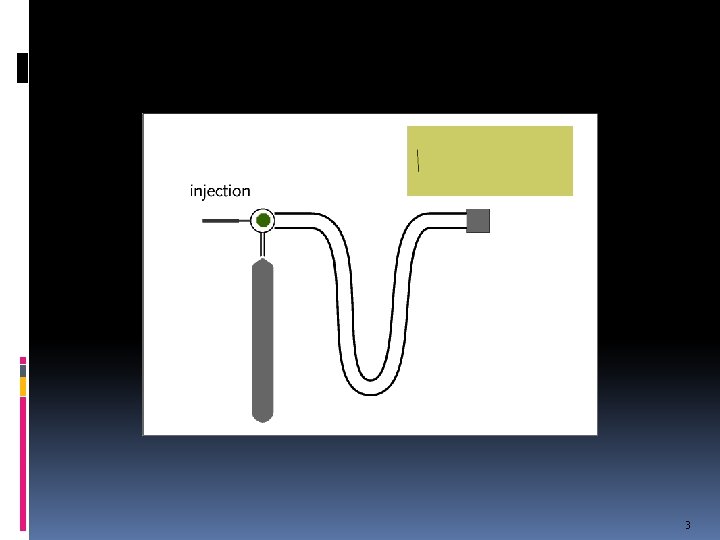
Chromatography Is a technique used to separate and identify the components of a mixture. Works by allowing the molecules present in the mixture to distribute themselves between a mobile and a stationary phase. mobile phase = solvent or gas stationary phase = column packing material 2

3

4

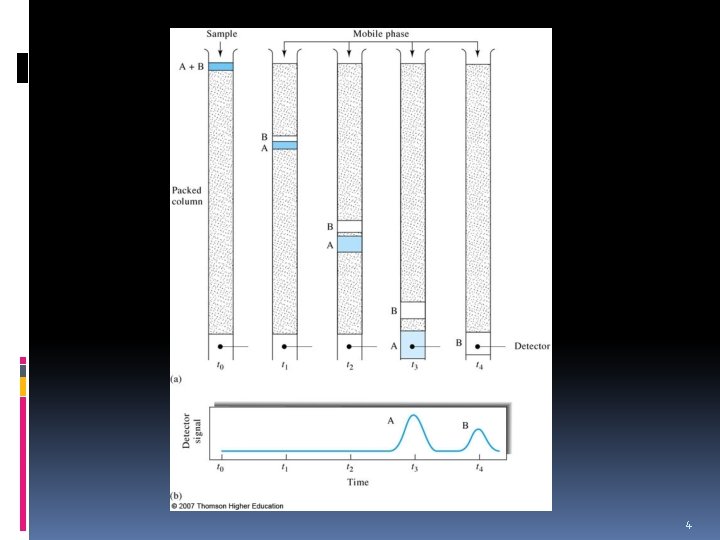
How separation occur? v Chromatography is a powerful separation method that is used to separate and identify the components of complex mixtures. v Works by allowing the molecules present in the mixture to distribute themselves between a stationary and a mobile phase to varying degrees. v Those components that are strongly retained by the stationary phase move slowly with the flow of mobile phase. 5

How separation occur? v In contrast, components that are weakly held by the stationary phase travel rapidly (fast). v As a consequence of these differences in mobility, sample components separate into discrete bands that can be analyzed qualitatively and/or quantitatively. 6

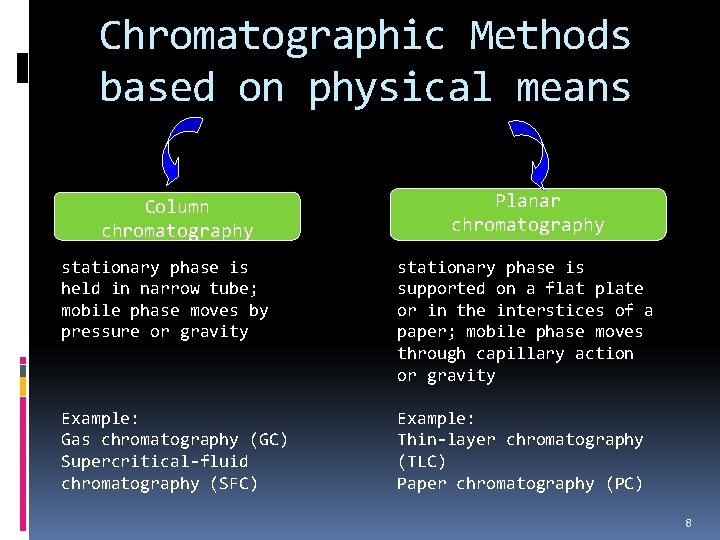
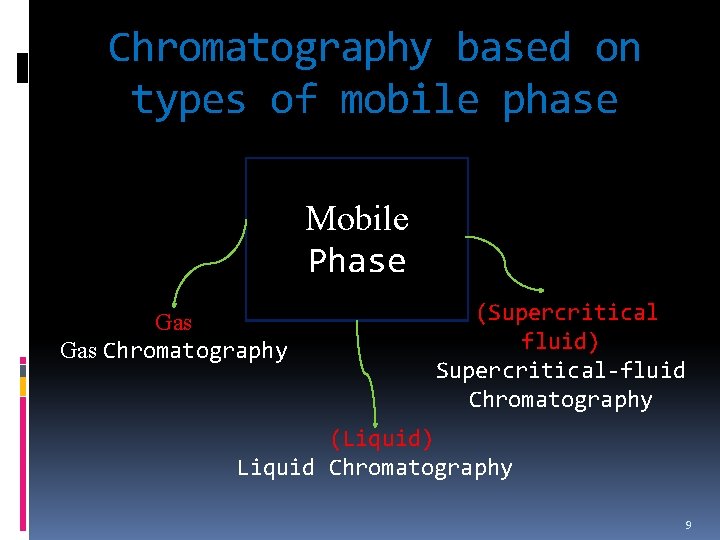
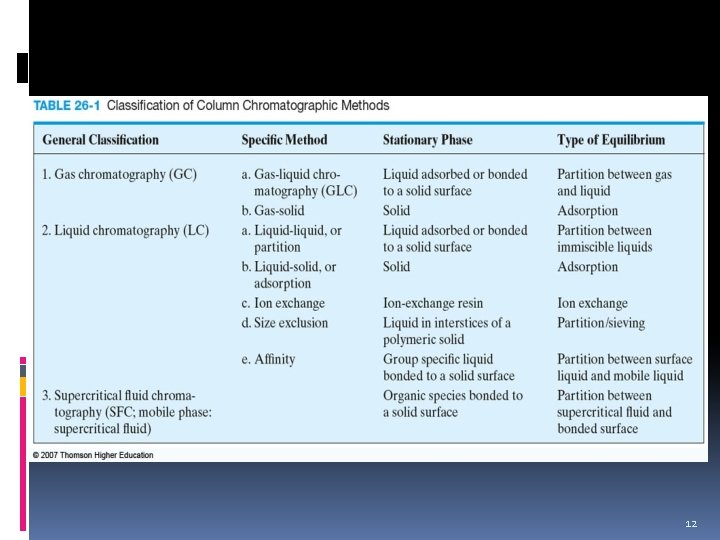
Classification of Chromatographic Methods 1. Based on physical means The way stationary and mobile phases are brought into contact. 2. Based on the types of mobile phase Either gas, liquid or supercritical fluid. 3. Based on the kinds of equilibria involved in the in solute transfer between the phases Interaction of analyte between stationary and mobile phases. 7

Chromatographic Methods based on physical means Column chromatography Planar chromatography stationary phase is held in narrow tube; mobile phase moves by pressure or gravity stationary phase is supported on a flat plate or in the interstices of a paper; mobile phase moves through capillary action or gravity Example: Gas chromatography (GC) Supercritical-fluid chromatography (SFC) Example: Thin-layer chromatography (TLC) Paper chromatography (PC) 8

Chromatography based on types of mobile phase Mobile Phase Gas Chromatography (Supercritical fluid) Supercritical-fluid Chromatography (Liquid) Liquid Chromatography 9

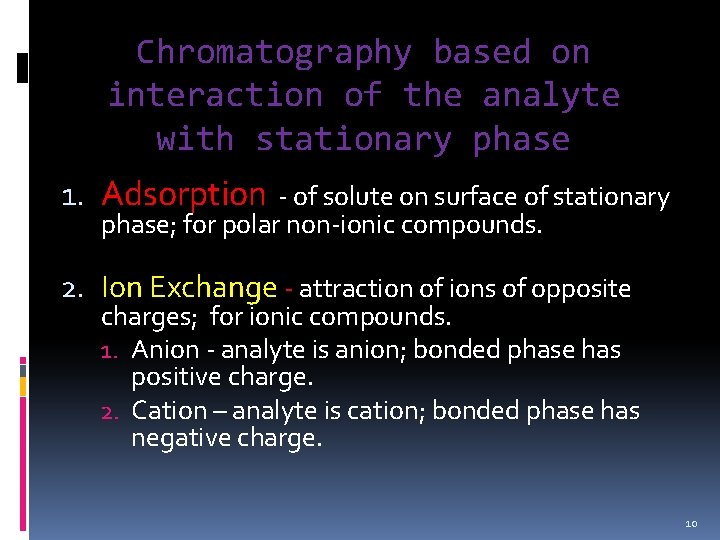
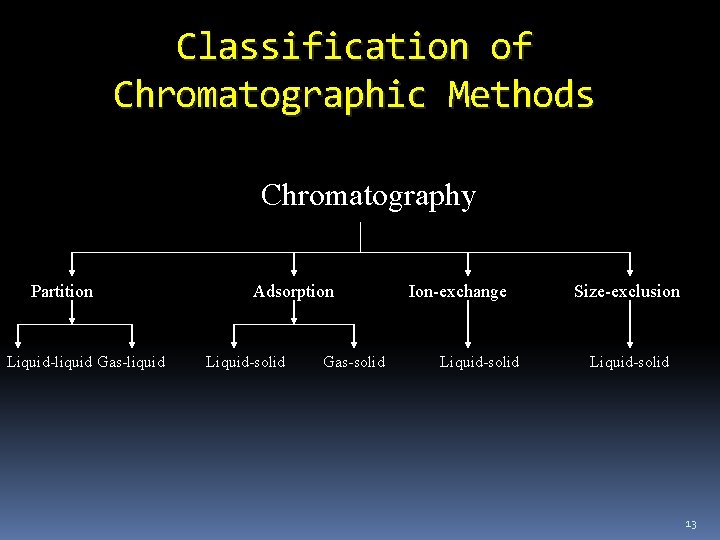
Chromatography based on interaction of the analyte with stationary phase 1. Adsorption - of solute on surface of stationary phase; for polar non-ionic compounds. 2. Ion Exchange - attraction of ions of opposite charges; for ionic compounds. 1. Anion - analyte is anion; bonded phase has positive charge. 2. Cation – analyte is cation; bonded phase has negative charge. 10

Chromatography based on interaction of the analyte with stationary phase 3. Partition - based on the relative solubility of analyte in mobile and stationary phases. a. Normal phase – stationary phase polar, the mobile phase nonpolar. b. Reverse phase– stationary phase nonpolar, the mobile phase polar. 4. Size Exclusion – separate molecules by size; sieving- stationary phase is a porous matrix. 11

12

Classification of Chromatographic Methods Chromatography Partition Liquid-liquid Gas-liquid Adsorption Liquid-solid Gas-solid Ion-exchange Liquid-solid Size-exclusion Liquid-solid 13

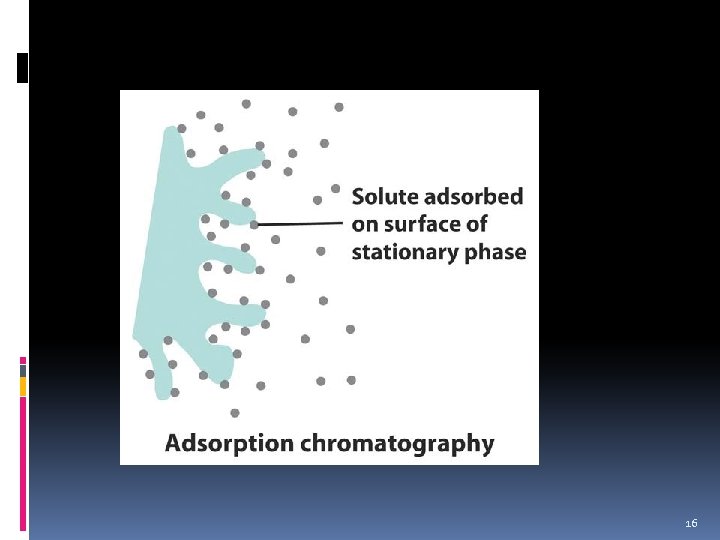
Adsorption Chromatography 14

Adsorption Chromatography Components of the mixture selectively adsorb (stick) on the surface of a finely divided solid stationary phase. v As mobile phase (gas/liquid) carries the mixture through the stationary phase, the components of the mixture stick to its surface with varying degrees of strength and thus separate. Stationary phase: solid v Mobile phase: gas or liquid 15

16

Partition Chromatography 17

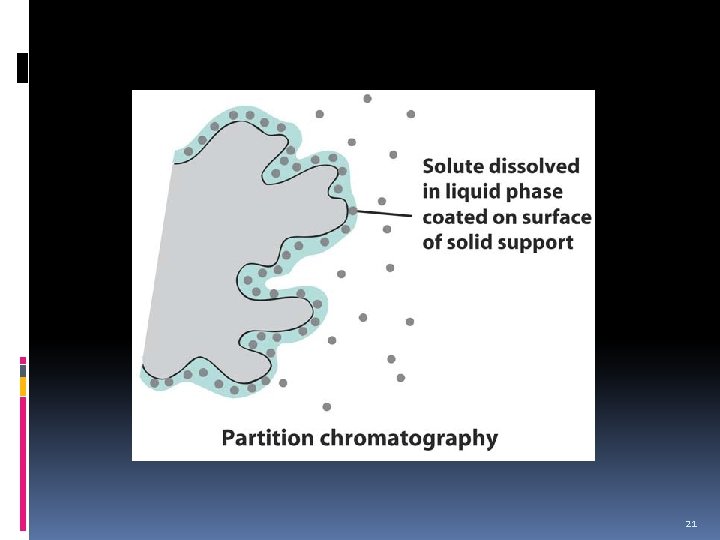
Partition Chromatography v Accomplished by selective and continuous transfer of the components of the mixture back and forth between a liquid stationary phase and a liquid mobile phase as the mobile phase liquid passes through the stationary phase liquid. Stationary phase: Mobile phase: liquid or gas 18

Partition Chromatography v Partitioning is a distribution (by dissolving) of the components between 2 immiscible phases. Seperations of the components will be based on relative solubilities of the components in the mobile and stationary phase. v Example of partitioning using polar stationary phase. Polar components will retain longer than the non-polar components. Non-polar components will move quickly through stationary phase and will elute first before the polar components and vice-versa. 19

Partition Chromatography The stationary phase actually consists of a thin film adsorbed (stuck) on or chemically bonded to the surface of a finely divided solid particles. v If the mobile phase is gas, the volatility (vapor pressure) and solubility in stationary phase plays an important role. v 20

21

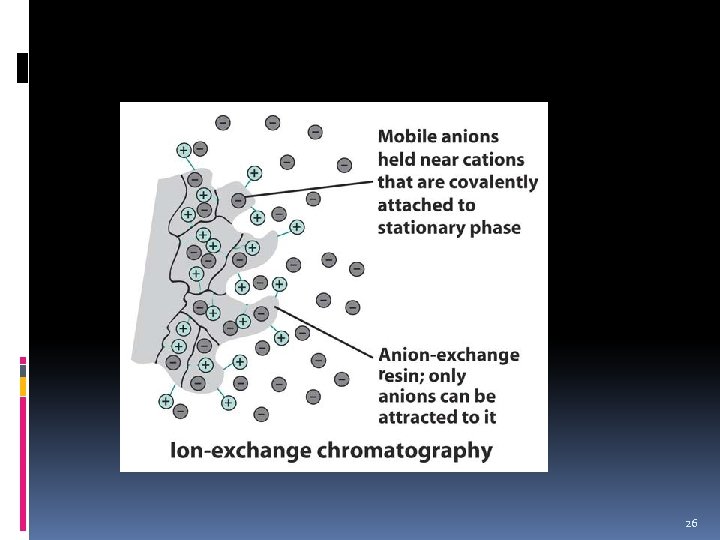
Ion-exchange Chromatography 22

Ion exchange Chromatography v Method for separating mixture of ions. v Sample is aqueous solution of inorganic ions or organic ions v Stationary phase are small polymer resin “beads” usually packed in a glass tube. a. These beads have ionic bonding sites on their surfaces which selectively exchange ions with certain mobile phase compositions as the mobile phase penetrates through it. 23

Ion exchange Chromatography v Ions that bond to the charged site on the resin bead are separated from organic or inorganic ions aqueous solution. v The process is repeated several times by changing of the mobile phase composition. 24

Ion exchange Chromatography v The process begin with initially running the analysis using a mobile phase with all the ions in the mixture. v The mobile phase is then change for several times in a stepwise fashion so that one kind of ion at a time is removed. v The process is repeated until complete separation achieved. 25

26

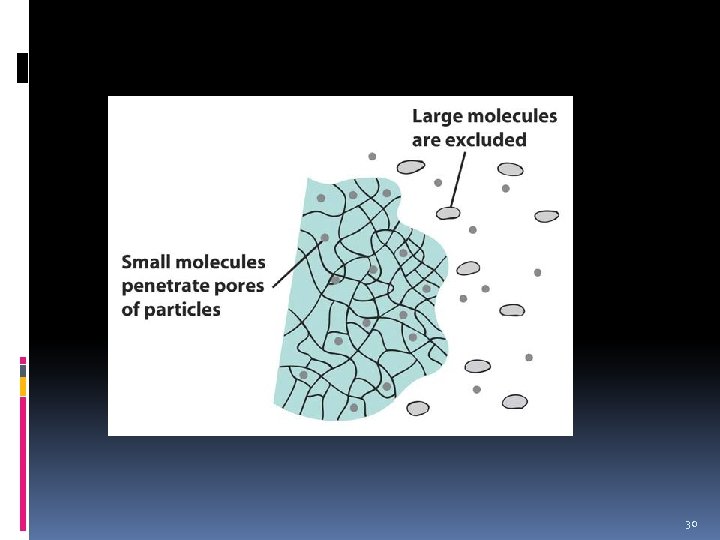
Size-exclusion Chromatography 27

Size exclusion Chromatography v Also called gel permeation chromatography. v Separation technique of dissolved species is based on the size of the components. v Stationary phase: porous polymer resin particles (molecular sieves). v The components to be separated enter the pores of these particles and are slowed down from progressing through this stationary phase. 28

Size exclusion Chromatography v Separation depends on the sizes of the pores relative to the sizes of the molecules to be separated. v Small particles are retarded to a greater extent than large particles (some of which may not enter the pores at all) and separation occurs. v Particles with size bigger than the pore size will be eluted first from the column. 29

30

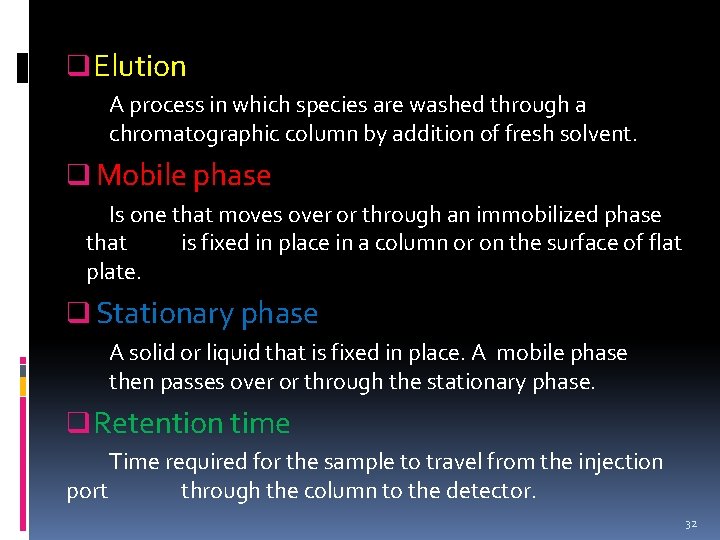
Terminologies in chromatography 31

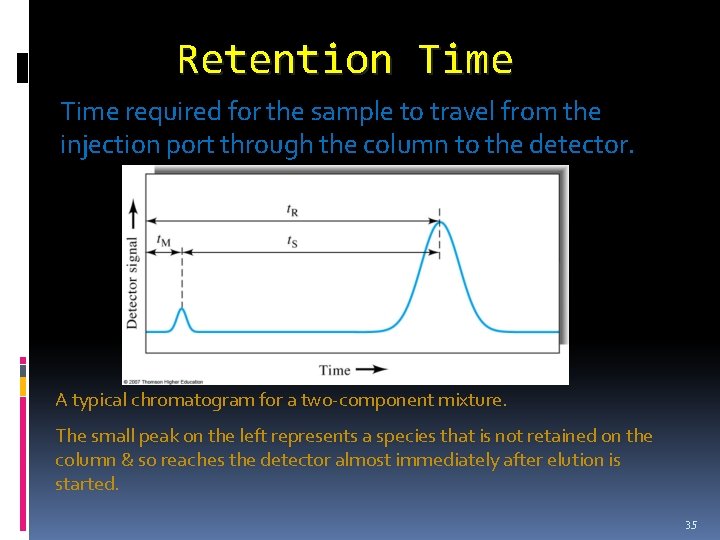
q Elution A process in which species are washed through a chromatographic column by addition of fresh solvent. q Mobile phase Is one that moves over or through an immobilized phase that is fixed in place in a column or on the surface of flat plate. q Stationary phase A solid or liquid that is fixed in place. A mobile phase then passes over or through the stationary phase. q Retention time Time required for the sample to travel from the injection port through the column to the detector. 32

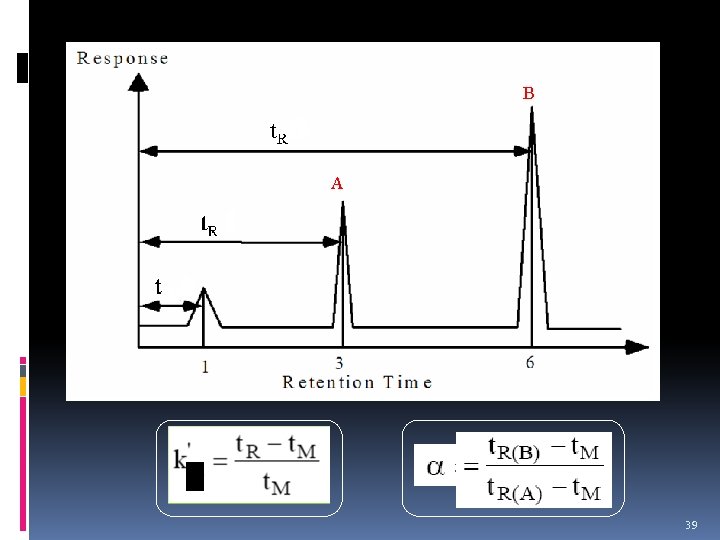
Migration Rates of Solutes q Distribution constant, K q Retention time, t. R q Capacity factor, k’ q Selectivity factor, α 33

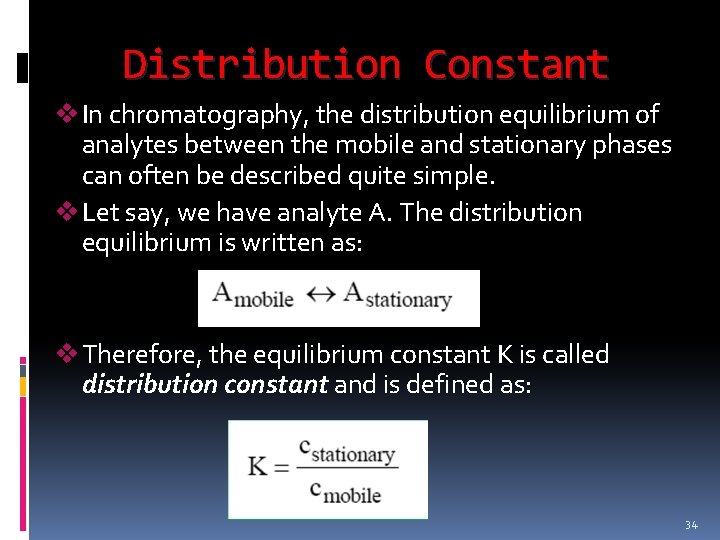
Distribution Constant v In chromatography, the distribution equilibrium of analytes between the mobile and stationary phases can often be described quite simple. v Let say, we have analyte A. The distribution equilibrium is written as: v Therefore, the equilibrium constant K is called distribution constant and is defined as: 34

Retention Time required for the sample to travel from the injection port through the column to the detector. A A typical chromatogram for a two-component mixture. The small peak on the left represents a species that is not retained on the column & so reaches the detector almost immediately after elution is started. 35

Dead Time, t. M v Defined as time taken for the unretained species to reach the detector. v Rate of migration of the unretained species is SIMILAR as the average rate of motion of mobile phase molecules. v So, t. M also can be expressed as the time required for an average molecule of the mobile phase to pass through the column. 36

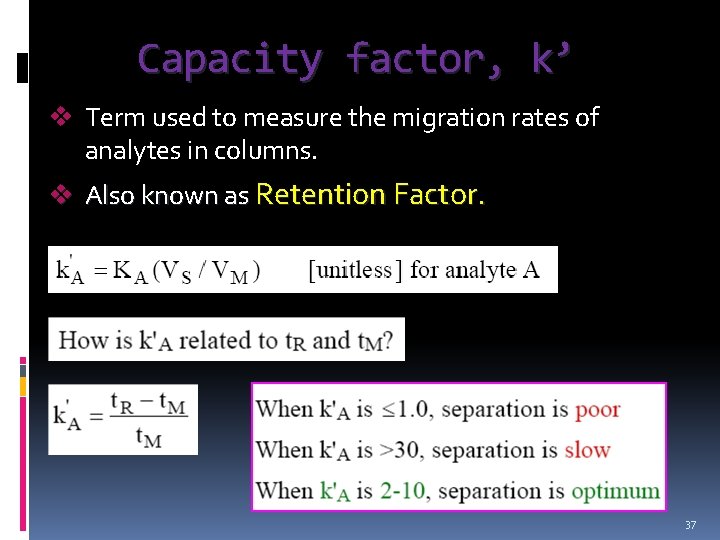
Capacity factor, k’ v Term used to measure the migration rates of analytes in columns. v Also known as Retention Factor. 37

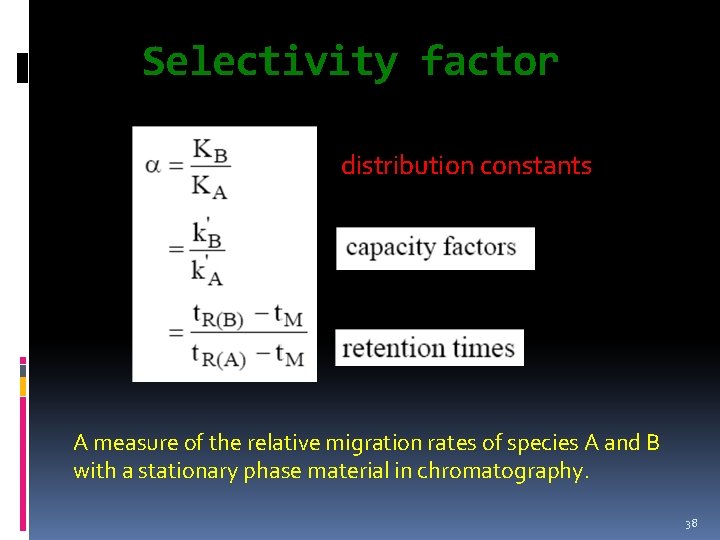
Selectivity factor is defined as: distribution constants A measure of the relative migration rates of species A and B with a stationary phase material in chromatography. 38

B A M 39

Column Efficiency Two related terms widely used as quantitative measures of chromatographic column efficiency are A. Plate height, H B. Number of theoretical plates, N 40

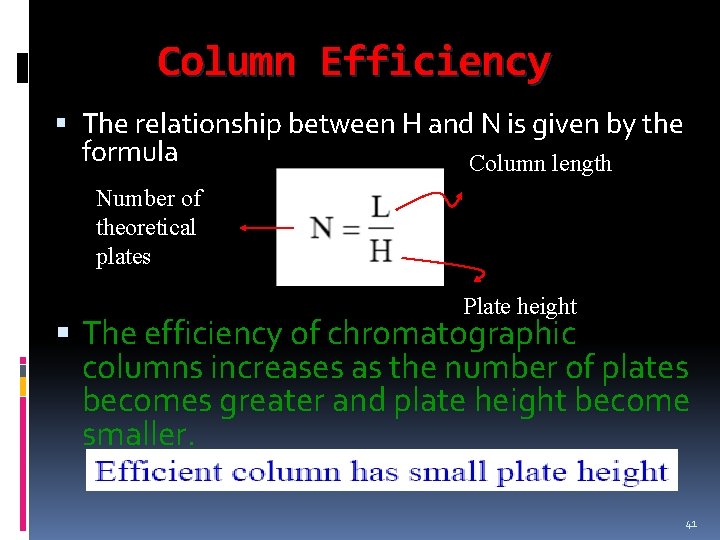
Column Efficiency The relationship between H and N is given by the formula Column length Number of theoretical plates Plate height The efficiency of chromatographic columns increases as the number of plates becomes greater and plate height become smaller. 41

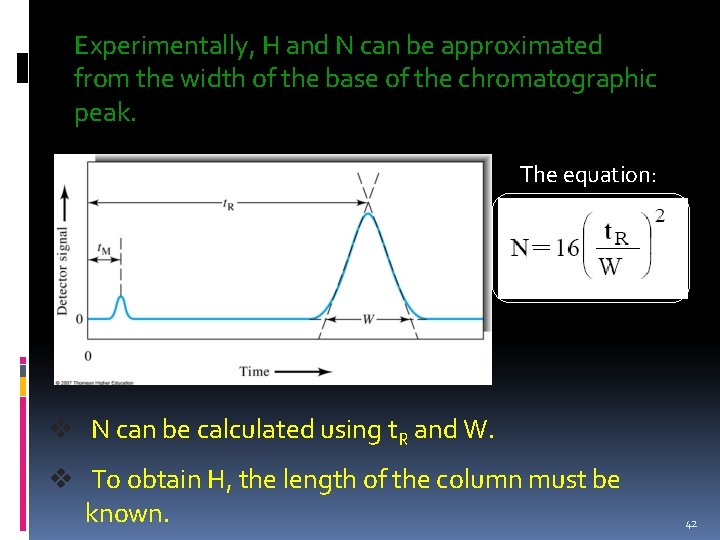
Experimentally, H and N can be approximated from the width of the base of the chromatographic peak. The equation: v N can be calculated using t. R and W. v To obtain H, the length of the column must be known. 42

Another method for approximating N is to determine W 1/2, the width of the peak at half its maximum height. t N = 5. 54 R W 1/2 2 43

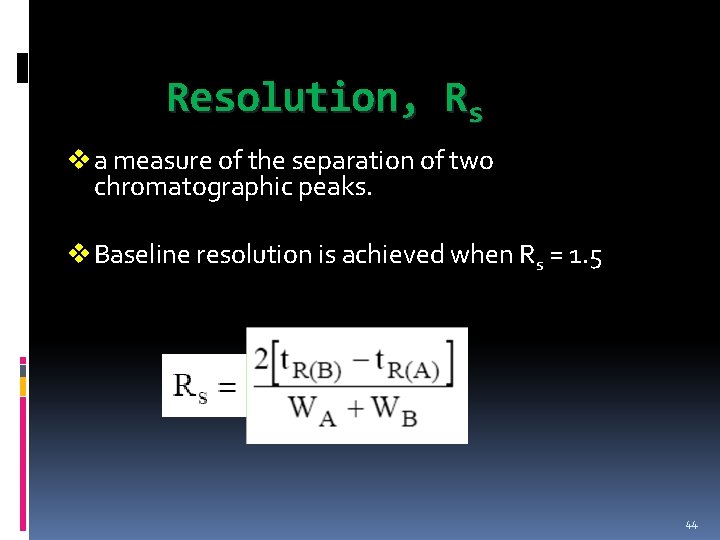
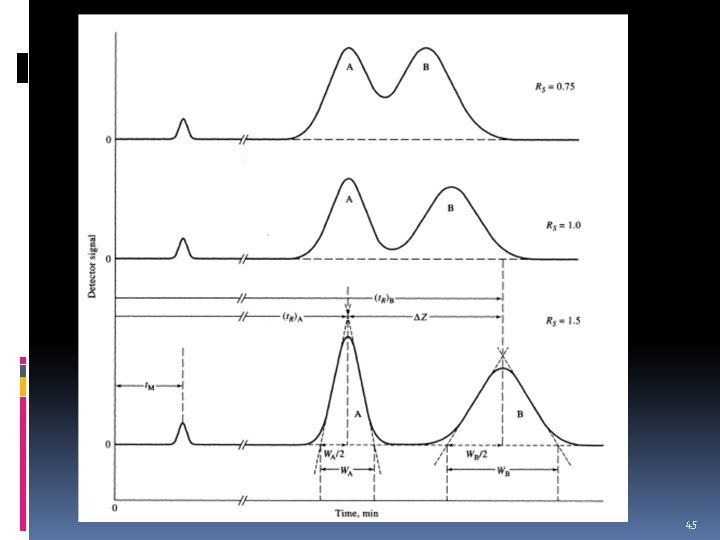
Resolution, Rs v a measure of the separation of two chromatographic peaks. v Baseline resolution is achieved when Rs = 1. 5 44

45

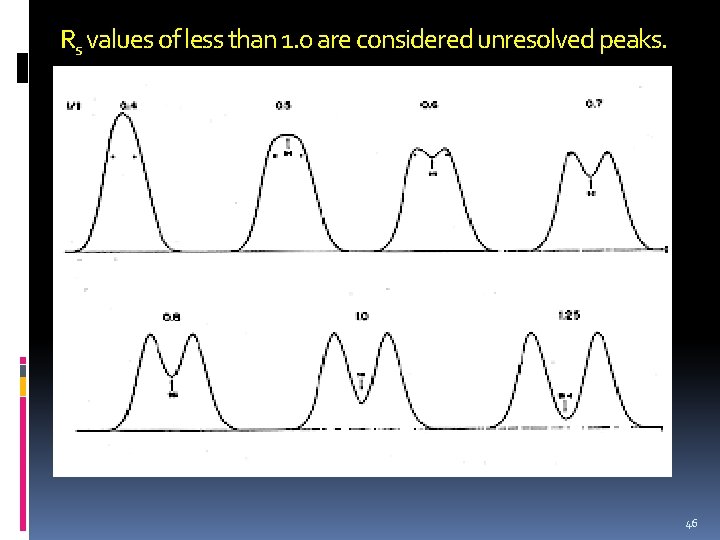
Rs values of less than 1. 0 are considered unresolved peaks. 46

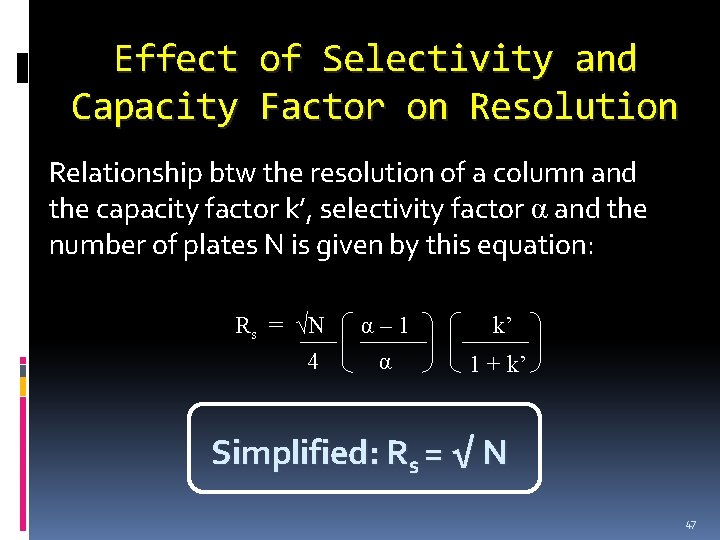
Effect Capacity of Selectivity and Factor on Resolution Relationship btw the resolution of a column and the capacity factor k’, selectivity factor α and the number of plates N is given by this equation: Rs = √N 4 α– 1 α k’ 1 + k’ Simplified: Rs = √ N 47

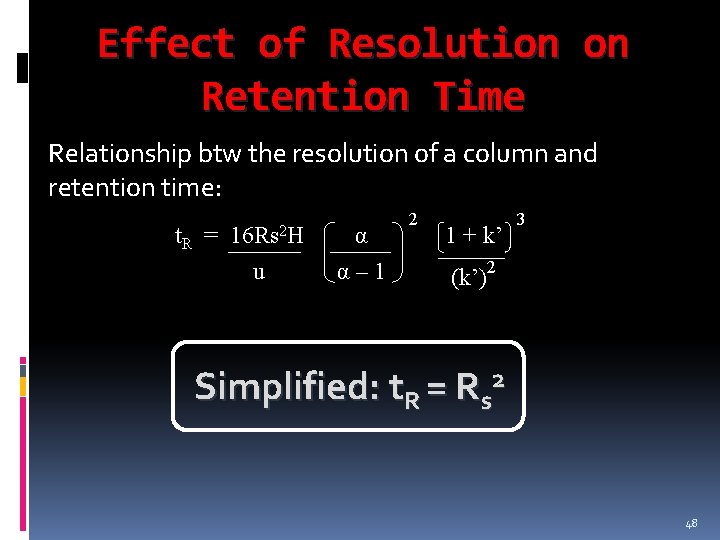
Effect of Resolution on Retention Time Relationship btw the resolution of a column and retention time: t. R = 16 Rs 2 H u α α– 1 2 1 + k’ 3 (k’)2 Simplified: t. R = Rs 2 48

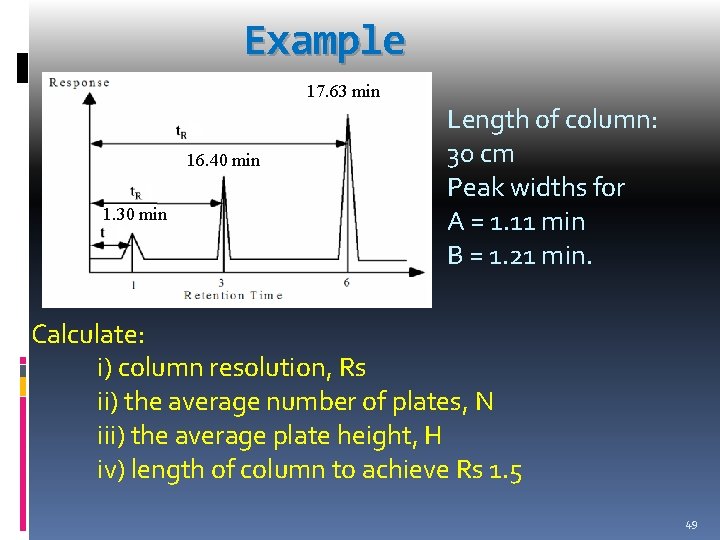
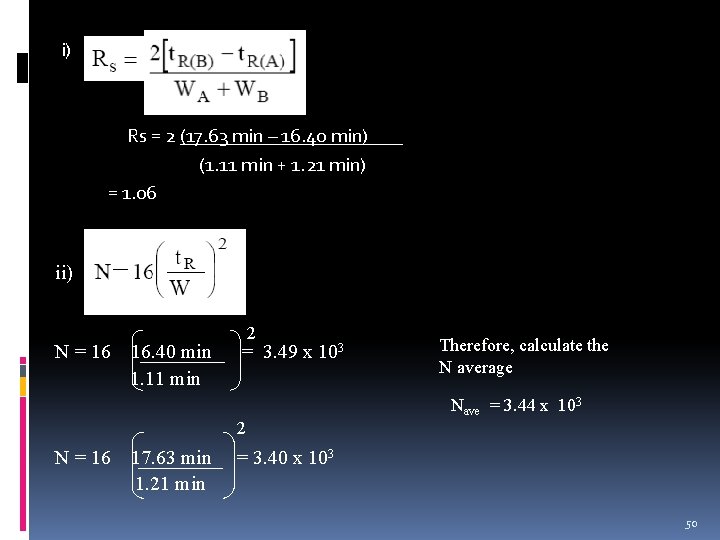
Example 17. 63 min 16. 40 min 1. 30 min Length of column: 30 cm Peak widths for A = 1. 11 min B = 1. 21 min. Calculate: i) column resolution, Rs ii) the average number of plates, N iii) the average plate height, H iv) length of column to achieve Rs 1. 5 49

i) Rs = 2 (17. 63 min – 16. 40 min) (1. 11 min + 1. 21 min) = 1. 06 ii) N = 16 16. 40 min 1. 11 min 2 = 3. 49 x 103 2 N = 16 17. 63 min 1. 21 min Therefore, calculate the N average Nave = 3. 44 x 103 = 3. 40 x 103 50

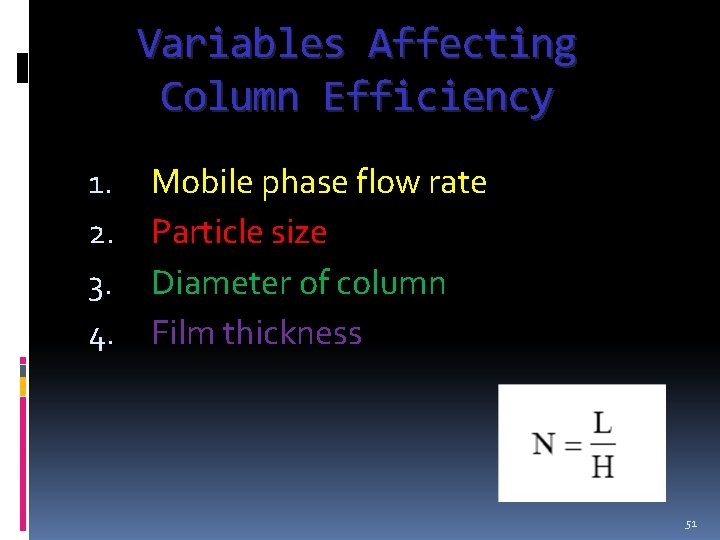
Variables Affecting Column Efficiency 1. 2. 3. 4. Mobile phase flow rate Particle size Diameter of column Film thickness 51

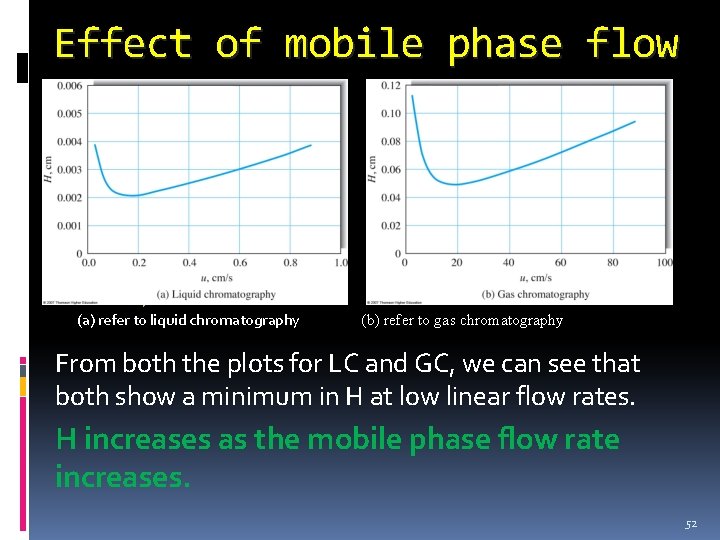
Effect of mobile phase flow (a) refer to liquid chromatography (b) refer to gas chromatography From both the plots for LC and GC, we can see that both show a minimum in H at low linear flow rates. H increases as the mobile phase flow rate increases. 52

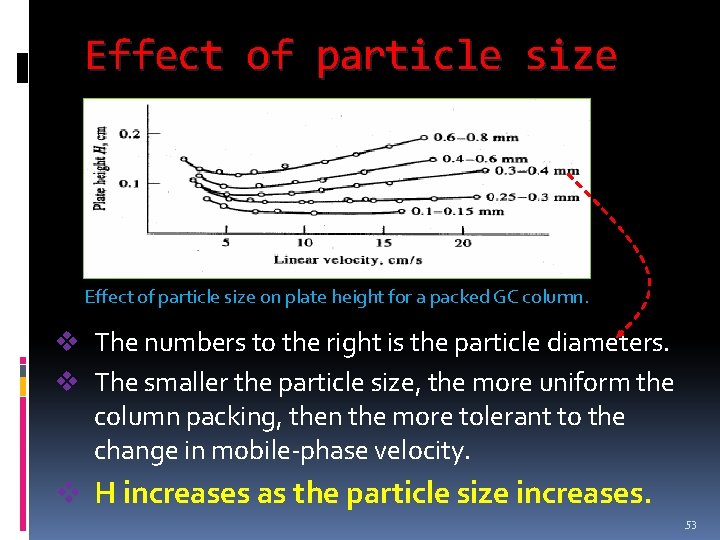
Effect of particle size on plate height for a packed GC column. v The numbers to the right is the particle diameters. v The smaller the particle size, the more uniform the column packing, then the more tolerant to the change in mobile-phase velocity. v H increases as the particle size increases. 53

Effect of diameter of the column v For packed column, the most important variable that affect column efficiency is the diameter of the particles that making up the packing. v While for open tubular column, the diameter of the column itself is an important variable. v The mobile-phase mass-transfer coefficient is known to be inversely proportional to the diffusion coefficient of the analyte in the mobile phase. 54

Effect of diameter of the column v Mass transfer coefficient is proportional to the square of the particle diameter of the packing material, d 2 p (packed column). v Mass transfer coefficient is proportional to the square of the column diameter, d 2 c (open tubular column). v As a conclusion, the bigger the column diameter, the smaller the diffusion coefficient. Therefore, we can say that increase in column diameter will increase the plate height. 55

Effect of film thickness v When stationary phase is an immobilized liquid, the mass-transfer coefficient is directly proportional to the square of the thickness of the film on the support particles d 2 f and inversely proportional to the diffusion coefficient of the solute in the film. v With thick films and smaller diffusion coefficient, analyte molecule travel slower. As a result, thick films will reduce the mass transfer rate and increase in plate height. 56

Applications of Chromatography q Qualitative analysis q Quantitative analysis 57

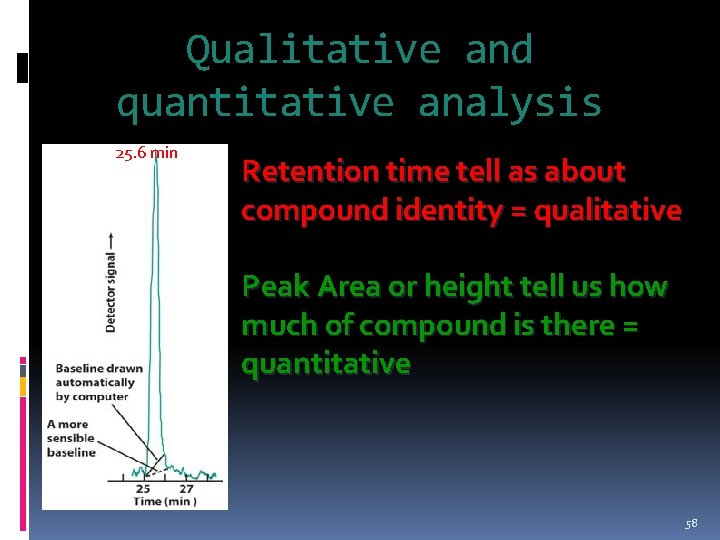
Qualitative and quantitative analysis 25. 6 min Retention time tell as about compound identity = qualitative Peak Area or height tell us how much of compound is there = quantitative 58

Qualitative analysis q Based on retention time q Provided the sample produce the peak at the same retention time as a standard under identical conditions. 59

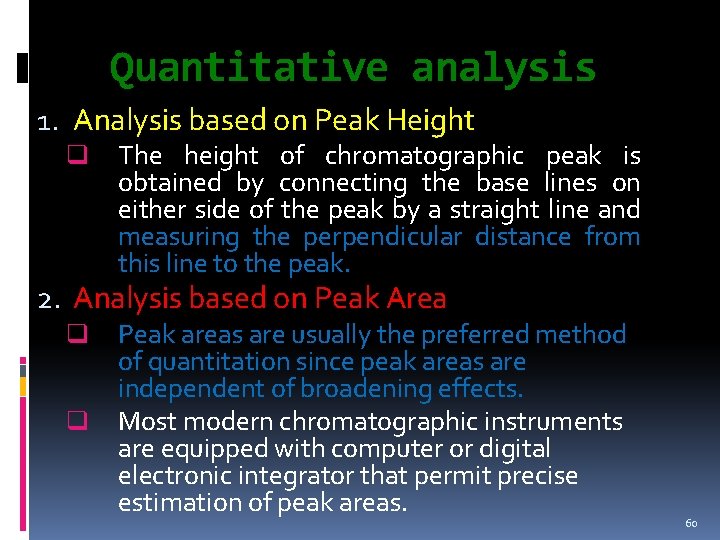
Quantitative analysis 1. Analysis based on Peak Height q The height of chromatographic peak is obtained by connecting the base lines on either side of the peak by a straight line and measuring the perpendicular distance from this line to the peak. 2. Analysis based on Peak Area q q Peak areas are usually the preferred method of quantitation since peak areas are independent of broadening effects. Most modern chromatographic instruments are equipped with computer or digital electronic integrator that permit precise estimation of peak areas. 60

3. Calibration method (also known as external method) q Involve preparation of series of standard solutions that approximate the composition of the unknown. q The peak heights or areas are plotted as a function of concentration. q The concentration of the component(s) to be analysed is determined by comparing the response(s) (peak(s)) obtained with the standard solutions. 61

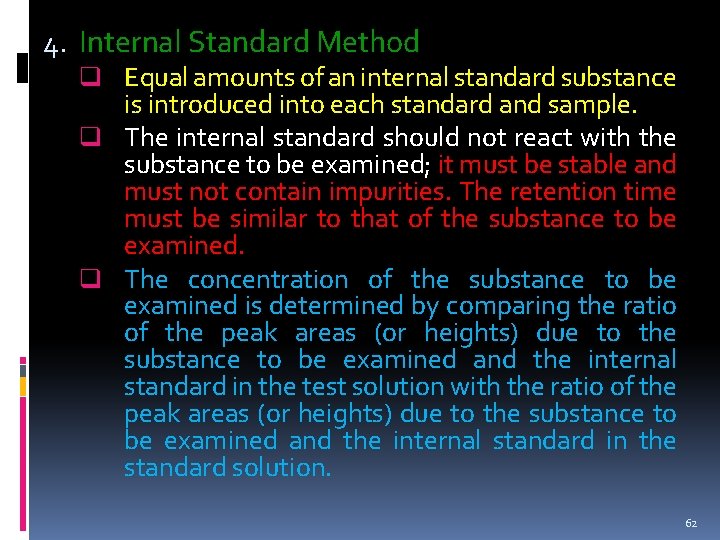
4. Internal Standard Method q Equal amounts of an internal standard substance is introduced into each standard and sample. q The internal standard should not react with the substance to be examined; it must be stable and must not contain impurities. The retention time must be similar to that of the substance to be examined. q The concentration of the substance to be examined is determined by comparing the ratio of the peak areas (or heights) due to the substance to be examined and the internal standard in the test solution with the ratio of the peak areas (or heights) due to the substance to be examined and the internal standard in the standard solution. 62

5. Area Normalization Method q In the normalization method, the areas of all eluted peaks is normalized. q The percentage content of one or more components of the substance to be examined is calculated by determining the area of the peak(s) as a percentage of the total area of all the peaks, excluding those due to solvents or any added reagents and those below the disregard limit. 63

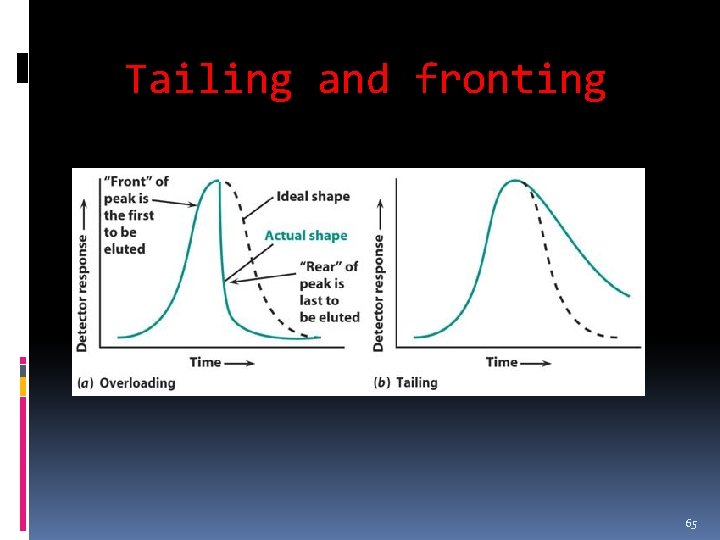
Tailing and fronting ü A common cause of tailing and fronting is a distribution constant that varies with concentration. ü Fronting also arises when the amount of sample introduced onto a column is too large. 64

Tailing and fronting 65