Gas Chromatography A Introduction Gas Chromatography GC is





- Slides: 19

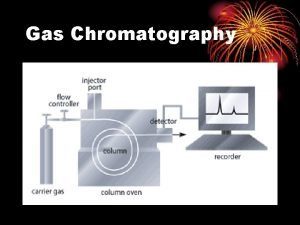
Gas Chromatography A. ) Introduction: Gas Chromatography (GC) is a chromatographic technique in which the mobile phase is a gas. GC is currently one of the most popular methods for separating and analyzing compounds. This is due to its high resolution, low limits of detection, speed, accuracy and reproducibility. GC can be applied to the separation of any compound that is either naturally volatile (i. e. , readily goes into the gas phase) or can be converted to a volatile derivative. This makes GC useful in the separation of a number of small organic and inorganic compounds. B. ) Equipment: A simple GC system consists of: 1. Gas source (with pressure and flow regulators) 2. Injector or sample application system 3. Chromatographic column (with oven for temperature control) 4. Detector & computer or recorder

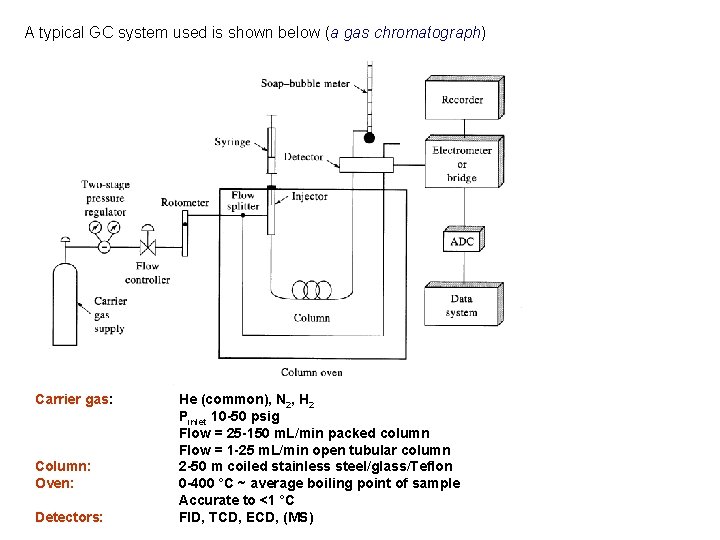
A typical GC system used is shown below (a gas chromatograph) Carrier gas: Column: Oven: Detectors: He (common), N 2, H 2 Pinlet 10 -50 psig Flow = 25 -150 m. L/min packed column Flow = 1 -25 m. L/min open tubular column 2 -50 m coiled stainless steel/glass/Teflon 0 -400 °C ~ average boiling point of sample Accurate to <1 °C FID, TCD, ECD, (MS)

C. ) Mobile Phase: GC separates solutes based on their different interactions with the mobile and stationary phases. - solute’s retention is determined mostly by its vapor pressure and volatility - solute’s retention is controlled by its interaction with the stationary phase - gas mobile phase has much lower density ‚decreased chance for interacting with solute ‚increased chance that solid or liquid stationary phase interacts with solute Carrier gas – main purpose of the gas in GC is to move the solutes along the column, mobile phase is often referred to as carrier gas. Common carrier gas: include He, Ar, H 2, N 2 Carrier Gas or Mobile phase does not affect solute retention, but does affect: 1. ) Desired efficiency for the GC System - low molecular weight gases (He, H 2) larger diffusion coefficients - low molecular weight gases faster, more efficient separations 2. ) Stability of column and solutes - H 2 or O 2 can react with functional groups on solutes and stationary phase or with surfaces of the injector, connections and detector 3. ) Response of the detector - thermal conductor requires H 2 or He - other detectors require specific carrier gas

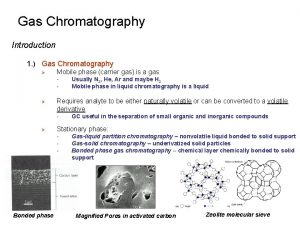
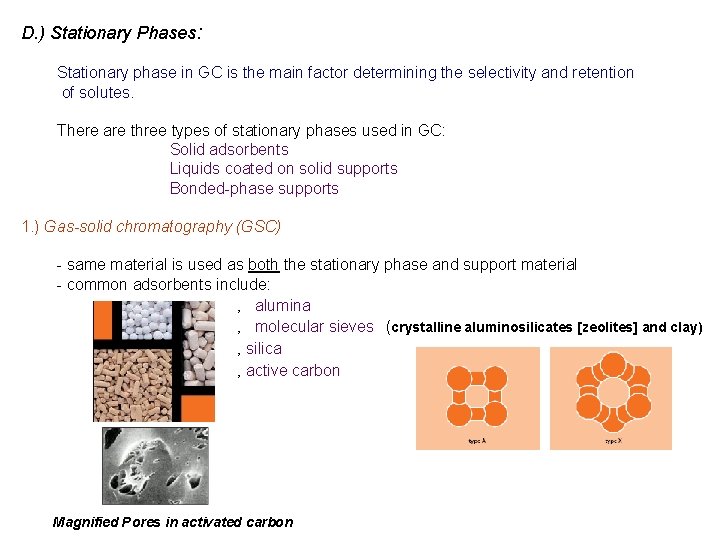
D. ) Stationary Phases: Stationary phase in GC is the main factor determining the selectivity and retention of solutes. There are three types of stationary phases used in GC: Solid adsorbents Liquids coated on solid supports Bonded-phase supports 1. ) Gas-solid chromatography (GSC) - same material is used as both the stationary phase and support material - common adsorbents include: ‚ alumina ‚ molecular sieves (crystalline aluminosilicates [zeolites] and clay) ‚ silica ‚ active carbon Magnified Pores in activated carbon

Gas-solid chromatography (GSC): advantages: - long column lifetimes - ability to retain and separate some compounds not easily resolved by other GC methods ‚ geometrical isomers ‚ permanent gases disadvantage: - very strong retention of low volatility or polar solutes - catalytic changes that can occur on GSC supports - GSC supports have a range of chemical and physical environments ‚ different strength retention sites ‚ non-symmetrical peaks ‚ variable retention times

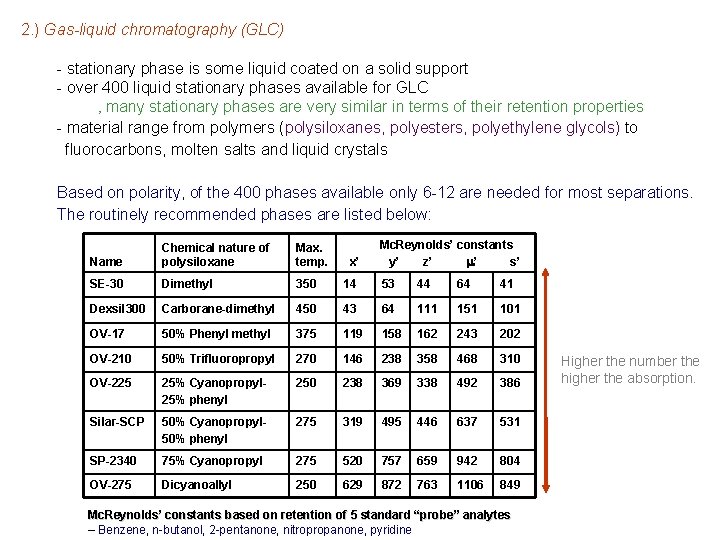
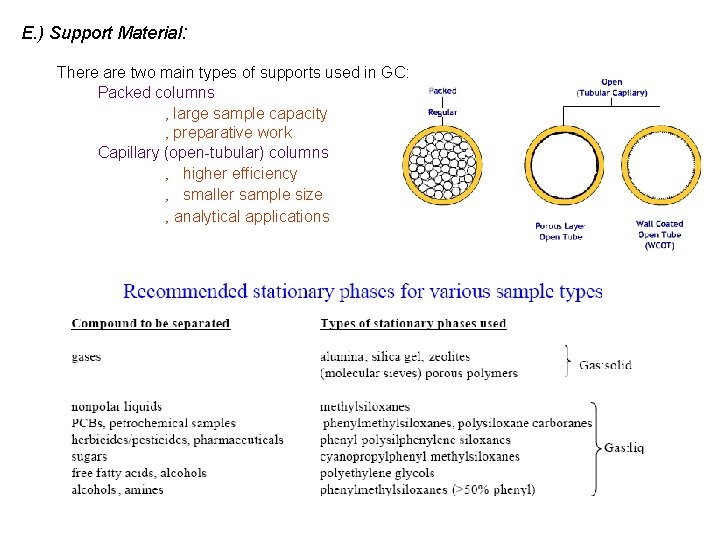
2. ) Gas-liquid chromatography (GLC) - stationary phase is some liquid coated on a solid support - over 400 liquid stationary phases available for GLC ‚ many stationary phases are very similar in terms of their retention properties - material range from polymers (polysiloxanes, polyesters, polyethylene glycols) to fluorocarbons, molten salts and liquid crystals Based on polarity, of the 400 phases available only 6 -12 are needed for most separations. The routinely recommended phases are listed below: Mc. Reynolds’ constants y’ z’ m’ s’ Name Chemical nature of polysiloxane Max. temp. SE-30 Dimethyl 350 14 53 44 64 41 Dexsil 300 Carborane-dimethyl 450 43 64 111 151 101 OV-17 50% Phenyl methyl 375 119 158 162 243 202 OV-210 50% Trifluoropropyl 270 146 238 358 468 310 OV-225 25% Cyanopropyl 25% phenyl 250 238 369 338 492 386 Silar-SCP 50% Cyanopropyl 50% phenyl 275 319 495 446 637 531 SP-2340 75% Cyanopropyl 275 520 757 659 942 804 OV-275 Dicyanoallyl 250 629 872 763 1106 849 x’ Mc. Reynolds’ constants based on retention of 5 standard “probe” analytes – Benzene, n-butanol, 2 -pentanone, nitropropanone, pyridine Higher the number the higher the absorption.

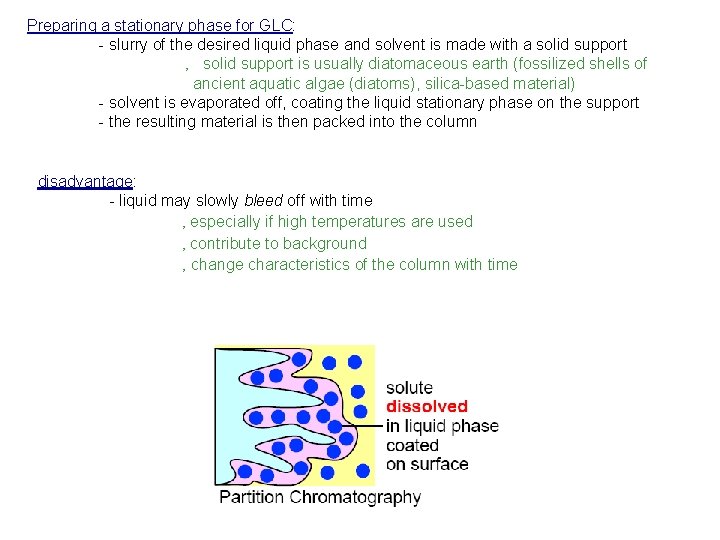
Preparing a stationary phase for GLC: - slurry of the desired liquid phase and solvent is made with a solid support ‚ solid support is usually diatomaceous earth (fossilized shells of ancient aquatic algae (diatoms), silica-based material) - solvent is evaporated off, coating the liquid stationary phase on the support - the resulting material is then packed into the column disadvantage: - liquid may slowly bleed off with time ‚ especially if high temperatures are used ‚ contribute to background ‚ change characteristics of the column with time

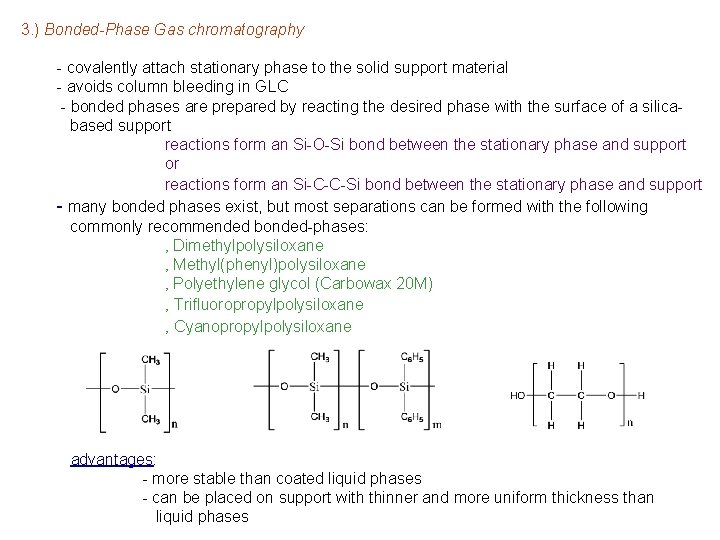
3. ) Bonded-Phase Gas chromatography - covalently attach stationary phase to the solid support material - avoids column bleeding in GLC - bonded phases are prepared by reacting the desired phase with the surface of a silicabased support reactions form an Si-O-Si bond between the stationary phase and support or reactions form an Si-C-C-Si bond between the stationary phase and support - many bonded phases exist, but most separations can be formed with the following commonly recommended bonded-phases: ‚ Dimethylpolysiloxane ‚ Methyl(phenyl)polysiloxane ‚ Polyethylene glycol (Carbowax 20 M) ‚ Trifluoropropylpolysiloxane ‚ Cyanopropylpolysiloxane advantages: - more stable than coated liquid phases - can be placed on support with thinner and more uniform thickness than liquid phases

E. ) Support Material: There are two main types of supports used in GC: Packed columns ‚ large sample capacity ‚ preparative work Capillary (open-tubular) columns ‚ higher efficiency ‚ smaller sample size ‚ analytical applications

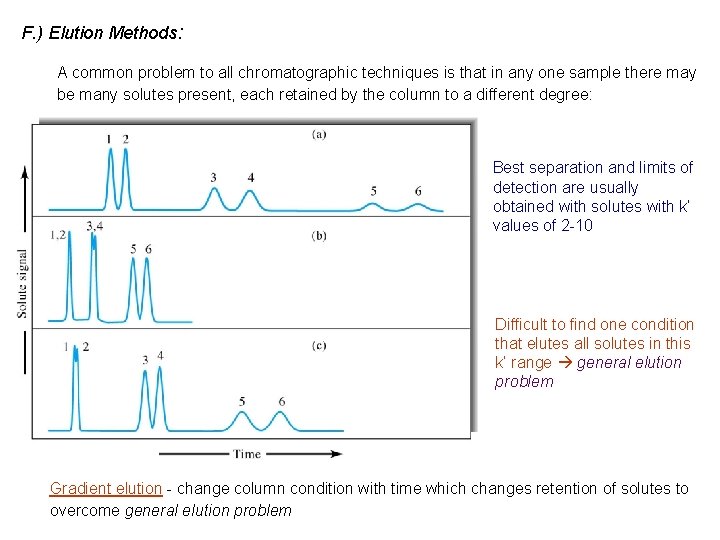
F. ) Elution Methods: A common problem to all chromatographic techniques is that in any one sample there may be many solutes present, each retained by the column to a different degree: Best separation and limits of detection are usually obtained with solutes with k’ values of 2 -10 Difficult to find one condition that elutes all solutes in this k’ range general elution problem Gradient elution - change column condition with time which changes retention of solutes to overcome general elution problem

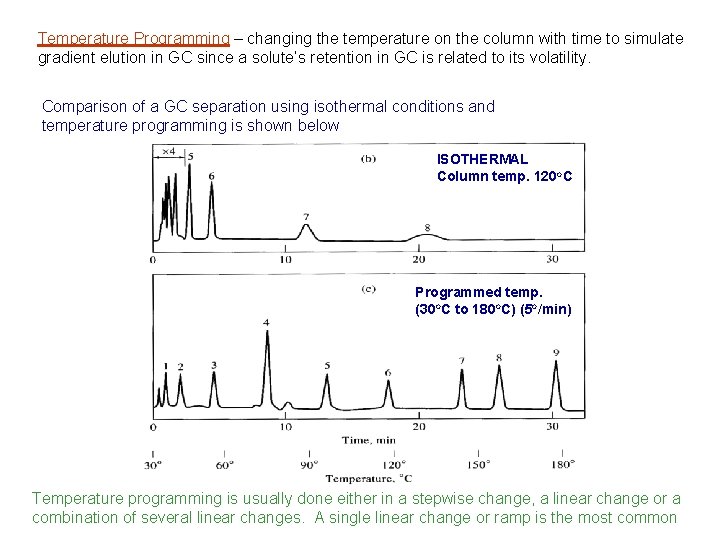
Temperature Programming – changing the temperature on the column with time to simulate gradient elution in GC since a solute’s retention in GC is related to its volatility. Comparison of a GC separation using isothermal conditions and temperature programming is shown below ISOTHERMAL Column temp. 120 o. C Programmed temp. (30 o. C to 180 o. C) (5 o/min) Temperature programming is usually done either in a stepwise change, a linear change or a combination of several linear changes. A single linear change or ramp is the most common

G. ) GC Detectors: The following devices are common types of GC detectors: 1. Thermal Conductivity Detector (TCD) 2. Flame Ionization Detector (FID) 3. Nitrogen-phosphorus Detector 4. Electron Capture Detector (ECD) 5. Mass Spectrometers (discussed later in the course) The choice of detector will depend on the analyte and how the GC method is being used (i. e. , analytical or preparative scale) 1. ) Thermal Conductivity Detector (TCD) - katherometer or hot-wire detector - first universal detector developed for GC Process - measures a bulk property of the mobile phase leaving the column. - measures ability to conduct heat away from a hot-wire (i. e. , thermal conductivity) - thermal conductivity changes with presence of other components in the mobile phase

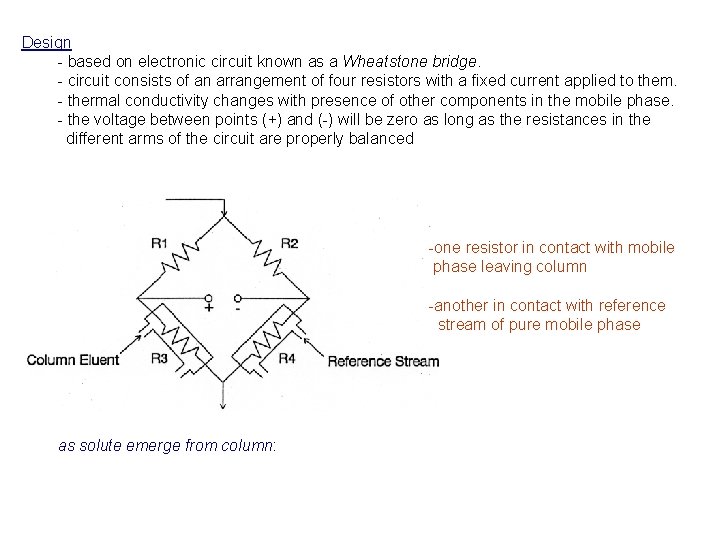
Design - based on electronic circuit known as a Wheatstone bridge. - circuit consists of an arrangement of four resistors with a fixed current applied to them. - thermal conductivity changes with presence of other components in the mobile phase. - the voltage between points (+) and (-) will be zero as long as the resistances in the different arms of the circuit are properly balanced -one resistor in contact with mobile phase leaving column -another in contact with reference stream of pure mobile phase as solute emerge from column:

Considerations - mobile phase must have very different thermal conductivity then solutes being separated. - most compounds separated in GC have thermal conductivity of about 1 -4 X 10 -5. - H 2 and He are carrier gases with significantly different thermal conductivity values. - H 2 reacts with metal oxides present on the resistors, so not used advantages: - truly universal detector ‚ applicable to the detection of any compound in GC - non-destructive ‚ useful for detecting compounds from preparative-scale columns ‚ useful in combination with other types of GC detectors disadvantage: - detect mobile phase impurities - sensitive to changes in flow-rates - limit of detection ‚ ~ 10 -7 M ‚ much higher then other GC detectors

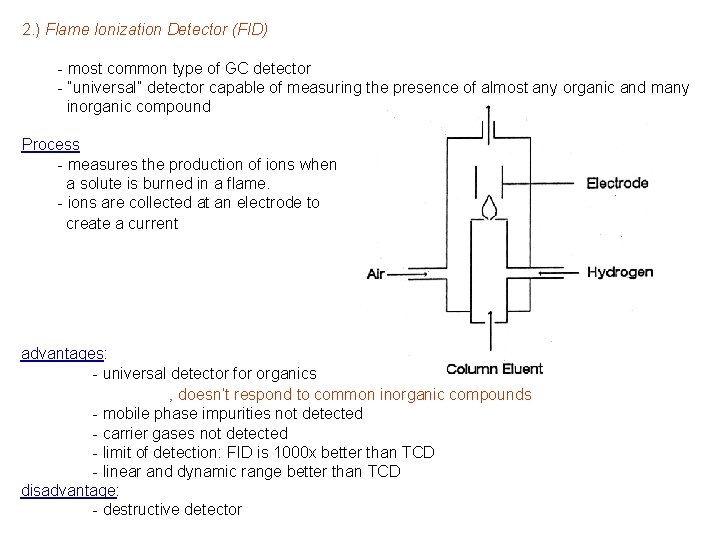
2. ) Flame Ionization Detector (FID) - most common type of GC detector - “universal” detector capable of measuring the presence of almost any organic and many inorganic compound Process - measures the production of ions when a solute is burned in a flame. - ions are collected at an electrode to create a current advantages: - universal detector for organics ‚ doesn’t respond to common inorganic compounds - mobile phase impurities not detected - carrier gases not detected - limit of detection: FID is 1000 x better than TCD - linear and dynamic range better than TCD disadvantage: - destructive detector

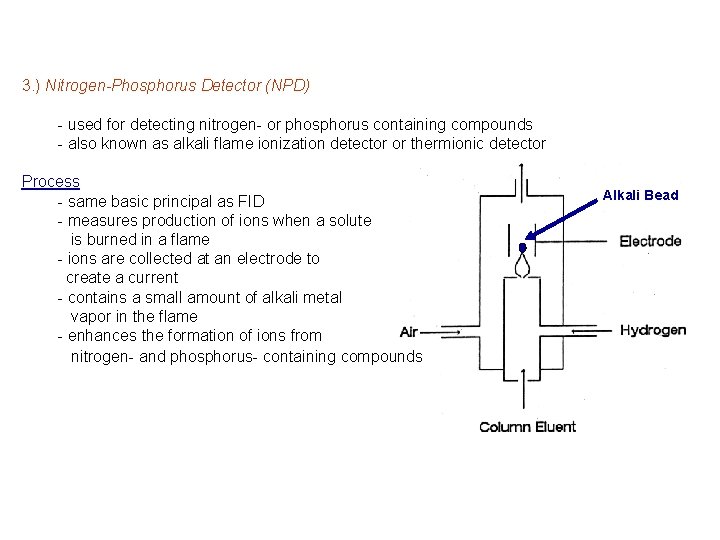
3. ) Nitrogen-Phosphorus Detector (NPD) - used for detecting nitrogen- or phosphorus containing compounds - also known as alkali flame ionization detector or thermionic detector Process - same basic principal as FID - measures production of ions when a solute is burned in a flame - ions are collected at an electrode to create a current - contains a small amount of alkali metal vapor in the flame - enhances the formation of ions from nitrogen- and phosphorus- containing compounds Alkali Bead

3. ) Nitrogen-Phosphorus Detector (NPD) advantages: - useful for environmental testing ‚ detection of organophosphate pesticides - useful for drug analysis ‚ determination of amine-containing or basic drugs - Like FID, does not detect common mobile phase impurities or carrier gases - limit of detection: NPD is 500 x better than FID in detecting nitrogen- and phosphorus- containing compounds - NPD more sensitive to other heterocompounds, such as sulfur-, halogen-, and arsenic- containing molecules disadvantage: - destructive detector - NPD is less sensitive to organic compounds compared to FID

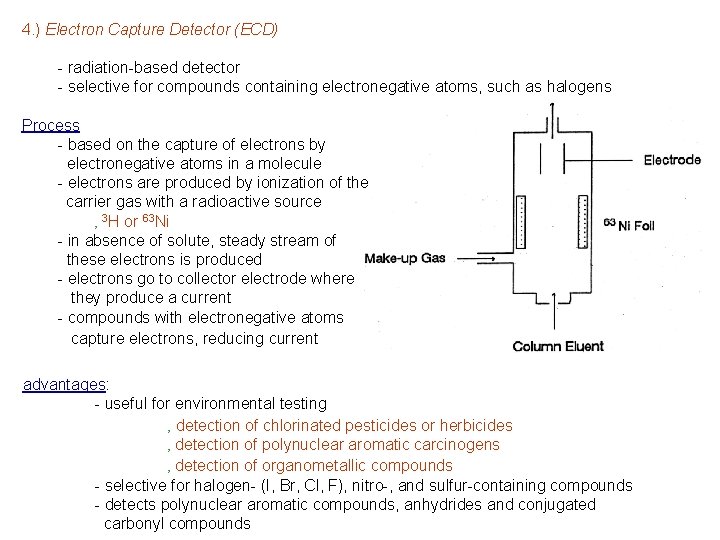
4. ) Electron Capture Detector (ECD) - radiation-based detector - selective for compounds containing electronegative atoms, such as halogens Process - based on the capture of electrons by electronegative atoms in a molecule - electrons are produced by ionization of the carrier gas with a radioactive source ‚ 3 H or 63 Ni - in absence of solute, steady stream of these electrons is produced - electrons go to collector electrode where they produce a current - compounds with electronegative atoms capture electrons, reducing current advantages: - useful for environmental testing ‚ detection of chlorinated pesticides or herbicides ‚ detection of polynuclear aromatic carcinogens ‚ detection of organometallic compounds - selective for halogen- (I, Br, Cl, F), nitro-, and sulfur-containing compounds - detects polynuclear aromatic compounds, anhydrides and conjugated carbonyl compounds

Example 13: (a) What properties should the stationary-phase liquid for GC possess? (b) What is the effect of stationary-phase film thickness on GC? (c) Why are GC stationary phases often bonded and cross-linked?
 Introduction of gas chromatography
Introduction of gas chromatography Van deemter
Van deemter Gradient vs isocratic elution
Gradient vs isocratic elution Principle of gc
Principle of gc Basic principle of gc
Basic principle of gc Gas liquid chromatography
Gas liquid chromatography Percent composition gas chromatography
Percent composition gas chromatography Stationary phase
Stationary phase Basic gas chromatography
Basic gas chromatography Gas chromatography history
Gas chromatography history Polarity paper chromatography
Polarity paper chromatography Contoh perhitungan rf
Contoh perhitungan rf Gas chromatography
Gas chromatography Gas chromatography
Gas chromatography Hplc slideshare
Hplc slideshare Gas chromatography theory
Gas chromatography theory Types of liquid chromatography
Types of liquid chromatography High performance liquid chromatography uses
High performance liquid chromatography uses Introduction of chromatography
Introduction of chromatography Introduction of chromatography
Introduction of chromatography