Paper Chromatography Identifying the components of a mixture





- Slides: 13

Paper Chromatography Identifying the components of a mixture

Chromatography is a method for separating mixtures into their components based on physical and/or chemical properties of the components. Developed around 1903 by Russian Mikhail Semenovich Tswett in which he separated plant pigments on “diatomaceous earth” with alcohol

Uses for Chromatography can be used to: • Qualitatively analyze the components of a mixture • Qualitatively identify the components of a mixture using known compounds • Quantitatively determine the amount of a component in a mixture using standard samples • Purify individual components by separating them from the other compounds in a mixture

The Basics • • Mixture is placed on stationary phase Mobile phase passes over the stationary phase Mobile phase dissolves the components Mobile phase carries the individual components a certain distance through the stationary phase, depending on their attraction to both of the phases

Solvent Front Chromatographed Spot Rf ratio of spot distance to front Putsolvent the spotted paper distance tank in a developing Origin line Solvent

Principles of Paper Chromatography • Capillary Action – the movement of liquid within the spaces of a porous material due to the forces of adhesion, cohesion, and surface tension. The liquid is able to move up the filter paper because its attraction to itself is stronger than the force of gravity. • Solubility – the degree to which a material (solute) dissolves into a solvent. Solutes dissolve into solvents that have similar properties. This allows different solutes to be separated by different combinations of solvents. Separation of components depends on both their solubility in the mobile phase and their differential affinity to the mobile phase and the stationary phase.

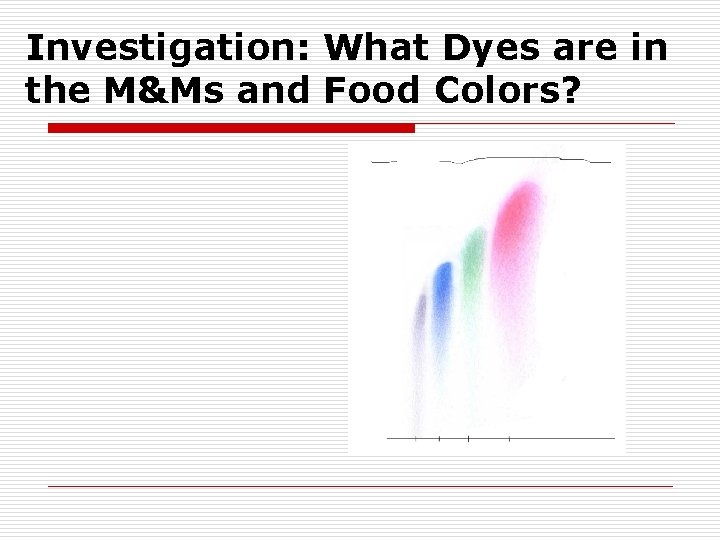
Investigation: What Dyes are in the M&Ms and Food Colors?

Preparing the Chromatography Paper • Obtain an 7 x 8 cm of chromatography paper • With a pencil (why? ), draw an origin line 1 cm above the bottom edge of the strip. Near the top, ID the chromatogram • Mark the origin line with a lane mark each cm. No lane can be closer than about 1 cm from the edge

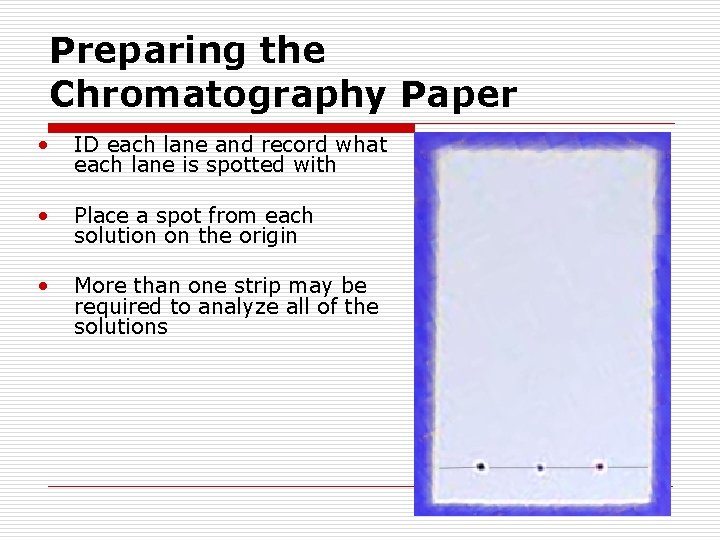
Preparing the Chromatography Paper • ID each lane and record what each lane is spotted with • Place a spot from each solution on the origin • More than one strip may be required to analyze all of the solutions

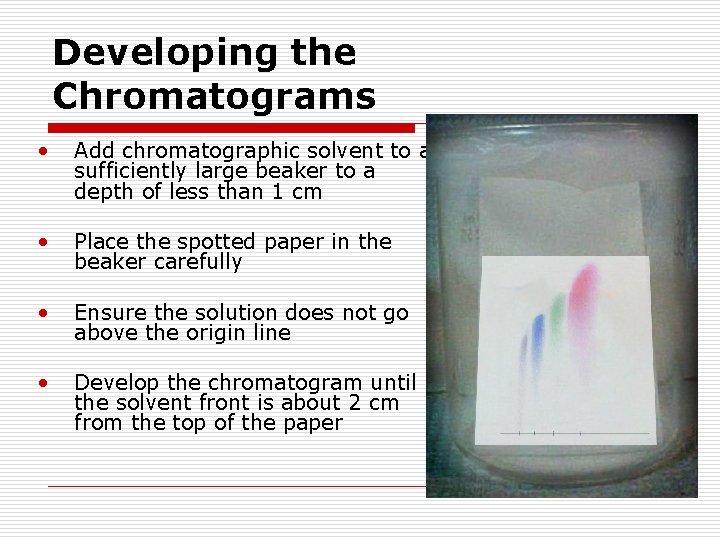
Developing the Chromatograms • Add chromatographic solvent to a sufficiently large beaker to a depth of less than 1 cm • Place the spotted paper in the beaker carefully • Ensure the solution does not go above the origin line • Develop the chromatogram until the solvent front is about 2 cm from the top of the paper

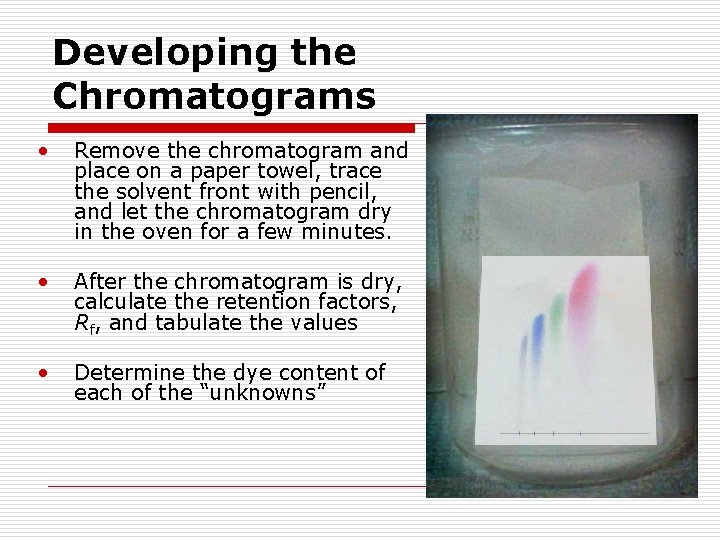
Developing the Chromatograms • Remove the chromatogram and place on a paper towel, trace the solvent front with pencil, and let the chromatogram dry in the oven for a few minutes. • After the chromatogram is dry, calculate the retention factors, Rf, and tabulate the values • Determine the dye content of each of the “unknowns”

Considerations • Small spots are generally better but harder to see after developing • UV lamps, if available can (sun)burn your eyes but UV is stopped by the plastic of your goggles • UV lamps are low power but extended exposure can cause a mild (sun)burn • Don’t eat the M&Ms – they’ve been in the lab awhile

Writing Procedure Proposals • • • Clearly state your experimental methods and how those procedures will yield solutions to the problems addressed in the investigation. State what data you plan to collect and how you will analyze the data. Include proposed data tables. State all the materials you plan to use including, if possible, concentrations and quantities. Describe the safety hazards associated with your analysis and appropriate precautions you will take to avoid personal injury. Each team member must sign and date the procedure proposal before turning it in.