Colligative Properties Kausar Ahmad Kulliyyah of Pharmacy http
























- Slides: 50

Colligative Properties Kausar Ahmad Kulliyyah of Pharmacy http: //staff. iiu. edu. my/akausar PHM 1153 Physical Pharmacy 1 2010/11 1

Contents Lecture 1 • • Effect of solute on vapour pressure Boiling point elevation Freezing point depression Osmotic pressure Lecture 2 • Raoult’s law • Deviation from Raoult’s law Lecture 3 • Distillation • Azeotropic mixtures • Clausius-Clapeyron equation PHM 1153 Physical Pharmacy 1 2010/11 2

Introduction Solutions have different properties than either the pure solvent or the solute. • a solution of sugar in water is neither crystalline like sugar nor tasteless like water. Some of the properties unique to solutions depend only on the number of dissolved particles and not their identity. PHM 1153 Physical Pharmacy 1 2010/11 3

Definition Colligative properties • Properties of a liquid that may be altered by the presence of a solute. • Depend only on the amount of solute in a solution • Does not depend on the identity of the solute Non-colligative properties • Depend on the identity of the dissolved solute and the solvent PHM 1153 Physical Pharmacy 1 2010/11 4

Colligative vs Non-colligative Compare 1. 0 M aqueous sugar solution to a 0. 5 M solution of salt (Na. Cl) in water. both solutions have the same number of dissolved particles any difference in the properties of those two solutions is due to a non-colligative property. Both have the same freezing point, boiling point, vapor pressure, and osmotic pressure PHM 1153 Physical Pharmacy 1 2010/11 5

Non-Colligative Properties Sugar solution is sweet and salt solution is salty. Therefore, the taste of the solution is not a colligative property. Another non-colligative property is the color of a solution. • A 0. 5 M solution of Cu. SO 4 is bright blue in contrast to the colorless salt and sugar solutions. Other non-colligative properties include viscosity, surface tension, and solubility. PHM 1153 Physical Pharmacy 1 2010/11 6

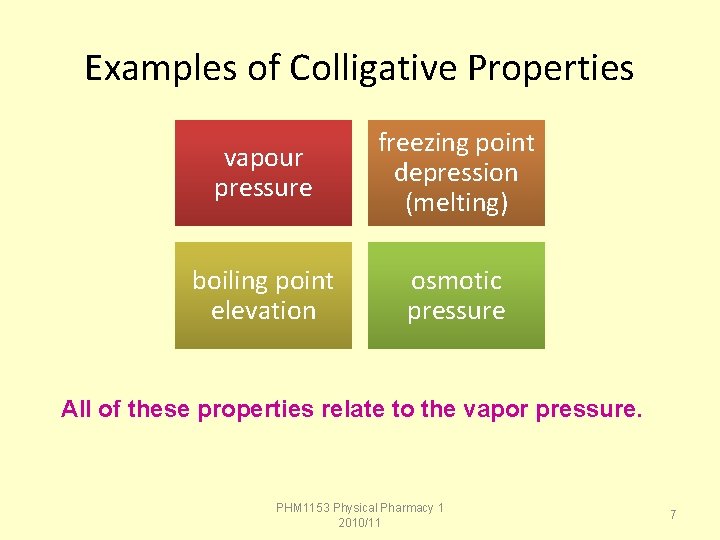
Examples of Colligative Properties vapour pressure freezing point depression (melting) boiling point elevation osmotic pressure All of these properties relate to the vapor pressure. PHM 1153 Physical Pharmacy 1 2010/11 7

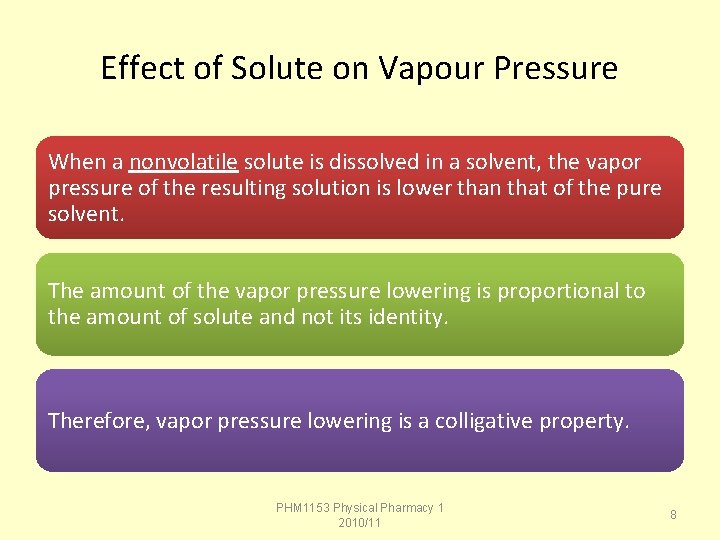
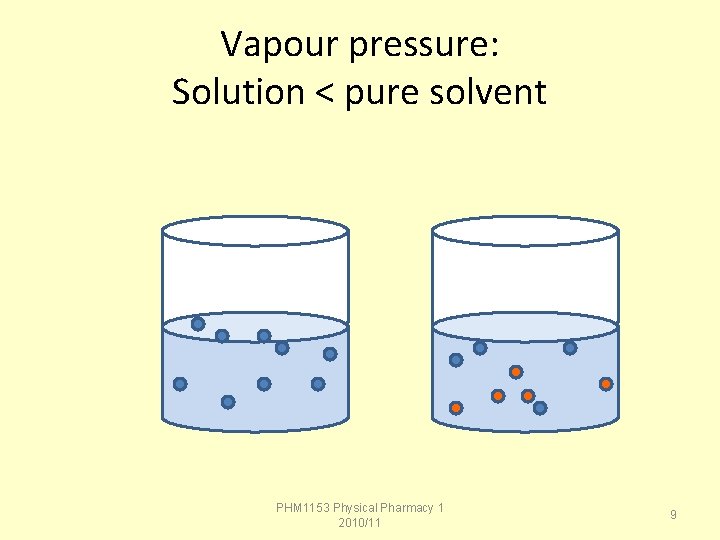
Effect of Solute on Vapour Pressure When a nonvolatile solute is dissolved in a solvent, the vapor pressure of the resulting solution is lower than that of the pure solvent. The amount of the vapor pressure lowering is proportional to the amount of solute and not its identity. Therefore, vapor pressure lowering is a colligative property. PHM 1153 Physical Pharmacy 1 2010/11 8

Vapour pressure: Solution < pure solvent PHM 1153 Physical Pharmacy 1 2010/11 9

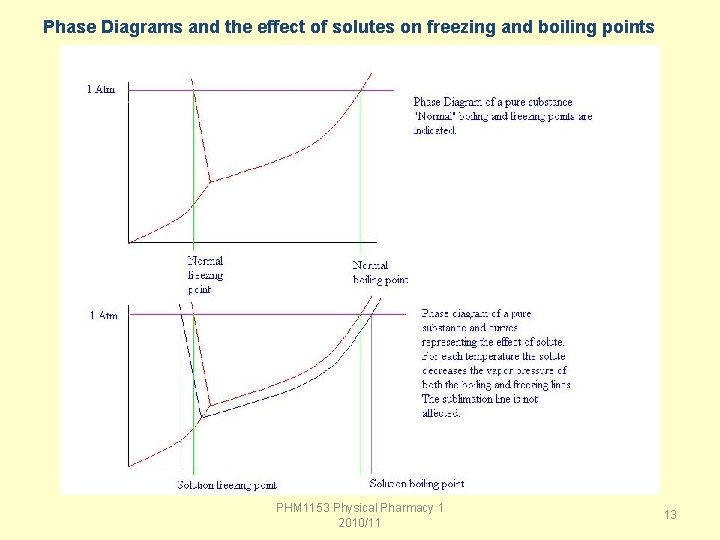
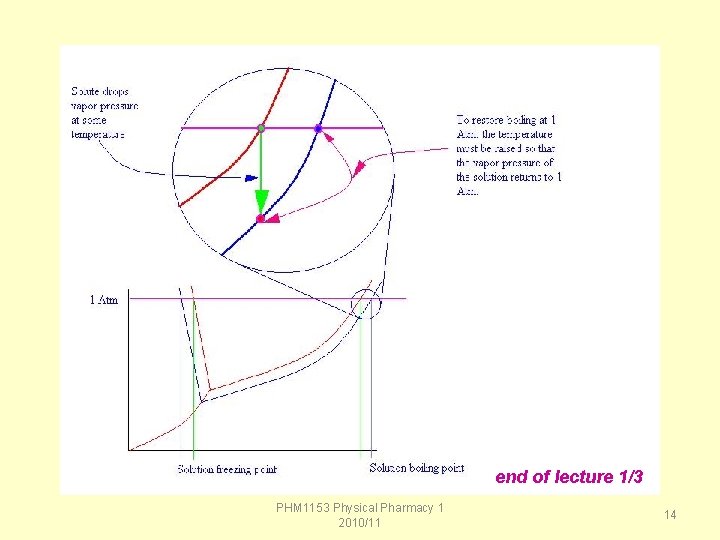
Boiling point elevation is a colligative property related to vapor pressure lowering. The boiling point is defined as the temperature at which the vapor pressure of a liquid equals the atmospheric pressure. Due to vapor pressure lowering, a solution will require a higher temperature to reach its boiling point than the pure solvent. PHM 1153 Physical Pharmacy 1 2010/11 10

Freezing Point Every liquid has a freezing point - the temperature at which a liquid undergoes a phase change from liquid to solid. When solutes are added to a liquid, forming a solution, the solute molecules disrupt the formation of crystals of the solvent. That disruption in the freezing process results in a depression of the freezing point for the solution relative to the pure solvent. PHM 1153 Physical Pharmacy 1 2010/11 11

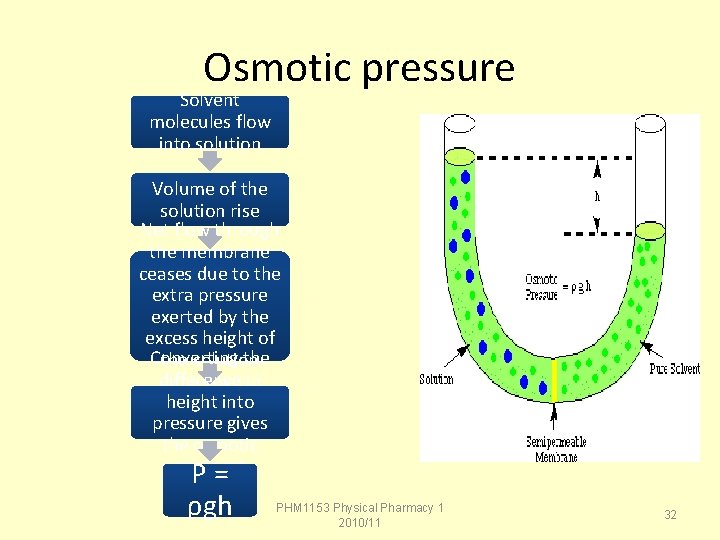
Osmotic Pressure When a solution is separated from a volume of pure solvent by a semi -permeable membrane that allows only the passage of solvent molecules, the height of the solution begins to rise. The value of the height difference between the two compartments reflects a property called the osmotic pressure of a solution. PHM 1153 Physical Pharmacy 1 2010/11 12

Phase Diagrams and the effect of solutes on freezing and boiling points PHM 1153 Physical Pharmacy 1 2010/11 13

end of lecture 1/3 PHM 1153 Physical Pharmacy 1 2010/11 14

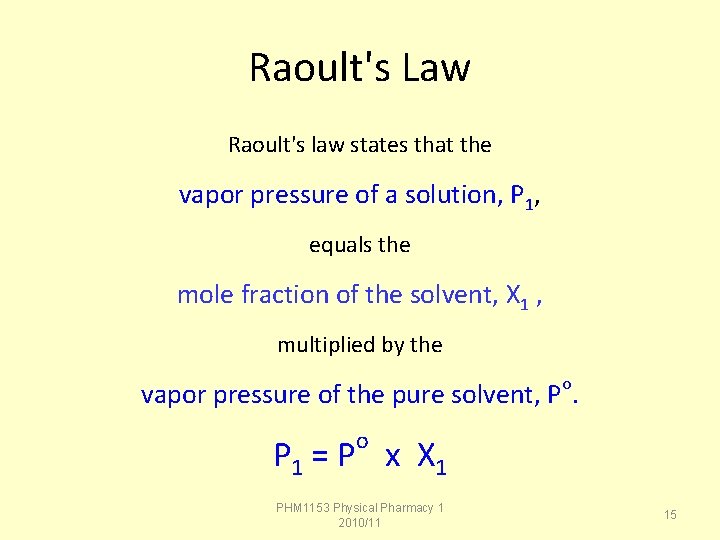
Raoult's Law Raoult's law states that the vapor pressure of a solution, P 1, equals the mole fraction of the solvent, X 1 , multiplied by the vapor pressure of the pure solvent, Po. o P 1 = P x X 1 PHM 1153 Physical Pharmacy 1 2010/11 15

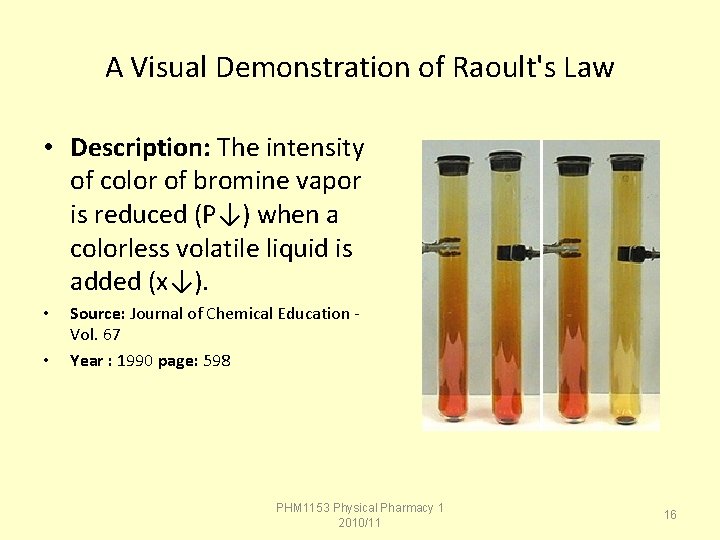
A Visual Demonstration of Raoult's Law • Description: The intensity of color of bromine vapor is reduced (P↓) when a colorless volatile liquid is added (x↓). • • Source: Journal of Chemical Education Vol. 67 Year : 1990 page: 598 PHM 1153 Physical Pharmacy 1 2010/11 16

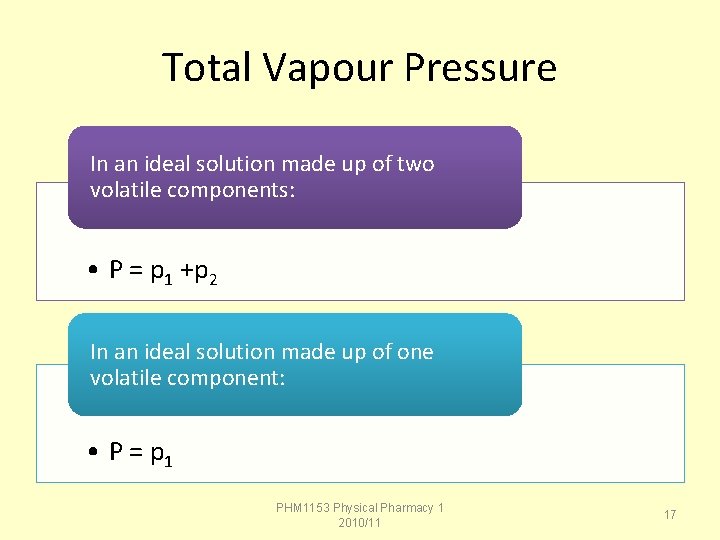
Total Vapour Pressure In an ideal solution made up of two volatile components: • P = p 1 +p 2 In an ideal solution made up of one volatile component: • P = p 1 PHM 1153 Physical Pharmacy 1 2010/11 17

Ideal & Non-Ideal/Real Solutions that obey Raoult's law are called ideal solutions because they behave exactly as we would predict. Solutions that show a deviation from Raoult's law are called non-ideal solutions or real solutions because they deviate from the expected behavior. PHM 1153 Physical Pharmacy 1 2010/11 18

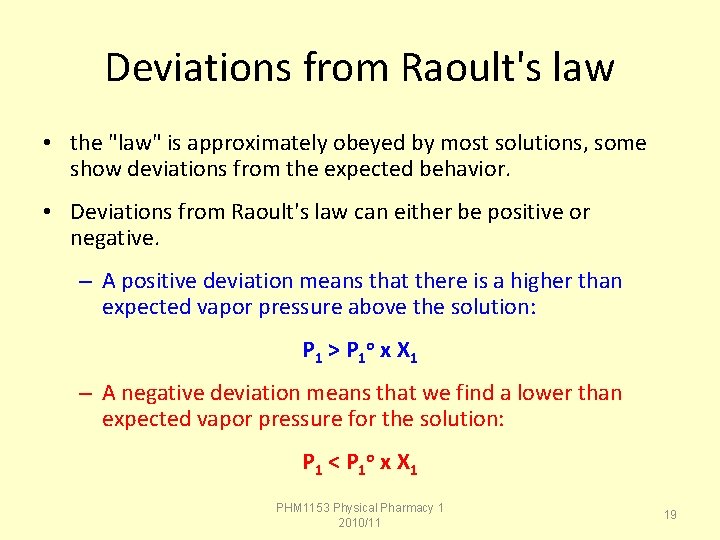
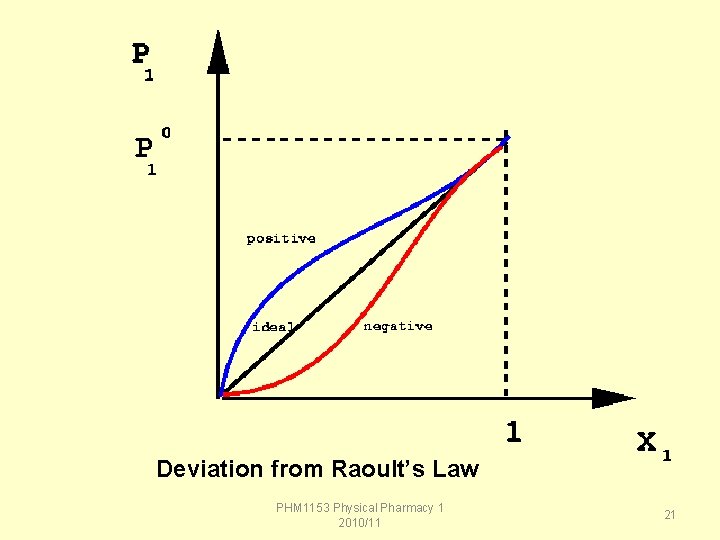
Deviations from Raoult's law • the "law" is approximately obeyed by most solutions, some show deviations from the expected behavior. • Deviations from Raoult's law can either be positive or negative. – A positive deviation means that there is a higher than expected vapor pressure above the solution: P 1 > P 1 o x X 1 – A negative deviation means that we find a lower than expected vapor pressure for the solution: P 1 < P 1 o x X 1 PHM 1153 Physical Pharmacy 1 2010/11 19

Reason for the deviation • In vapor pressure lowering we assumed that the solute did not interact with the solvent at all. • If the solute is strongly held by the solvent, the solution will show a negative deviation from Raoult's law, because the solvent will find it more difficult to escape from solution. • If the solute and solvent are not as tightly bound to each other as they are to themselves, then the solution will show a positive deviation from Raoult's law because the solvent molecules will find it easier to escape from solution into the gas phase. PHM 1153 Physical Pharmacy 1 2010/11 20

Deviation from Raoult’s Law PHM 1153 Physical Pharmacy 1 2010/11 21

Vapor Pressure of a Mixture: Raoult's Law • The measurement of pressure exerted by a vapour is demonstrated using barometers. • Vapor pressure varies with the strength of the intermolecular forces in the liquid. • We can calculate the vapor pressure of a mixture using Raoult's law. Source: http: //www. upscale. utoronto. ca/General. Interest/ Harrison/Manometer. html PHM 1153 Physical Pharmacy 1 2010/11 22

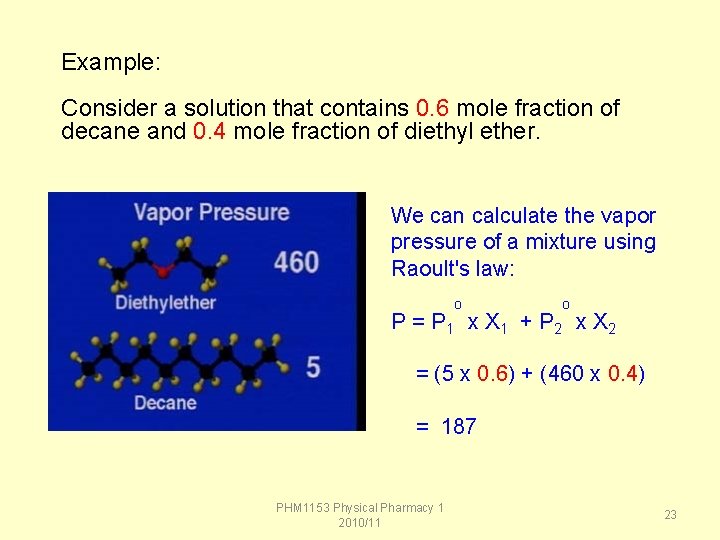
Example: Consider a solution that contains 0. 6 mole fraction of decane and 0. 4 mole fraction of diethyl ether. We can calculate the vapor pressure of a mixture using Raoult's law: o o P = P 1 x X 1 + P 2 x X 2 = (5 x 0. 6) + (460 x 0. 4) = 187 PHM 1153 Physical Pharmacy 1 2010/11 23

Factors that affect the magnitude of the changes in melting point and boiling point. Concentration Effect of ionic compounds PHM 1153 Physical Pharmacy 1 2010/11 24

Effect of ionic compounds • 0. 1 mole of an ionic compound such as Na. Cl, , has an effect on the melting and boiling points that is almost twice what we would observe for 0. 1 mole sugar. – salt ionizes into Na+ and Cl- ions in water – these ions act as independent particles on the vapor pressure of water. – Thus Na. Cl, Na. NO 3, and Ca. CO 3 would have multipliers of 2, while Na 2 SO 4, and Ca. Cl 2 would have multipliers of 3. PHM 1153 Physical Pharmacy 1 2010/11 25

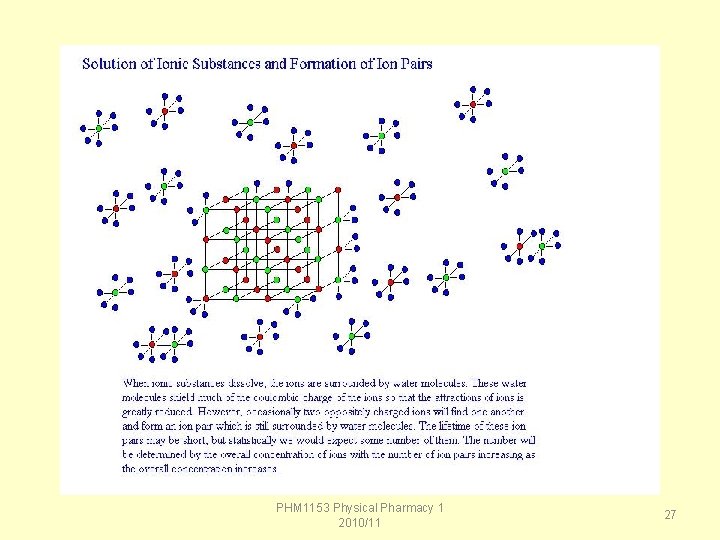
Van’t Hoff factor • In dilute solutions, ionic compounds have simple multiple effects, but as the solution concentration increases the multiplier effect diminishes. • This phenomenon was first discovered by van't Hoff and is generally called the van't Hoff factor. • As concentration increases, some of the ions floating in solution find one another and form ion pairs, in which two oppositely charged ions briefly stick together and act as a single particle. • the higher the concentration, the more likely it is that two ions will find one another. PHM 1153 Physical Pharmacy 1 2010/11 26

PHM 1153 Physical Pharmacy 1 2010/11 27

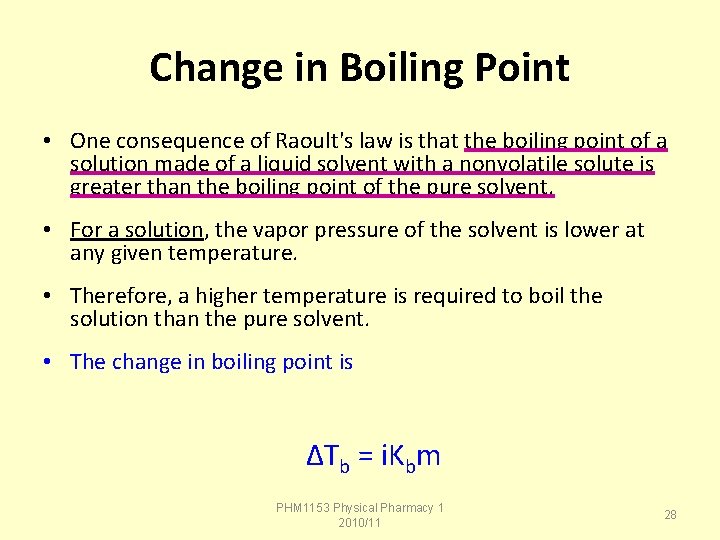
Change in Boiling Point • One consequence of Raoult's law is that the boiling point of a solution made of a liquid solvent with a nonvolatile solute is greater than the boiling point of the pure solvent. • For a solution, the vapor pressure of the solvent is lower at any given temperature. • Therefore, a higher temperature is required to boil the solution than the pure solvent. • The change in boiling point is ΔTb = i. Kbm PHM 1153 Physical Pharmacy 1 2010/11 28

ΔTb = i. Kbm m is molality • because molality is temperature independent. Kb is a boiling point elevation constant • that depends on the particular solvent used. i is the van't Hoff factor • number of dissociated moles of particles per mole of solute. • i=1 for all non-electrolyte solutes and equals the total number of ions released for electrolytes. • Na 2 SO 4 is 3 because that salt releases three moles of ions per mole of the salt. PHM 1153 Physical Pharmacy 1 2010/11 29

Change in Freezing Point • In order for a liquid to freeze it must achieve a very ordered state that results in the formation of a crystal. • If there are impurities in the liquid, i. e. solutes, the liquid is inherently less ordered. • Therefore, a solution is more difficult to freeze than the pure solvent so a lower temperature is required to freeze the liquid. • The change in freezing point is ΔTf = -i. Kfm – Note that the sign of the change in freezing point is negative because the freezing point of the solution is less than that of the pure solvent. PHM 1153 Physical Pharmacy 1 2010/11 30

Molal Boiling Point Elevation and Freezing Point Depression Constants at 1 Atm pressure Compound Normal bp Kb (o. C/m) Normal fp Kf (o. C/m) Water, H 2 O 100. 0 0. 52 0. 0 1. 86 Benzene, C 6 H 6 80. 1 2. 53 5. 5 5. 12 Ethanol, C 2 H 5 OH 78. 4 1. 22 -114. 6 1. 99 Carbon tetrachloride, CCl 4 76. 8 5. 02 -22. 3 29. 8 Chloroform, CHCl 3 3. 63 -63. 5 4. 68 61. 2 Thus the boiling point of water would increase 0. 52 o. C for a one molal solution, while the freezing point of this solution would decrease by 1. 86 o. C. PHM 1153 Physical Pharmacy 1 2010/11 31

Osmotic pressure Solvent molecules flow into solution Volume of the solution rise Net flow through the membrane ceases due to the extra pressure exerted by the excess height of Converting the solution difference chamber. in height into pressure gives the osmotic pressure P= ρgh PHM 1153 Physical Pharmacy 1 2010/11 32

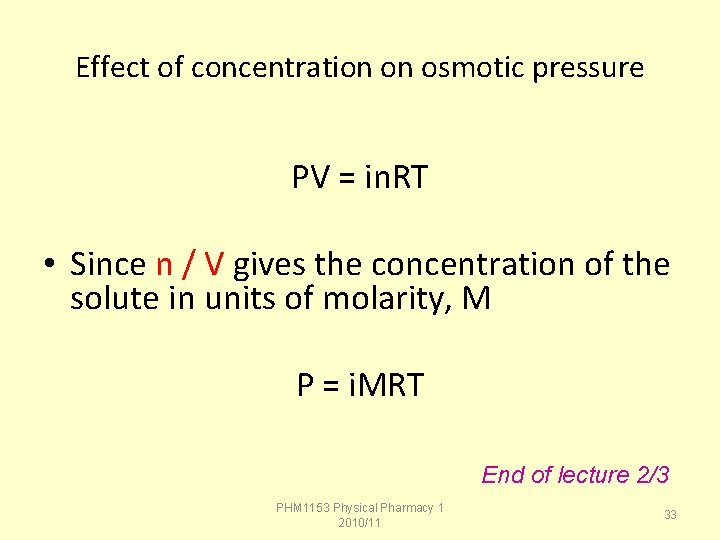
Effect of concentration on osmotic pressure PV = in. RT • Since n / V gives the concentration of the solute in units of molarity, M P = i. MRT End of lecture 2/3 PHM 1153 Physical Pharmacy 1 2010/11 33

Application of Raoult’s Law Distillation PHM 1153 Physical Pharmacy 1 2010/11 34

DISTILLATION Distillation is the process of boiling & condensing the vapour back to liquid A simple distillation: • To change from liquid to vapour by boiling • To change from vapour to liquid by condensing PHM 1153 Physical Pharmacy 1 2010/11 35

Distillation of Binary Mixture Ideal Solution • When a liquid and its vapour are in equilibrium, vapour is richer in the more volatile component compared to liquid mixture • At equilibrium, liquid and vapour phase can be separated analysed PHM 1153 Physical Pharmacy 1 2010/11 36

Binary Mixtures Obeying Raoult’s Law Ideal Solution • Attractive forces between molecules of different component equal those of the same component. A-B = A-A = B-B – Without a maximum or minimum at intermediate compositions. • No change in properties of components (except dilution to form solution). • Vapour pressure, refractive index, surface tension and viscosity of solution are averages of properties of pure individual constituents. • No heat evolved or absorbed during mixing process. • No change in solution temperature. • No shrinkage or expansion. • Final properties of solution are additive properties of individual constituents. • E. g. methyl alcohol-water, benzene-toluene, methanol-ethanol. PHM 1153 Physical Pharmacy 1 2010/11 37

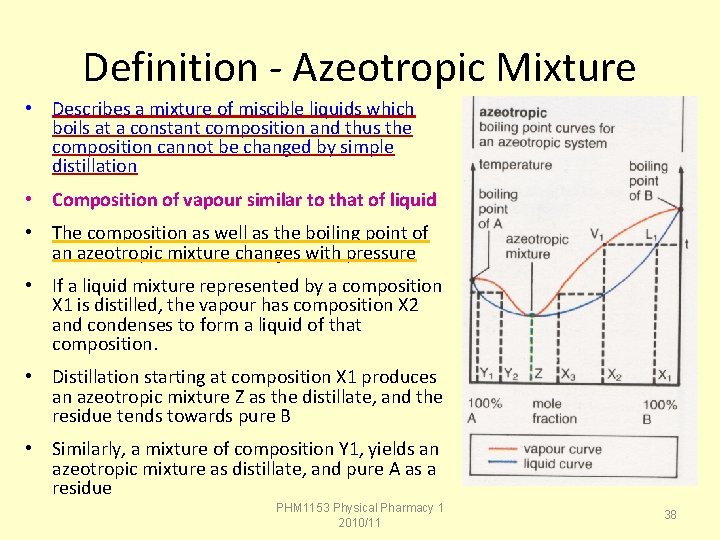
Definition - Azeotropic Mixture • Describes a mixture of miscible liquids which boils at a constant composition and thus the composition cannot be changed by simple distillation • Composition of vapour similar to that of liquid • The composition as well as the boiling point of an azeotropic mixture changes with pressure • If a liquid mixture represented by a composition X 1 is distilled, the vapour has composition X 2 and condenses to form a liquid of that composition. • Distillation starting at composition X 1 produces an azeotropic mixture Z as the distillate, and the residue tends towards pure B • Similarly, a mixture of composition Y 1, yields an azeotropic mixture as distillate, and pure A as a residue PHM 1153 Physical Pharmacy 1 2010/11 38

Positive Deviation from Raoult’s Law Vapour pressure greater than expected from Raoult’s law. The two components differ in properties e. g. polarity Attractive forces: A-B < A-A or B-B • Escaping tendency higher A max is found in vapour pressure-composition curve Presence of azeotropic mixtures lead to min boiling point Examples: PHM 1153 Physical Pharmacy 1 1. ethyl, isopropyl, n-propyl alcohol 2010/11 - water, 39

Positively deviated solution mixture with a minimum boiling point • The mixture shows positive deviation from Raoult’s law • A minimum boiling point is obtained • The solution has an azeotropic mixture whose vapour pressure is the highest and boiling point is the lowest • example of a positive azeotrope is 95. 6% ethanol and 4. 4% water (by weight). Ethanol boils at 78. 4°C, water boils at 100°C, but the azeotrope boils at 78. 1°C PHM 1153 Physical Pharmacy 1 2010/11 40

Distillation of positive azeotrope 95. 6% ethanol and 4. 4% water • distillation of any mixture will result in the distillate being closer in composition to the azeotrope than the starting mixture. • E. g. if a 50/50 mixture of ethanol and water is distilled once, the distillate will be 80% ethanol and 20% water i. e. closer to the azeotropic mixture than the original. • Distilling the 80/20% mixture produces a distillate that is 87% ethanol and 13% water. • Further repeated distillations will produce mixtures that are progressively closer to the azeotropic ratio of 95. 5/4. 5%. • increasing distillations will not give distillate that exceeds the azeotropic ratio. PHM 1153 Physical Pharmacy 1 2010/11 41

Negative Deviation from Raoult’s Law Vapour pressure of solution less than that expected from Raoult’s law Attractive forces: A-B > A-A or B-B • Escaping tendency lower A min is found in vapour pressure – composition curve Presence of azeotropic mixtures lead to max boiling point E. g. pyridine-acetic acid, chloroform-acetone (formation of loose compounds from hydrogen bonding), formation of hydrates, nitric acid-water, sulfuric PHM 1153 Physical Pharmacy 1 acid-water. 42 2010/11

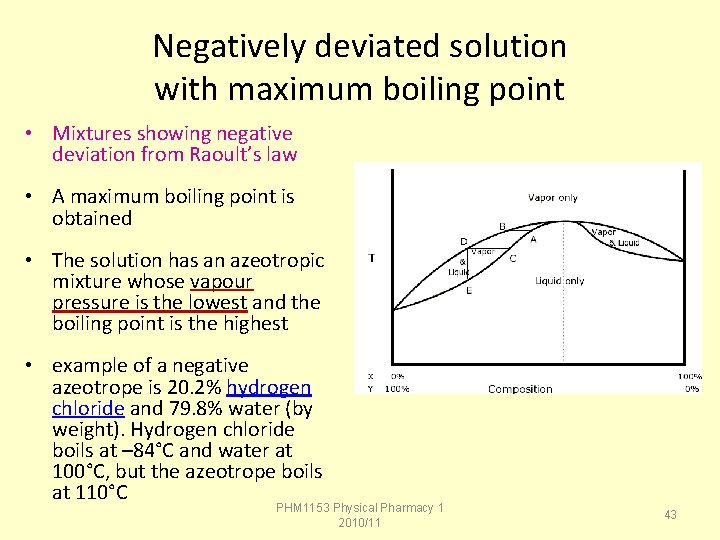
Negatively deviated solution with maximum boiling point • Mixtures showing negative deviation from Raoult’s law • A maximum boiling point is obtained • The solution has an azeotropic mixture whose vapour pressure is the lowest and the boiling point is the highest • example of a negative azeotrope is 20. 2% hydrogen chloride and 79. 8% water (by weight). Hydrogen chloride boils at – 84°C and water at 100°C, but the azeotrope boils at 110°C PHM 1153 Physical Pharmacy 1 2010/11 43

Distillation of negative azeotrope 20. 2% hydrogen chloride and 79. 8% water • distillation of any mixture of those constituents will result in the residue being closer in composition to the azeotrope than the original mixture. • E. g. hydrochloric acid solution contains less than 20. 2% hydrogen chloride, • boiling the mixture will give a solution that is richer in hydrogen chloride than the original. • If the solution initially contains more than 20. 2% hydrogen chloride, boiling will give a solution that is poorer in hydrogen chloride than the original. • Boiling of any hydrochloric acid solution long enough will cause the solution left behind to approach the azeotropic ratio. PHM 1153 Physical Pharmacy 1 2010/11 44

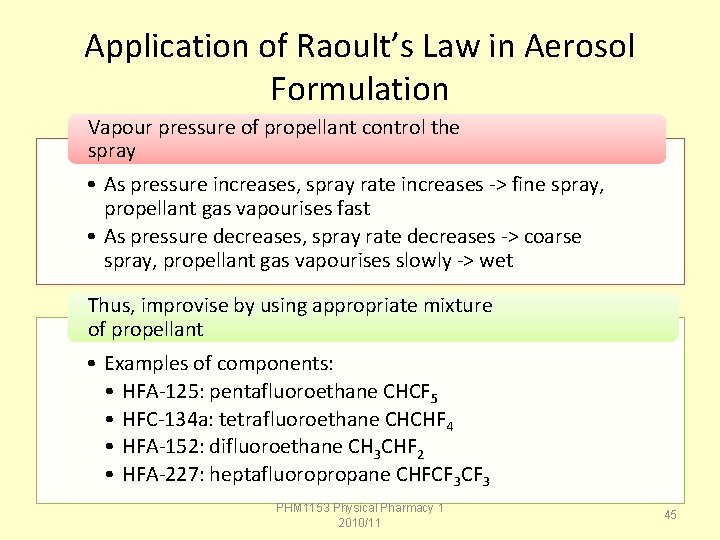
Application of Raoult’s Law in Aerosol Formulation Vapour pressure of propellant control the spray • As pressure increases, spray rate increases -> fine spray, propellant gas vapourises fast • As pressure decreases, spray rate decreases -> coarse spray, propellant gas vapourises slowly -> wet Thus, improvise by using appropriate mixture of propellant • Examples of components: • HFA-125: pentafluoroethane CHCF 5 • HFC-134 a: tetrafluoroethane CHCHF 4 • HFA-152: difluoroethane CH 3 CHF 2 • HFA-227: heptafluoropropane CHFCF 3 PHM 1153 Physical Pharmacy 1 2010/11 45

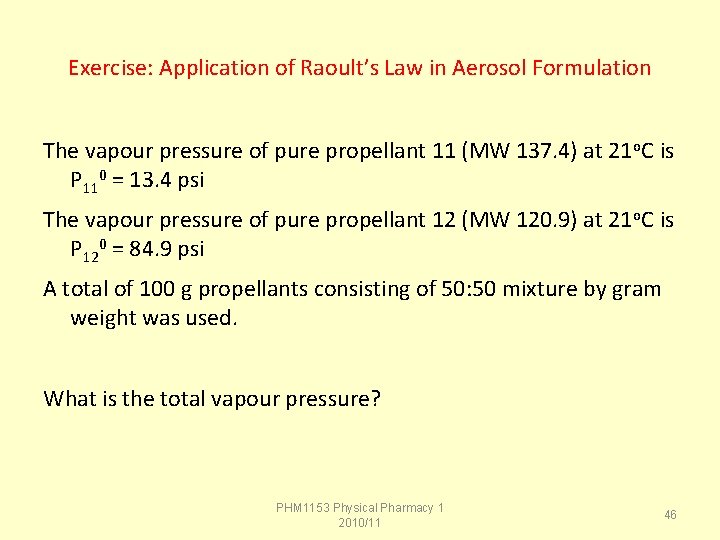
Exercise: Application of Raoult’s Law in Aerosol Formulation The vapour pressure of pure propellant 11 (MW 137. 4) at 21 o. C is P 110 = 13. 4 psi The vapour pressure of pure propellant 12 (MW 120. 9) at 21 o. C is P 120 = 84. 9 psi A total of 100 g propellants consisting of 50: 50 mixture by gram weight was used. What is the total vapour pressure? PHM 1153 Physical Pharmacy 1 2010/11 46

Variation of Vapour Pressure with Temperature As T ↑, energy of molecules ↑ More escape into vapour phase increase in vapour pressure PHM 1153 Physical Pharmacy 1 2010/11 Clausius. Clapeyron Equation 47

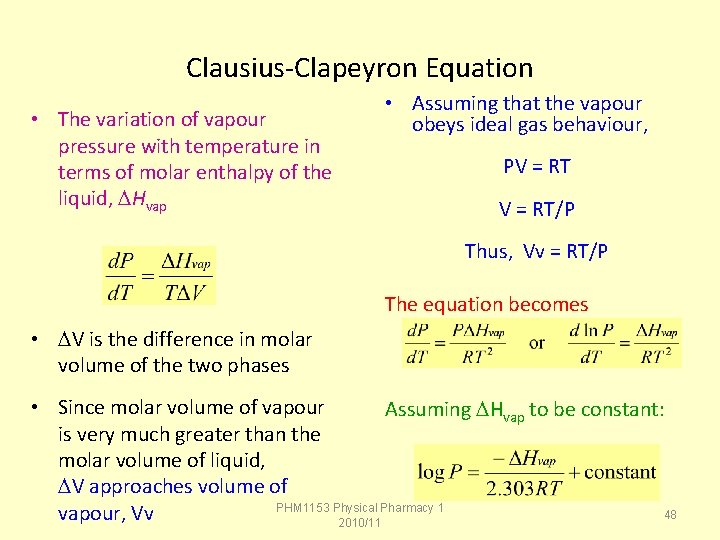
Clausius-Clapeyron Equation • The variation of vapour pressure with temperature in terms of molar enthalpy of the liquid, Hvap • Assuming that the vapour obeys ideal gas behaviour, PV = RT/P Thus, Vv = RT/P The equation becomes • V is the difference in molar volume of the two phases • Since molar volume of vapour Assuming Hvap to be constant: is very much greater than the molar volume of liquid, V approaches volume of PHM 1153 Physical Pharmacy 1 48 vapour, Vv 2010/11

Application of Clausius-Clapeyron Equation • To estimate vapour pressure at any temperature. • To calculate enthalpy of vaporisation based on the slope of plot • To study phase transition – important to determine the extent of weight loss during processing or testing. End lecture 3/3 PHM 1153 Physical Pharmacy 1 2010/11 49

References The required texts you shall seek, The recommended ones you may flirt, Revise! You’d better be quick! When you get your grades you won’t be hurt! PHM 1153 Physical Pharmacy 1 2010/11 50
 Kausar ahmad
Kausar ahmad Ahmad ibrahim kulliyyah of laws
Ahmad ibrahim kulliyyah of laws Kulliyyah of pharmacy
Kulliyyah of pharmacy Applications of molality in pharmacy
Applications of molality in pharmacy Ms 2424:2012
Ms 2424:2012 Kulliyyah of islamic revealed knowledge
Kulliyyah of islamic revealed knowledge 4 colligative properties
4 colligative properties Elemental analysis of an unknown pure substance
Elemental analysis of an unknown pure substance Colligative property definition
Colligative property definition Colligative property definition
Colligative property definition Colligative properties regents questions
Colligative properties regents questions Colligative properties of milk
Colligative properties of milk Non colligative properties
Non colligative properties Colligative properties worksheet
Colligative properties worksheet Properties of l
Properties of l Freezing point depression examples in real life
Freezing point depression examples in real life 4 colligative properties
4 colligative properties Colligative properties depend on
Colligative properties depend on Calculations involving colligative properties
Calculations involving colligative properties Three colligative properties
Three colligative properties Applications of colligative properties in foods
Applications of colligative properties in foods Colligative properties calculator
Colligative properties calculator Ions in aqueous solutions and colligative properties
Ions in aqueous solutions and colligative properties Calculating molar mass using colligative properties
Calculating molar mass using colligative properties Colligative properties notes
Colligative properties notes Colligative properties osmotic pressure
Colligative properties osmotic pressure Colligative properties of seawater
Colligative properties of seawater Aulton
Aulton Abbassamento crioscopico
Abbassamento crioscopico Proprietà colligative delle soluzioni
Proprietà colligative delle soluzioni Le proprietà colligative
Le proprietà colligative Which of the following involves a colligative property
Which of the following involves a colligative property Propriété colligative des solutions
Propriété colligative des solutions Costanti ebullioscopiche e crioscopiche
Costanti ebullioscopiche e crioscopiche Abbassamento crioscopico
Abbassamento crioscopico Le soluzioni zanichelli
Le soluzioni zanichelli Http //mbs.meb.gov.tr/ http //www.alantercihleri.com
Http //mbs.meb.gov.tr/ http //www.alantercihleri.com Siat.ung.ac.id krs
Siat.ung.ac.id krs Extensive and intensive properties
Extensive and intensive properties Chemical property definition
Chemical property definition Vostrine
Vostrine Risala asbab-i-baghawat-i-hind
Risala asbab-i-baghawat-i-hind Zak ahmad
Zak ahmad Dr fawad randhawa
Dr fawad randhawa Jawad ahmad md
Jawad ahmad md Nur ahmad husin
Nur ahmad husin Gayatiri
Gayatiri Civil responsibility of mentally ill person
Civil responsibility of mentally ill person Seitz filter funnel
Seitz filter funnel Dr nur ahmad tabri
Dr nur ahmad tabri Nur ahmad husin
Nur ahmad husin