SIZE EXCLUSION CHROMATOGRAPHY Kausar Ahmad Kulliyyah of Pharmacy









- Slides: 36

SIZE EXCLUSION CHROMATOGRAPHY Kausar Ahmad Kulliyyah of Pharmacy, IIUM http: //staff. iiu. edu. my/akausar 1 Physical Pharmacy 2

Contents Principles underlying chromatographic techniques Retention mechanism ○ Diffusion Fick’s law Types of size exclusion chromatography Gel permeation Gel filtration 2 Applications Reliability of results Physical Pharmacy 2

Objective of Separation Proteins are extracted from animals and humans as a mixture in a serum of body fluids. To study a specific protein, like an antibody, hormone, or enzyme, need to separate from the mix. 3 Physical Pharmacy 2

Some examples of separative techniques 4 1. Solvent extraction 2. Chromatography 3. Precipitation 4. Recrystallisation 5. Electrophoresis Physical Pharmacy 2

Examples of chromatographic techniques 5 1. Ion-exchange chromatography 2. Size-exclusion chromatography 3. Paper chromatography 4. Thin layer chromatography 5. Affinity chromatography Physical Pharmacy 2

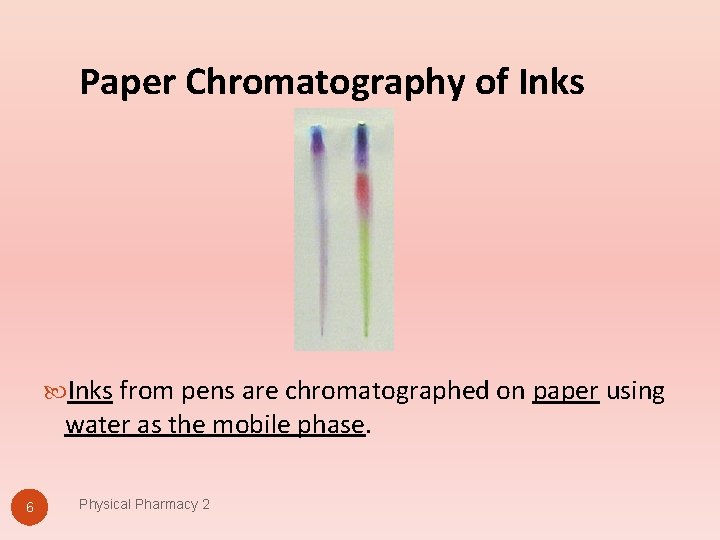
Paper Chromatography of Inks from pens are chromatographed on paper using water as the mobile phase. 6 Physical Pharmacy 2

Chromatography of spinach extract Spinach extract is separated by thin layer chromatography into chlorophyll and B-carotene 7 Physical Pharmacy 2

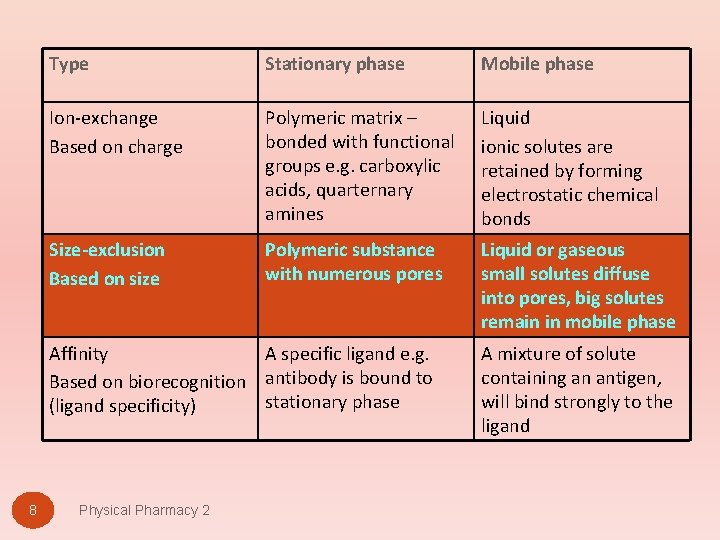
8 Type Stationary phase Mobile phase Ion-exchange Based on charge Polymeric matrix – bonded with functional groups e. g. carboxylic acids, quarternary amines Liquid ionic solutes are retained by forming electrostatic chemical bonds Size-exclusion Based on size Polymeric substance with numerous pores Liquid or gaseous small solutes diffuse into pores, big solutes remain in mobile phase Affinity A specific ligand e. g. Based on biorecognition antibody is bound to stationary phase (ligand specificity) A mixture of solute containing an antigen, will bind strongly to the ligand Physical Pharmacy 2

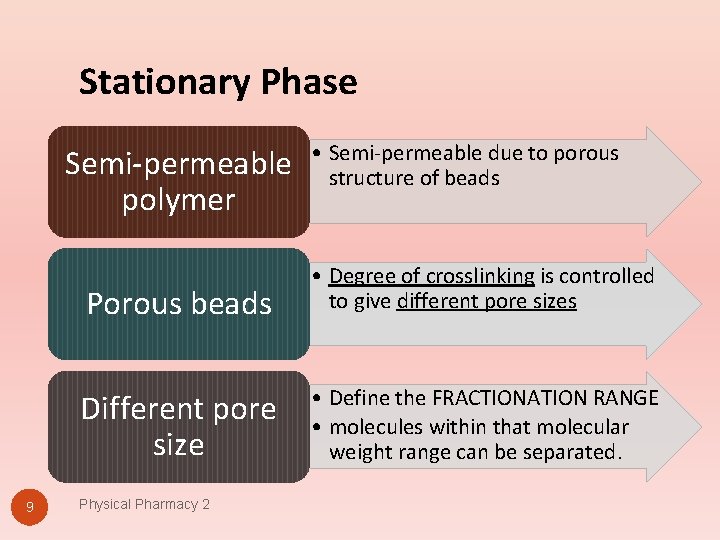
Stationary Phase Semi-permeable polymer 9 • Semi-permeable due to porous structure of beads Porous beads • Degree of crosslinking is controlled to give different pore sizes Different pore size • Define the FRACTIONATION RANGE • molecules within that molecular weight range can be separated. Physical Pharmacy 2

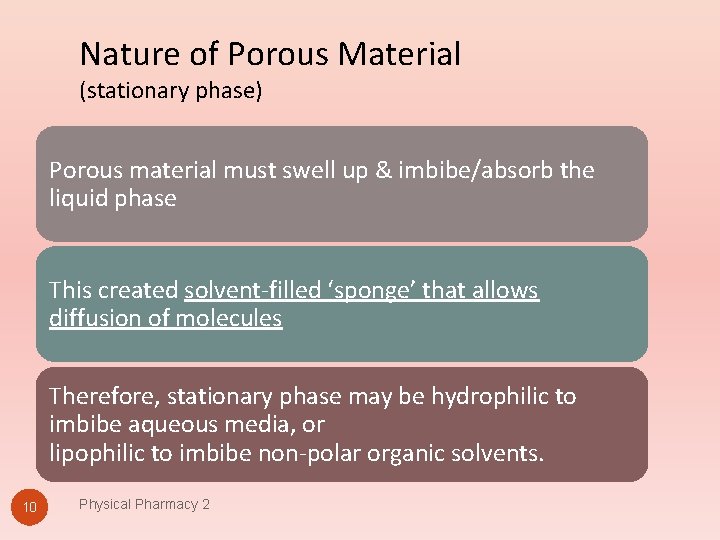
Nature of Porous Material (stationary phase) Porous material must swell up & imbibe/absorb the liquid phase This created solvent-filled ‘sponge’ that allows diffusion of molecules Therefore, stationary phase may be hydrophilic to imbibe aqueous media, or lipophilic to imbibe non-polar organic solvents. 10 Physical Pharmacy 2

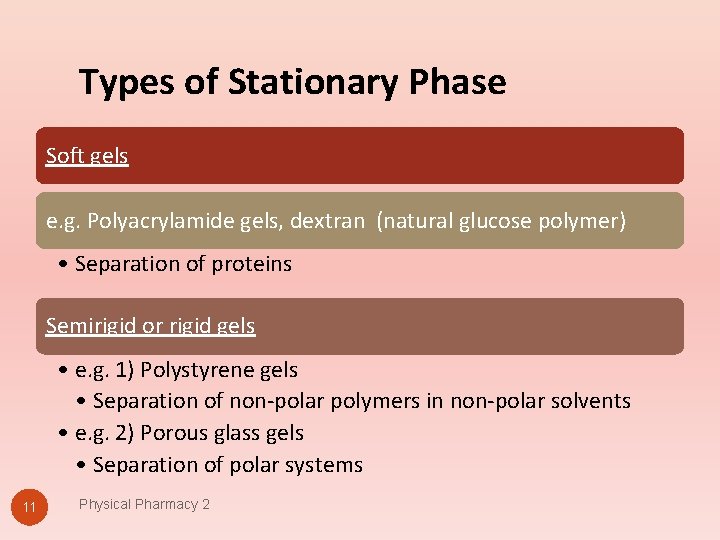
Types of Stationary Phase Soft gels e. g. Polyacrylamide gels, dextran (natural glucose polymer) • Separation of proteins Semirigid or rigid gels • e. g. 1) Polystyrene gels • Separation of non-polar polymers in non-polar solvents • e. g. 2) Porous glass gels • Separation of polar systems 11 Physical Pharmacy 2

Soft gels Before column is packed, gel is imbibed by enough liquid to completely swell. • These gels are used with aqueous mobile phase. Once column is packed, the composition of the mobile phase cannot be altered to prevent shrinkage or bursting of the packed column. • Because of low structural strength, they cannot be used under high pressure. classified as gel filtration. 12 Physical Pharmacy 2

Semirigid Gels Made from crosslinked polystyrene, glass beads or alkylated dextran. Used for separation of organic-soluble polymers. Non-aqueous mobile phases e. g. chloroform, acetone, pyridine or tetrahydrofuran. Classified as gel permeation. 13 Physical Pharmacy 2

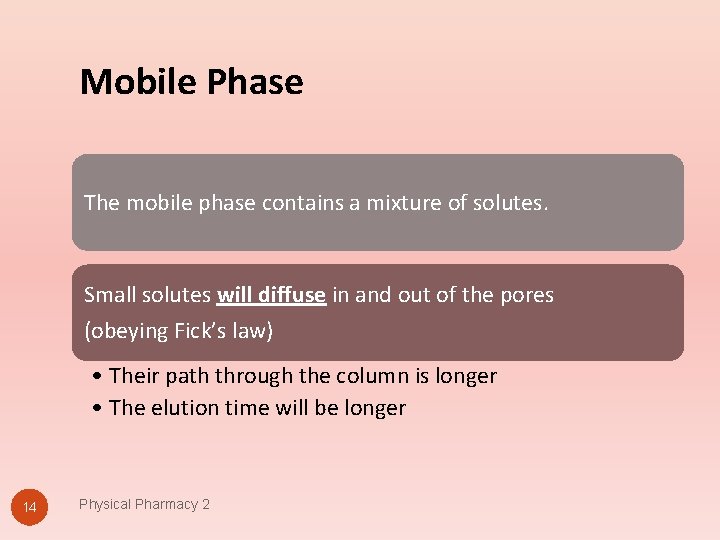
Mobile Phase The mobile phase contains a mixture of solutes. Small solutes will diffuse in and out of the pores (obeying Fick’s law) • Their path through the column is longer • The elution time will be longer 14 Physical Pharmacy 2

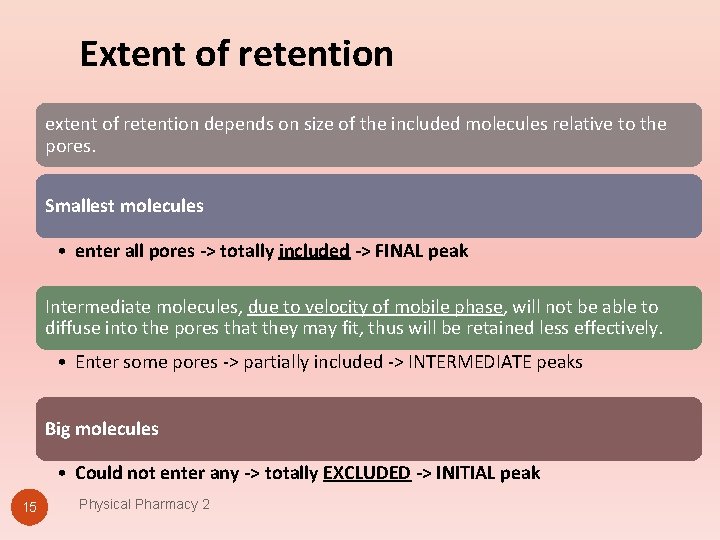
Extent of retention extent of retention depends on size of the included molecules relative to the pores. Smallest molecules • enter all pores -> totally included -> FINAL peak Intermediate molecules, due to velocity of mobile phase, will not be able to diffuse into the pores that they may fit, thus will be retained less effectively. • Enter some pores -> partially included -> INTERMEDIATE peaks Big molecules • Could not enter any -> totally EXCLUDED -> INITIAL peak 15 Physical Pharmacy 2

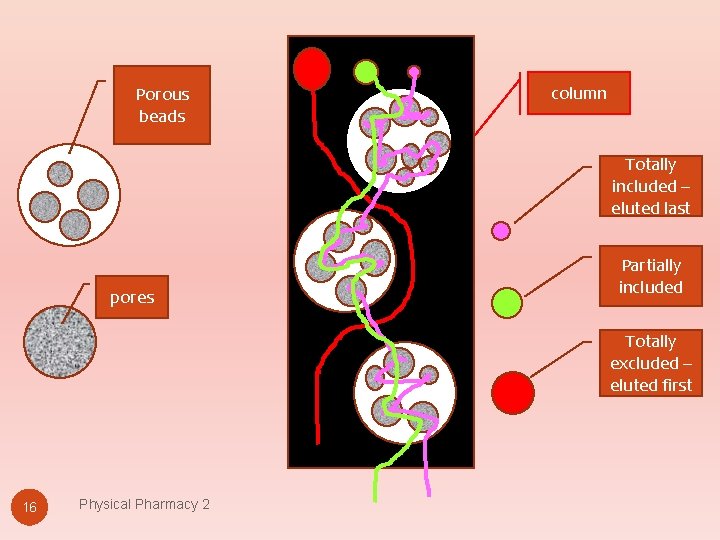
Porous beads column Totally included – eluted last pores Partially included Totally excluded – eluted first 16 Physical Pharmacy 2

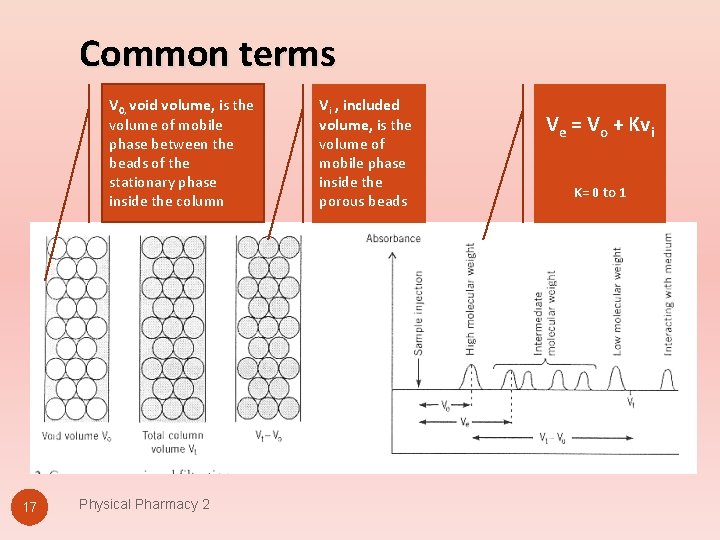
Common terms V 0, void volume, is the volume of mobile phase between the beads of the stationary phase inside the column 17 Physical Pharmacy 2 Vi , included volume, is the volume of mobile phase inside the porous beads Ve = Vo + Kvi K= 0 to 1

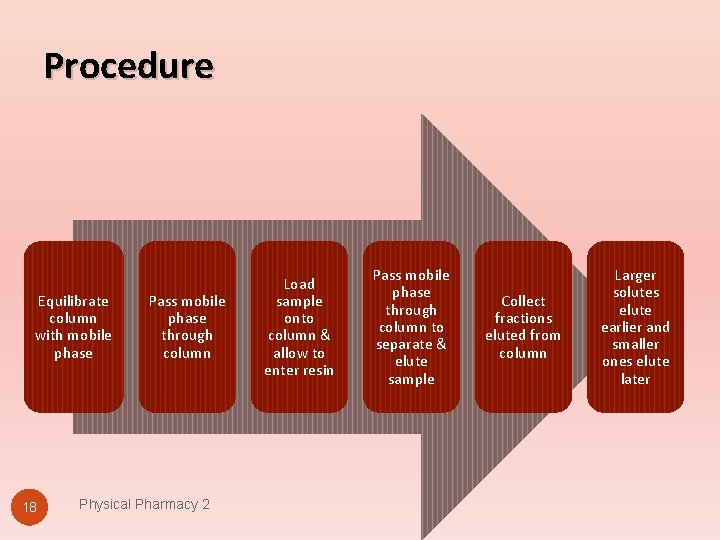
Procedure Equilibrate column with mobile phase 18 Pass mobile phase through column Physical Pharmacy 2 Load sample onto column & allow to enter resin Pass mobile phase through column to separate & elute sample Collect fractions eluted from column Larger solutes elute earlier and smaller ones elute later

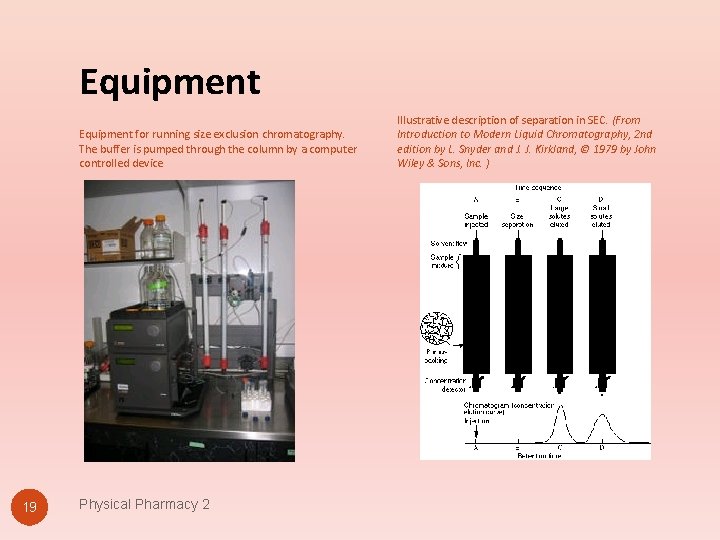
Equipment for running size exclusion chromatography. The buffer is pumped through the column by a computer controlled device 19 Physical Pharmacy 2 Illustrative description of separation in SEC. (From Introduction to Modern Liquid Chromatography, 2 nd edition by L. Snyder and J. J. Kirkland, © 1979 by John Wiley & Sons, Inc. )

Applications 20 Fractionation Desalting Concentration Molecular weight determination Physical Pharmacy 2

Desalting Necessary for purification of biochemicals. Due to techniques involving buffers and precipitating reagents. Gel with low exclusion limit (MW 1000 -2000) is used. Short column and high flow rate can be used because of the vast difference in size of solutes and contaminants. 21 Macromolecules will be eluted with little dilution and salts retained on the column. Physical Pharmacy 2

Concentration of Dilute Solutions Exclusion limit of gels less than MW of solutes. Solution is mixed with a small quantity of dry gel that will absorb 10 to 20 times its weight in water. Some salts and small molecules are taken up also. Final macromolecules in a solution of almost unchanged p. H and ionic strength but significantly decreased volume. 22 Physical Pharmacy 2

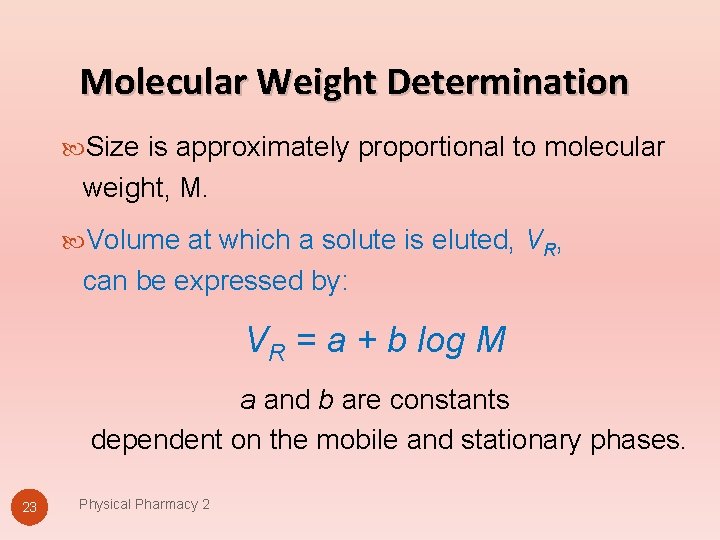
Molecular Weight Determination Size is approximately proportional to molecular weight, M. Volume at which a solute is eluted, VR, can be expressed by: VR = a + b log M a and b are constants dependent on the mobile and stationary phases. 23 Physical Pharmacy 2

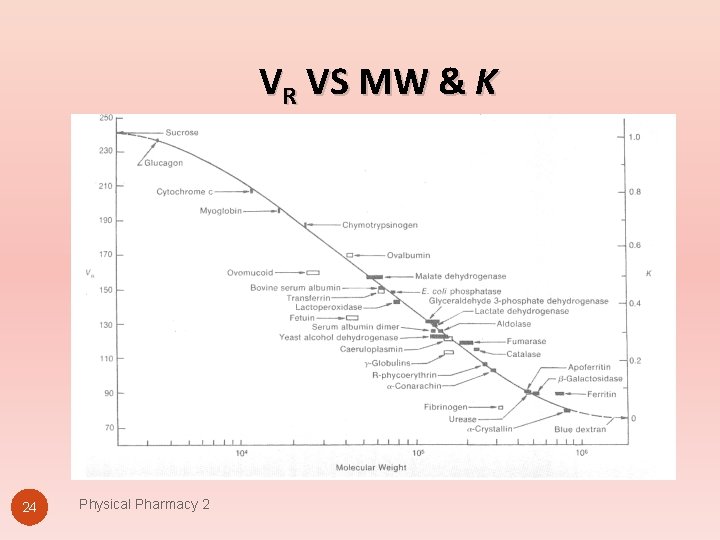
VR VS MW & K 24 Physical Pharmacy 2

Partition coefficient, K K=0 • glutamate dehydrogenase (totally excluded), K = 1 • cytochrome c (totally included) 0 > K > 1 25 Physical Pharmacy 2 • other proteins, which are within the fractionation range for the column.

Separation based on size - precaution Proteins are separated according to their molecular weight • because this is the major contribution to molecular size. However, the shape will affect its apparent size in solution. Hence, gel filtration is NOT recommended for separating proteins with only a small difference in molecular weight. 26 Physical Pharmacy 2

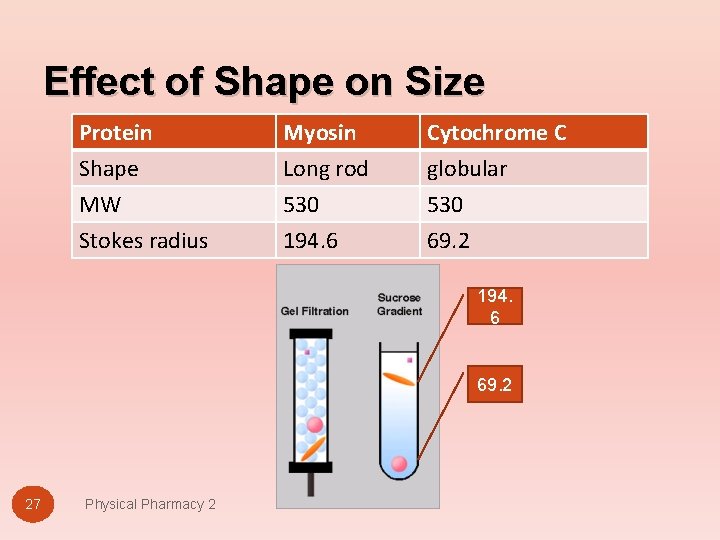
Effect of Shape on Size Protein Shape MW Stokes radius Myosin Long rod 530 194. 6 Cytochrome C globular 530 69. 2 194. 6 69. 2 27 Physical Pharmacy 2

Advantages of Gel Filtration Can handle biomolecules that are sensitive to changes in p. H, concentration of metal ions or harsh environmental conditions. Separations can be performed in the presence of essential ions, detergents, urea, , at high or low ionic strength, at 37 °C or in the cold room according to the requirements of the experiment. 28 Physical Pharmacy 2

Columns and Detectors Detection of the solute zones as they emerge from the column can be achieved by spectrophotometric monitoring of the eluate by measurement of refractive index of eluate Collection of aliquots for later analysis Ø Mobile phase is allowed to flow by gravity Ø Very high flow rate not suitable for soft gels 29 Physical Pharmacy 2

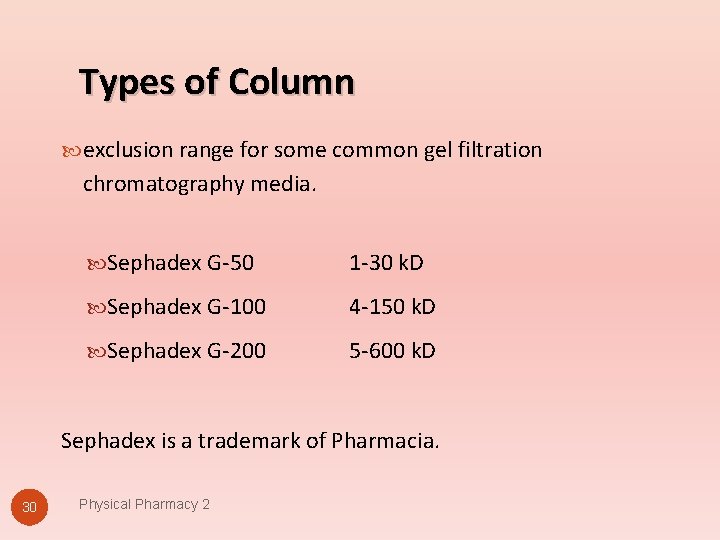
Types of Column exclusion range for some common gel filtration chromatography media. Sephadex G-50 1 -30 k. D Sephadex G-100 4 -150 k. D Sephadex G-200 5 -600 k. D Sephadex is a trademark of Pharmacia. 30 Physical Pharmacy 2

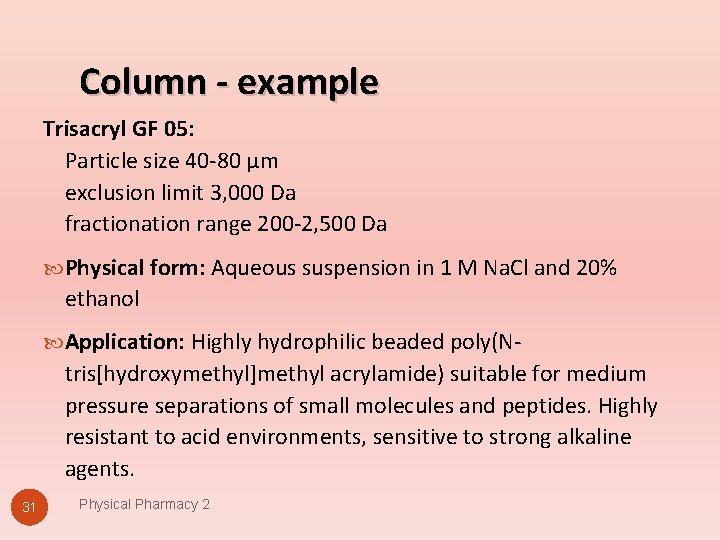
Column - example Trisacryl GF 05: Particle size 40 -80 µm exclusion limit 3, 000 Da fractionation range 200 -2, 500 Da Physical form: Aqueous suspension in 1 M Na. Cl and 20% ethanol Application: Highly hydrophilic beaded poly(N- tris[hydroxymethyl]methyl acrylamide) suitable for medium pressure separations of small molecules and peptides. Highly resistant to acid environments, sensitive to strong alkaline agents. 31 Physical Pharmacy 2

How to check reliability? Calibrate Use standards Choice of standards depends on application Available in low and high molecular weight ranges supplied lyophilized in individual vials. Kits include Blue Dextran 2000 to determine the column void volume and to check column packing. 32 Physical Pharmacy 2

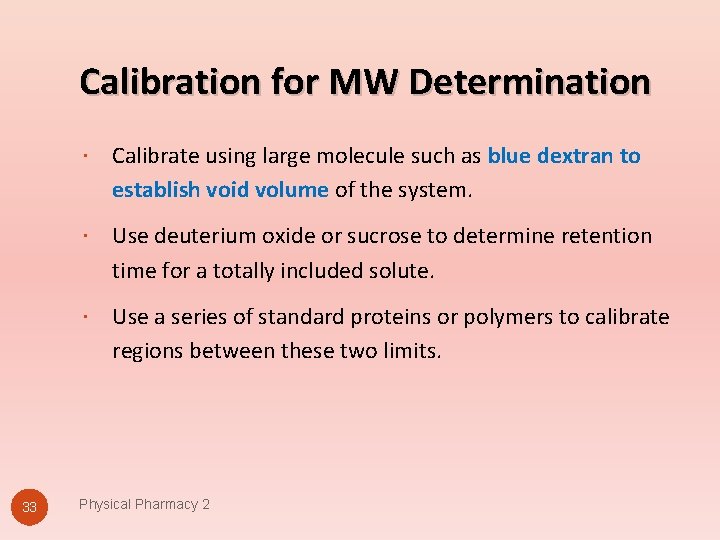
Calibration for MW Determination 33 Calibrate using large molecule such as blue dextran to establish void volume of the system. Use deuterium oxide or sucrose to determine retention time for a totally included solute. Use a series of standard proteins or polymers to calibrate regions between these two limits. Physical Pharmacy 2

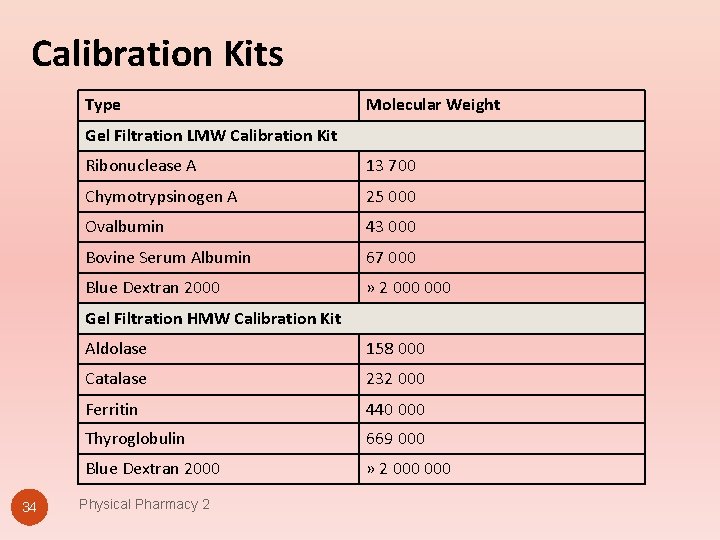
Calibration Kits Type Molecular Weight Gel Filtration LMW Calibration Kit Ribonuclease A 13 700 Chymotrypsinogen A 25 000 Ovalbumin 43 000 Bovine Serum Albumin 67 000 Blue Dextran 2000 » 2 000 Gel Filtration HMW Calibration Kit 34 Aldolase 158 000 Catalase 232 000 Ferritin 440 000 Thyroglobulin 669 000 Blue Dextran 2000 » 2 000 Physical Pharmacy 2

Exercise Consider the separation of a mixture of glutamate dehydrogenase (MW 290, 000), lactate dehydrogenase (MW 140, 000), serum albumin (MW 67, 000), ovalbumin (MW 43, 000), cytochrome c (MW 12, 400) on a gel filtration column: fractionation range 15, 000 - 150, 000). 35 Physical Pharmacy 2

References AR Gennaro, Remington: The Science and Practice of Pharmacy 20 th Ed. , Lippincott Williams & Wilkins (2000) Part 4 DG Peters, JM Hayes, GM Hieftje, Chemical Separations and Measurements, Saunders, Philadelphia(1974) Chapter 17 Peter Atkins & Julio de Paula, Atkin’s Physical Chemistry 7 th Ed. , Oxford, New York (2002) Chapter 36 22 Physical Pharmacy 2
 Kausar ahmad
Kausar ahmad Ahmad ibrahim kulliyyah of laws
Ahmad ibrahim kulliyyah of laws Kulliyyah of pharmacy
Kulliyyah of pharmacy Size exclusion chromatography animation
Size exclusion chromatography animation Capacity factor formula
Capacity factor formula Principle of size exclusion chromatography
Principle of size exclusion chromatography Biolaxin
Biolaxin Kulliyyah of islamic revealed knowledge
Kulliyyah of islamic revealed knowledge Brushing method in size separation
Brushing method in size separation What are the advantages of size reduction
What are the advantages of size reduction Const char *s
Const char *s Allelic exclusion
Allelic exclusion Example of inclusion criteria
Example of inclusion criteria Niche partitioning
Niche partitioning Mutual exclusion in distributed systems
Mutual exclusion in distributed systems Niche definition
Niche definition Microempresa características
Microempresa características Exclusion critera
Exclusion critera Dijkstra mutual exclusion algorithm
Dijkstra mutual exclusion algorithm Allelic exclusion
Allelic exclusion Paternity exclusion
Paternity exclusion Competitive exclusion example
Competitive exclusion example Que representa
Que representa Electrons in an atom tend to assume the arrangement
Electrons in an atom tend to assume the arrangement Mutual exclusion in distributed system tutorialspoint
Mutual exclusion in distributed system tutorialspoint Chromatographie d'exclusion
Chromatographie d'exclusion What is the inclusion and exclusion criteria in research
What is the inclusion and exclusion criteria in research Hestia mdcalc
Hestia mdcalc Pauli exclusion principle
Pauli exclusion principle Singhals heuristic algorithm
Singhals heuristic algorithm Principe d exclusion de pauli
Principe d exclusion de pauli Inclusion exclusion principle
Inclusion exclusion principle Competitive exclusion occurs when _______.
Competitive exclusion occurs when _______. Pauli exclusion principle violation
Pauli exclusion principle violation Derangements formula
Derangements formula Orbital filling sequence and energy levels
Orbital filling sequence and energy levels Inclusion exclusion principle
Inclusion exclusion principle