Cream Formulation 1 KAUSAR AHMAD KULLIYYAH OF PHARMACY










- Slides: 38

Cream Formulation 1 KAUSAR AHMAD KULLIYYAH OF PHARMACY http: //staff. iium. edu. my/akausar PHM 4153 Dosage Design 2 2011/12

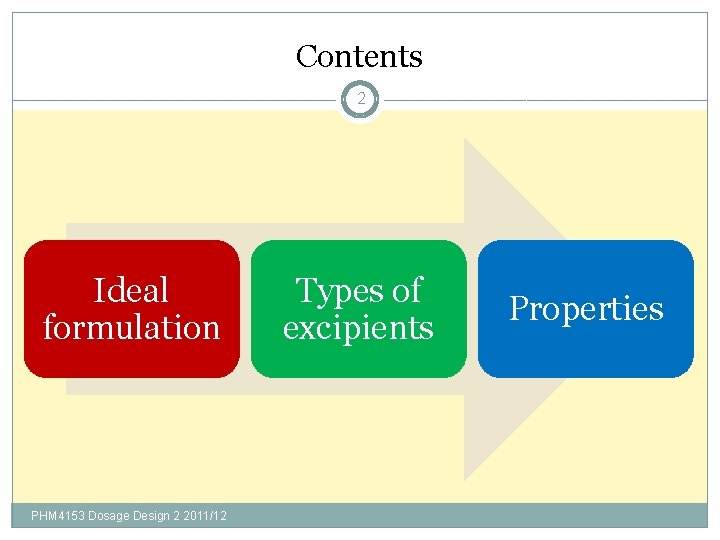
Contents 2 Ideal formulation PHM 4153 Dosage Design 2 2011/12 Types of excipients Properties

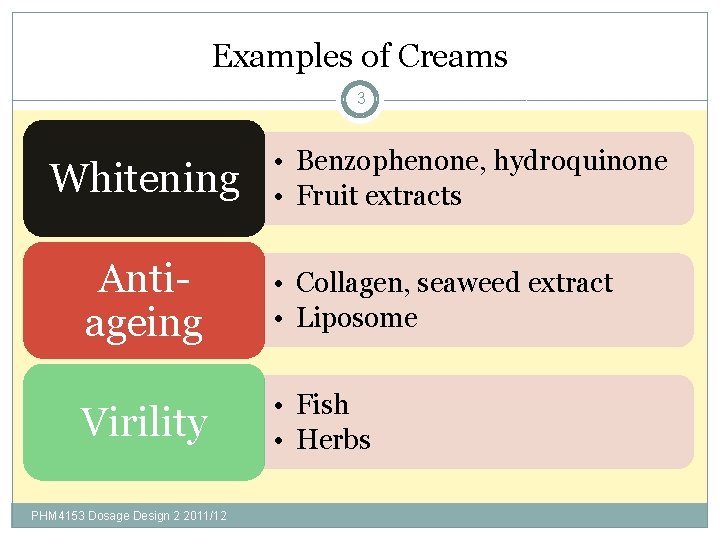
Examples of Creams 3 Whitening • Benzophenone, hydroquinone • Fruit extracts Antiageing • Collagen, seaweed extract • Liposome Virility • Fish • Herbs PHM 4153 Dosage Design 2 2011/12

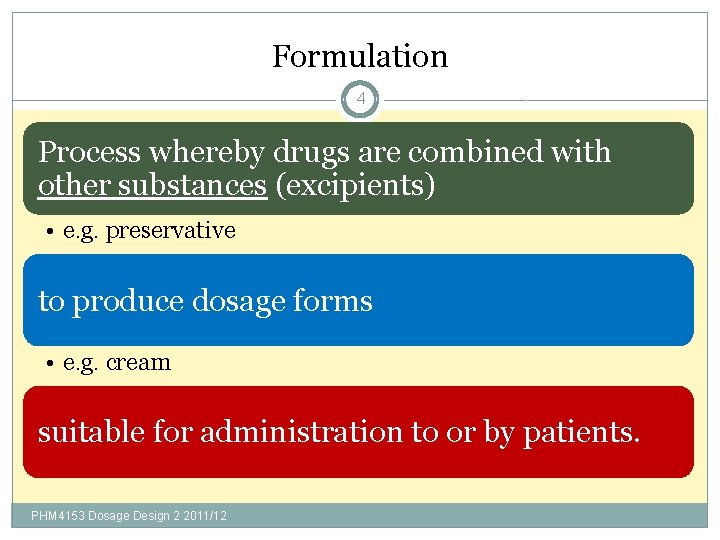
Formulation 4 Process whereby drugs are combined with other substances (excipients) • e. g. preservative to produce dosage forms • e. g. cream suitable for administration to or by patients. PHM 4153 Dosage Design 2 2011/12

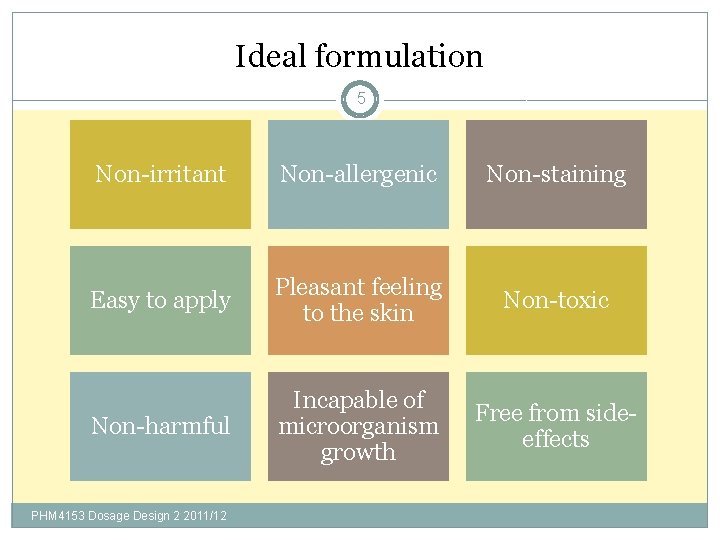
Ideal formulation 5 Non-irritant Non-allergenic Non-staining Easy to apply Pleasant feeling to the skin Non-toxic Non-harmful Incapable of microorganism growth Free from sideeffects PHM 4153 Dosage Design 2 2011/12

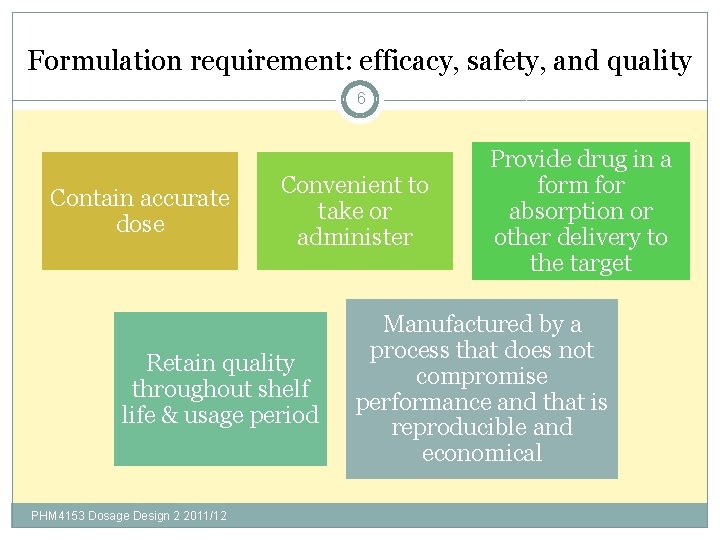
Formulation requirement: efficacy, safety, and quality 6 Contain accurate dose Convenient to take or administer Retain quality throughout shelf life & usage period PHM 4153 Dosage Design 2 2011/12 Provide drug in a form for absorption or other delivery to the target Manufactured by a process that does not compromise performance and that is reproducible and economical

Factors to be considered in formulation 7 Physicochemical properties Choice of vehicle • Waxes and oils or emulsions Categories of excipients • Provide essential part of the dosage form • Prevent degradation of the formulation Stability PHM 4153 Dosage Design 2 2011/12

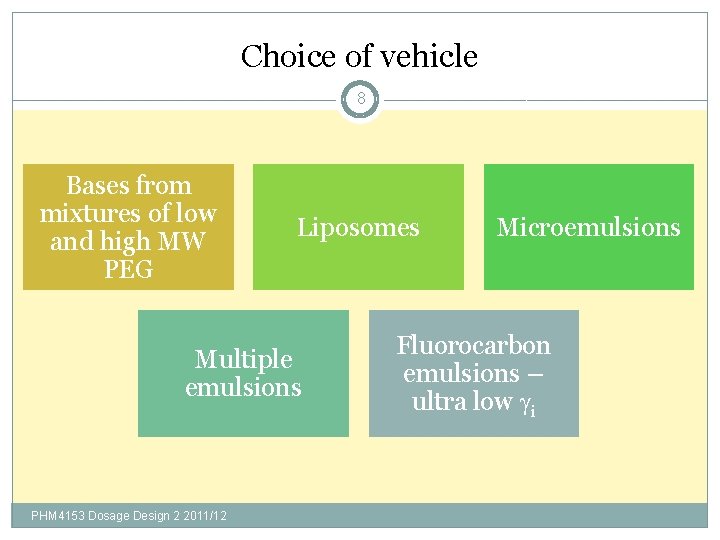
Choice of vehicle 8 Bases from mixtures of low and high MW PEG Liposomes Multiple emulsions PHM 4153 Dosage Design 2 2011/12 Microemulsions Fluorocarbon emulsions – ultra low i

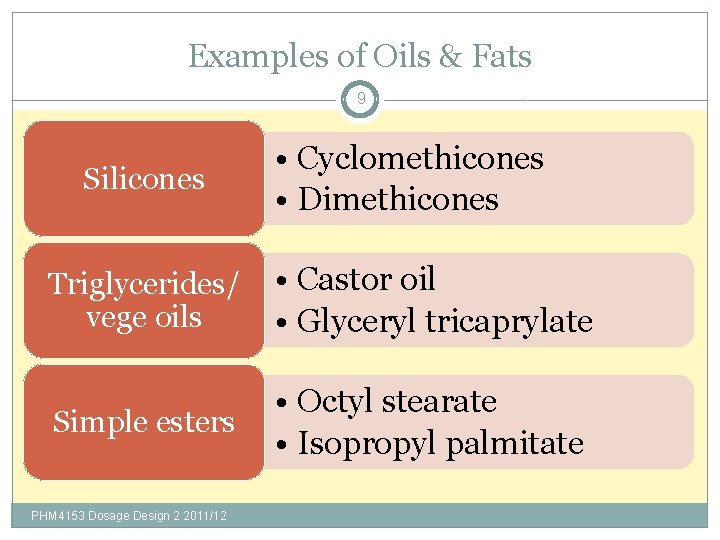
Examples of Oils & Fats 9 Silicones • Cyclomethicones • Dimethicones Triglycerides/ vege oils • Castor oil • Glyceryl tricaprylate Simple esters • Octyl stearate • Isopropyl palmitate PHM 4153 Dosage Design 2 2011/12

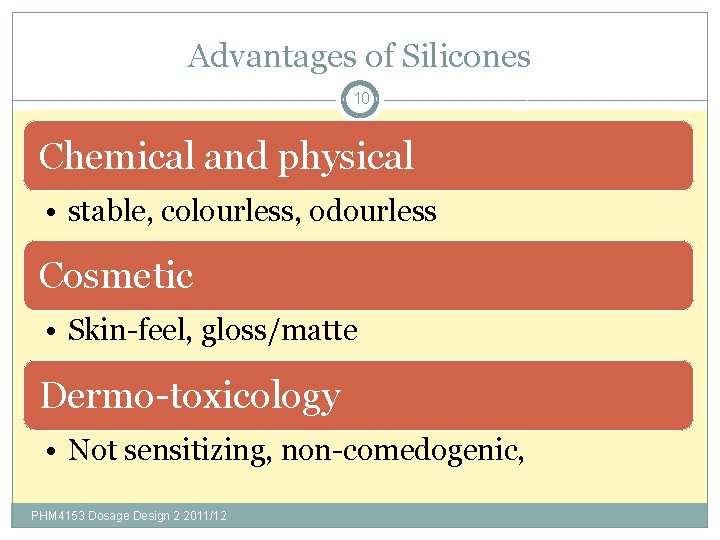
Advantages of Silicones 10 Chemical and physical • stable, colourless, odourless Cosmetic • Skin-feel, gloss/matte Dermo-toxicology • Not sensitizing, non-comedogenic, PHM 4153 Dosage Design 2 2011/12

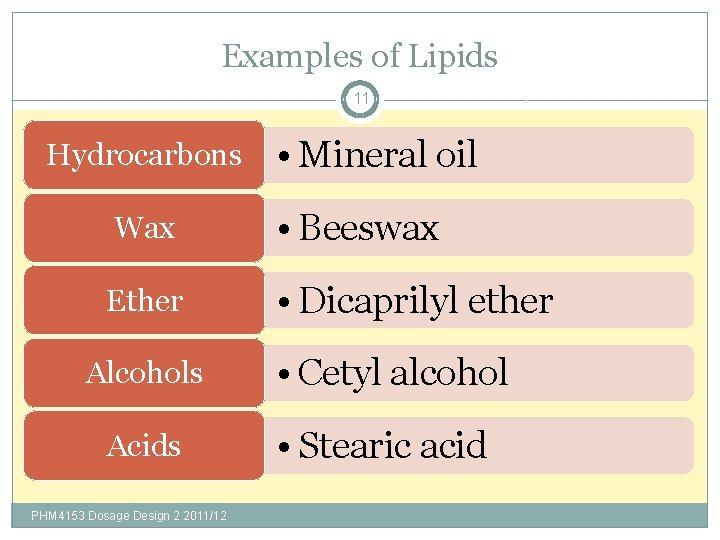
Examples of Lipids 11 Hydrocarbons • Mineral oil Wax • Beeswax Ether • Dicaprilyl ether Alcohols Acids PHM 4153 Dosage Design 2 2011/12 • Cetyl alcohol • Stearic acid

Choosing Oils 12 Properties Limitation �Emollient effect �Odour �Shine �Colour �Lubricity �Spreadability �Solvency �Drying PHM 4153 Dosage Design 2 2011/12 �Viscosity �Miscibility with other oils �Toxicity �Impurities �Cost

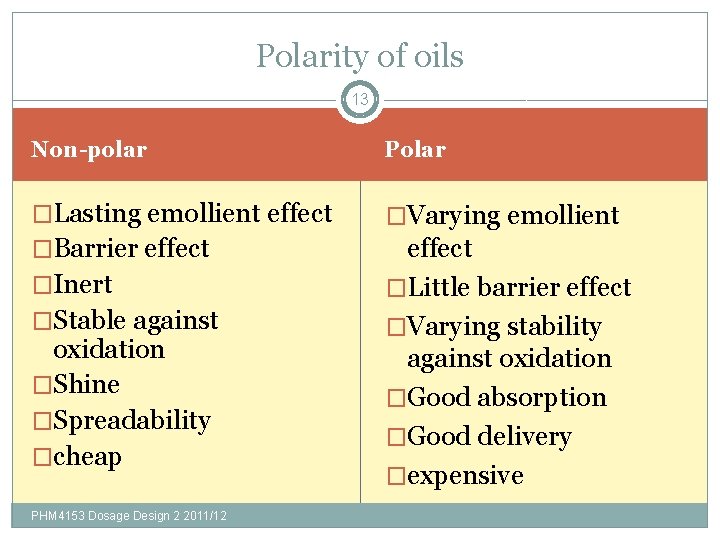
Polarity of oils 13 Non-polar Polar �Lasting emollient effect �Varying emollient �Barrier effect �Little barrier effect �Varying stability against oxidation �Good absorption �Good delivery �expensive �Inert �Stable against oxidation �Shine �Spreadability �cheap PHM 4153 Dosage Design 2 2011/12

Excipients 14 Other components other than API added to formulation PHM 4153 Dosage Design 2 2011/12

Categories of excipients 15 Provide essential parts of dosage form & enhance bioavailability • Emulsifiers • Viscosity modifier Prevent degradation of the formulation: protect, improve safety & enhance stability • Anti-oxidants • Anti-bacterials • Preservatives • UV absorbers Aid processing during manufacturing Assist product identification colour PHM 4153 Dosage Design 2 2011/12

Choosing excipients 16 physiological inertness commercially available at low cost physical and chemical stability absence of pathogenic microbial organisms conformance to regulatory agency requirements no interference with drug bioavailability PHM 4153 Dosage Design 2 2011/12

Emulsifiers 17 w/o o/w PHM 4153 Dosage Design 2 2011/12

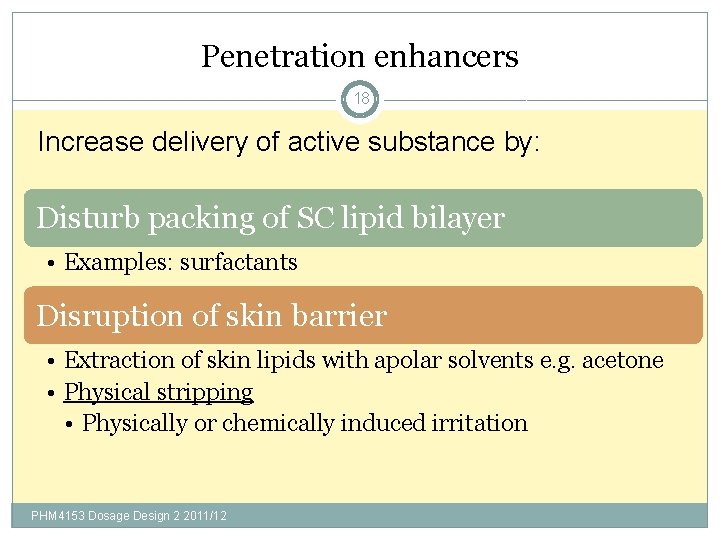
Penetration enhancers 18 Increase delivery of active substance by: Disturb packing of SC lipid bilayer • Examples: surfactants Disruption of skin barrier • Extraction of skin lipids with apolar solvents e. g. acetone • Physical stripping • Physically or chemically induced irritation PHM 4153 Dosage Design 2 2011/12

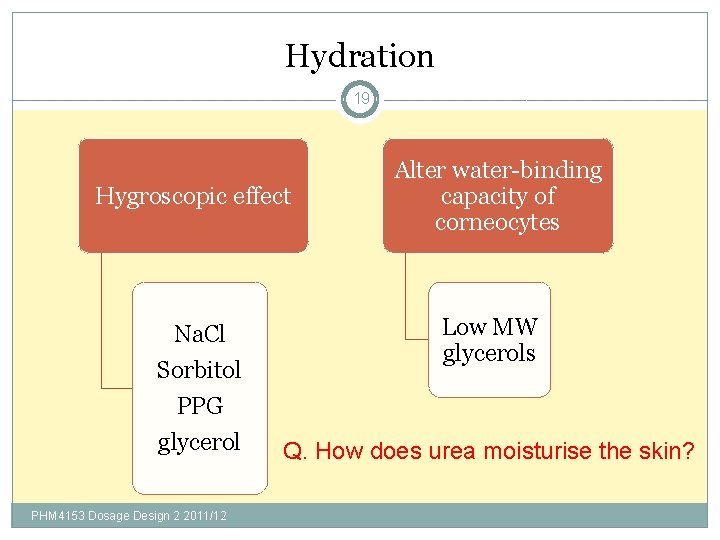
Hydration 19 Hygroscopic effect Na. Cl Sorbitol PPG glycerol PHM 4153 Dosage Design 2 2011/12 Alter water-binding capacity of corneocytes Low MW glycerols Q. How does urea moisturise the skin?

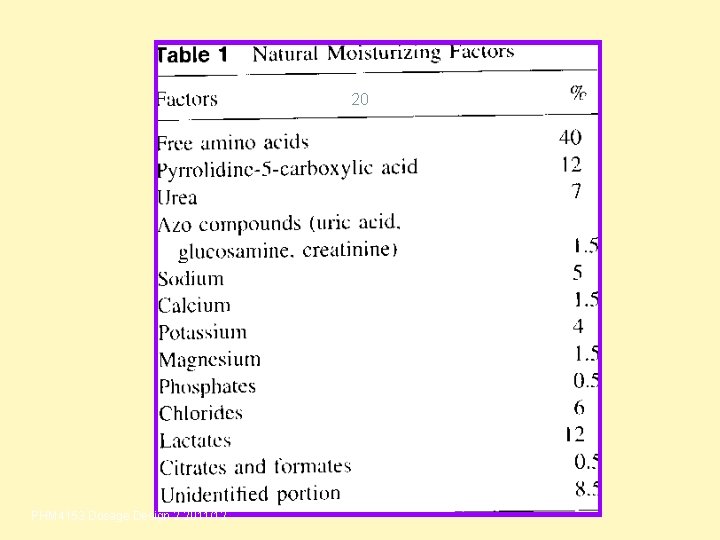
20 PHM 4153 Dosage Design 2 2011/12

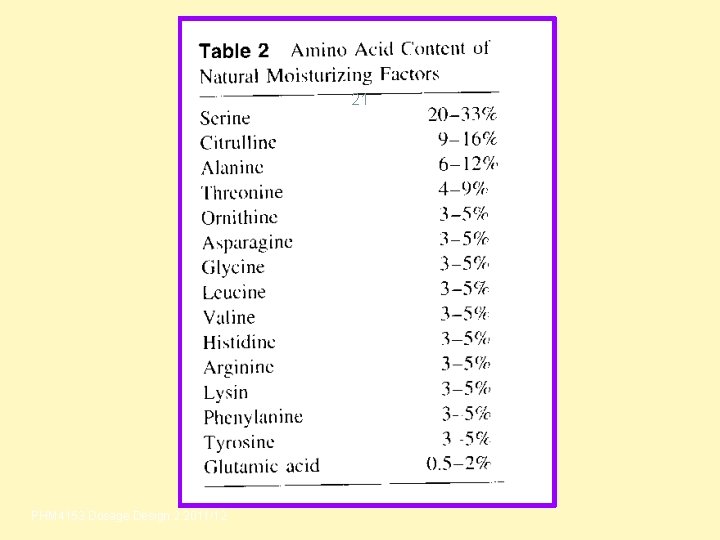
21 PHM 4153 Dosage Design 2 2011/12

p. H adjustment 22 Triethanolamine Nao. H PHM 4153 Dosage Design 2 2011/12

Preservatives 23 Sodium methyl/butyl/propyl paraben Imidazolidinyl urea PHM 4153 Dosage Design 2 2011/12

Anti-oxidant 24 Butyl hydroxy toluene Butyl hydroxy anisole PHM 4153 Dosage Design 2 2011/12

UV filters 25 Zinc oxide Titanium dioxide Benzophenone PHM 4153 Dosage Design 2 2011/12

Other types of excipients 26 Soothing • Allantoin Anti-free radicals • Polyphenols PHM 4153 Dosage Design 2 2011/12

Effects of excipients 27 texture and consistency phase behaviour of the component emulsifiers. PHM 4153 Dosage Design 2 2011/12 physicochemical properties rheological, thermal and microscopical

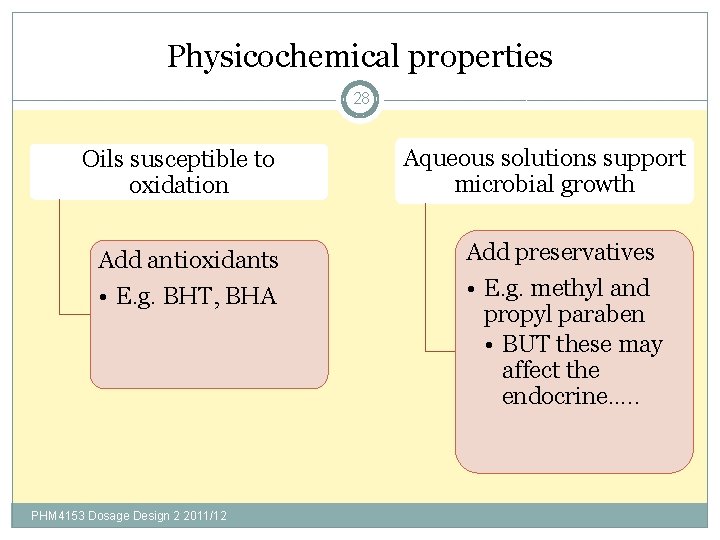
Physicochemical properties 28 Oils susceptible to oxidation Aqueous solutions support microbial growth Add antioxidants Add preservatives • E. g. BHT, BHA • E. g. methyl and propyl paraben • BUT these may affect the endocrine…. . PHM 4153 Dosage Design 2 2011/12

Physical and chemical properties of excipients 29 solubility hygroscopicity swelling hydration capacity particle size distribution bulk & tap density specific surface area complexation infrared spectrum microbes PHM 4153 Dosage Design 2 2011/12

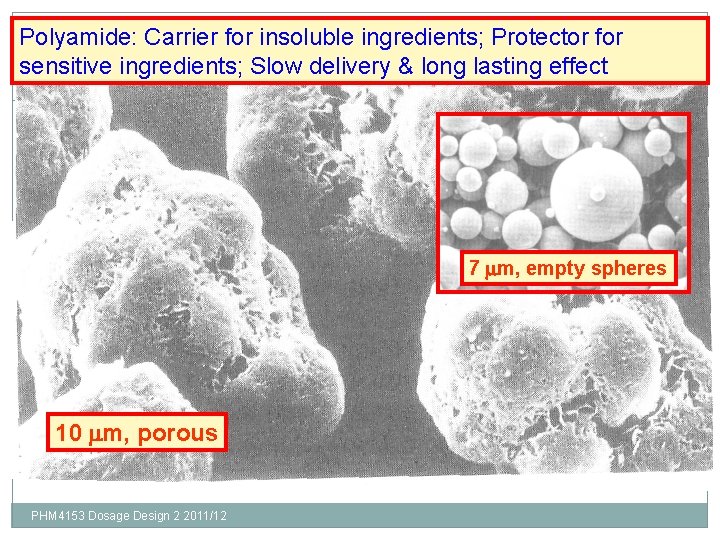
Polyamide: Carrier for insoluble ingredients; Protector for sensitive ingredients; Slow delivery & long lasting effect 30 7 m, empty spheres 10 m, porous PHM 4153 Dosage Design 2 2011/12

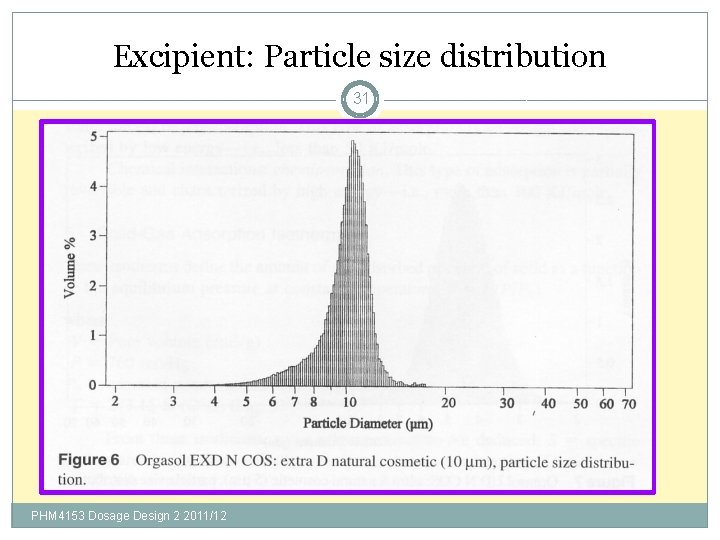
Excipient: Particle size distribution 31 PHM 4153 Dosage Design 2 2011/12

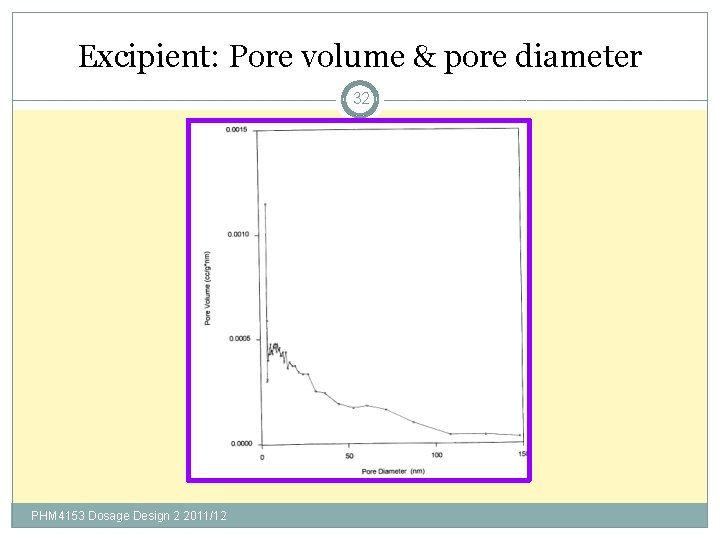
Excipient: Pore volume & pore diameter 32 PHM 4153 Dosage Design 2 2011/12

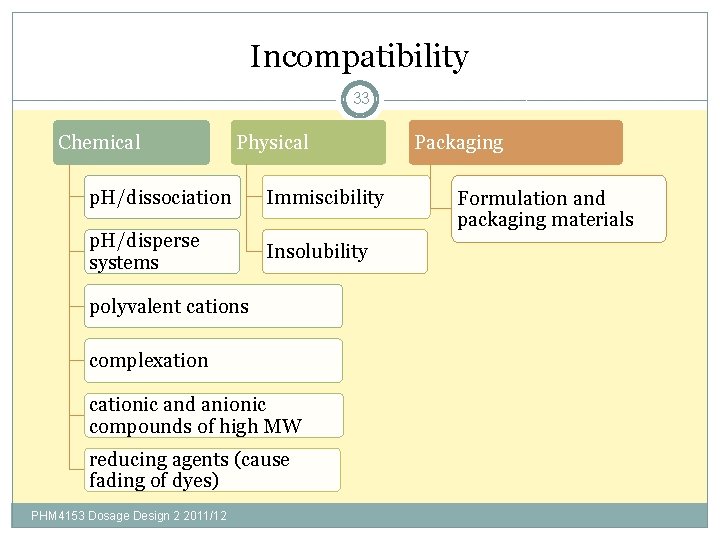
Incompatibility 33 Chemical Physical p. H/dissociation Immiscibility p. H/disperse systems Insolubility polyvalent cations complexation cationic and anionic compounds of high MW reducing agents (cause fading of dyes) PHM 4153 Dosage Design 2 2011/12 Packaging Formulation and packaging materials

Detection of Incompatibility 34 Cracked cream Hydrolysis or oxidation Discoloration Precipitation PHM 4153 Dosage Design 2 2011/12

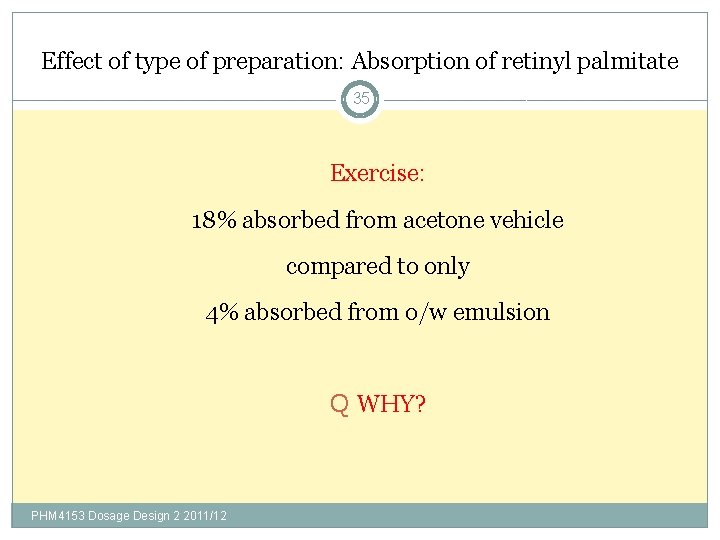
Effect of type of preparation: Absorption of retinyl palmitate 35 Exercise: 18% absorbed from acetone vehicle compared to only 4% absorbed from o/w emulsion Q WHY? PHM 4153 Dosage Design 2 2011/12

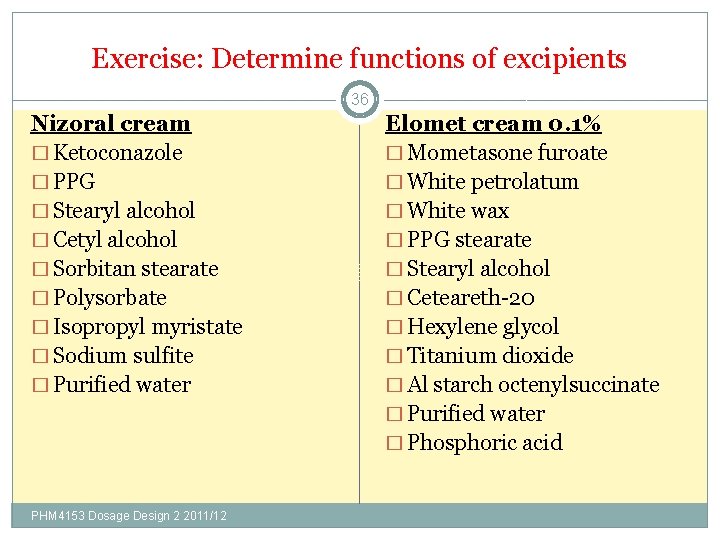
Exercise: Determine functions of excipients 36 Nizoral cream � Ketoconazole � PPG � Stearyl alcohol � Cetyl alcohol � Sorbitan stearate � Polysorbate � Isopropyl myristate � Sodium sulfite � Purified water PHM 4153 Dosage Design 2 2011/12 Elomet cream 0. 1% � Mometasone furoate � White petrolatum � White wax � PPG stearate � Stearyl alcohol � Ceteareth-20 � Hexylene glycol � Titanium dioxide � Al starch octenylsuccinate � Purified water � Phosphoric acid

References 37 Bugay, D. E. (1999). Pharmaceutical excipients : characterization by IR, Raman, and NMR spectroscopy. RS 201 E 87 B 931 P Kibbe, A. H. (2000). Handbook of pharmaceutical excipients. RS 201 E 87 H 236 K Rowe, R. C. , Sheskey, P. J. & Owen, S. C. (2006). Handbook of pharmaceutical excipients RS 201 E 87 H 236 K Rowe, R. C. (2009). Handbook of pharmaceutical excipients. RS 201 E 87 H 236 K PHM 4153 Dosage Design 2 2011/12

38 Some materials sourced from the following: http: //www. eastman. com/Markets/Pharmaceutical/Excipients_intro. asp http: //www. pharmaceutical-technology. com/contractors/materials/uniqema/ http: //www. pformulate. com/ http: //images. vertmarkets. com/CRLive/files/Downloads/89 FB 7970 -7376 -44 A 0 -B 6 B 6 -4 B 171 E 4 B 978 B/Insoluble. Kollidon. pdf Thank you to contributors. PHM 4153 Dosage Design 2 2011/12
 Kausar ahmad
Kausar ahmad Ahmad ibrahim kulliyyah of laws
Ahmad ibrahim kulliyyah of laws Kulliyyah of pharmacy
Kulliyyah of pharmacy Ms 2424:2012
Ms 2424:2012 Revealed knowledge adalah
Revealed knowledge adalah Why problem formulation follow goal formulation
Why problem formulation follow goal formulation Water removable bases
Water removable bases Emulsion definition pharmacy
Emulsion definition pharmacy Carpopedal spasm panic attack
Carpopedal spasm panic attack Nur ahmad husin
Nur ahmad husin Cstr in parallel
Cstr in parallel I said to him please lend me your pen change the narration
I said to him please lend me your pen change the narration Inovasi spbt
Inovasi spbt Legionella pneumonia
Legionella pneumonia Mian ahmad farhan
Mian ahmad farhan Yamin ahmad
Yamin ahmad Dr usama ahmad
Dr usama ahmad Zamri ahmad
Zamri ahmad Shahzad ahmad md
Shahzad ahmad md Salman ahmad awan
Salman ahmad awan Dr shahzad ahmad
Dr shahzad ahmad Antitussives
Antitussives Disadvantages of dsc
Disadvantages of dsc Dr waseem ibrahim
Dr waseem ibrahim Precapillary sphincter contraction
Precapillary sphincter contraction Dr adnan ahmad
Dr adnan ahmad Ahmad ibrahim secondary school timetable
Ahmad ibrahim secondary school timetable Ceper maksud
Ceper maksud Ahmad aulia jusuf
Ahmad aulia jusuf Jawad ahmad md
Jawad ahmad md Rabiah ahmad
Rabiah ahmad Hadi ahmad md
Hadi ahmad md Dr nur ahmad tabri
Dr nur ahmad tabri Ahmad kamal
Ahmad kamal Fouzia khursheed ahmad
Fouzia khursheed ahmad Aijaz ahmad jameson's rhetoric of otherness summary
Aijaz ahmad jameson's rhetoric of otherness summary Political services of sir syed ahmad khan
Political services of sir syed ahmad khan Ahmad rafay alam
Ahmad rafay alam Trofozit
Trofozit