Ion exchange chromatography What is ion exchange chromatography




- Slides: 16

Ion exchange chromatography

What is ion exchange chromatography ? • IEC is a technique for separating proteins according to their charge • Its easy of use and scale up capabilities • Large volumes be applied • High resolution and high capacity method

Principle Packing material • Resin are charged molecules • Securely bound to column by covalent bonds • Negatively or positively charged groups Anion exchanger • Positively charged beads “exchange” with negatively charged counter ions • Negatively charged molecules - “anions” Cation exchangers • Negatively charged beads exchange positive counter -ions (cations)


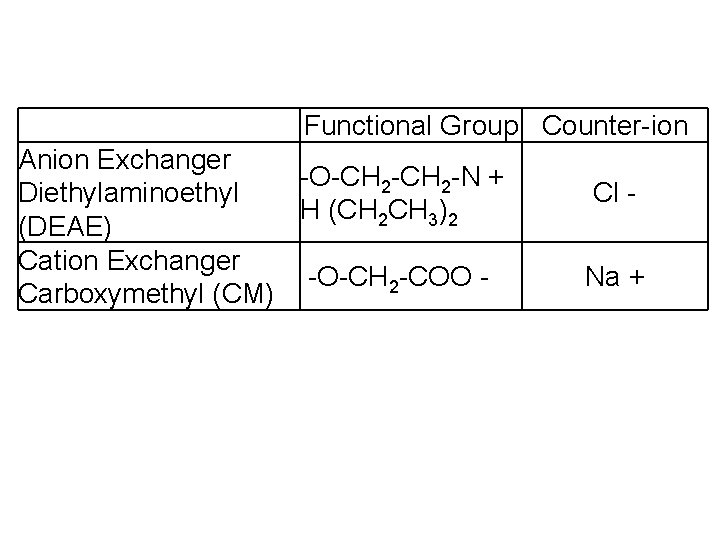
Functional Group Counter-ion Anion Exchanger -O-CH 2 -N + Diethylaminoethyl H (CH 2 CH 3)2 (DEAE) Cation Exchanger -O-CH 2 -COO Carboxymethyl (CM) Cl Na +

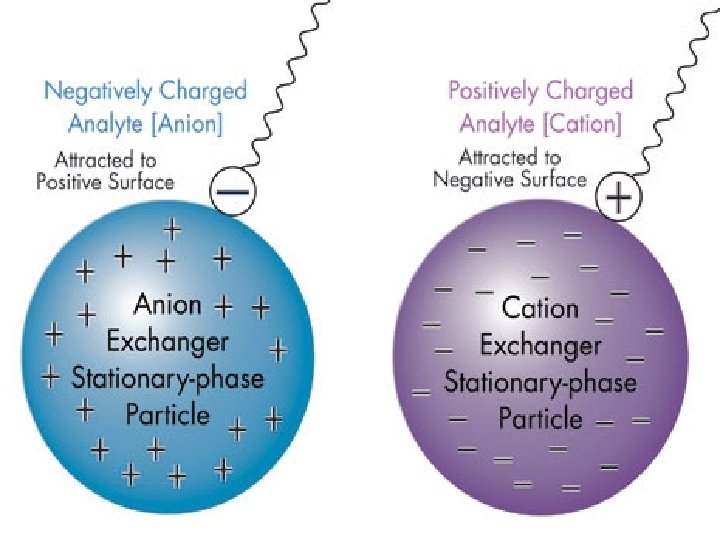
Separation of protein • Proteins are charged molecules • Interaction with the packing material depends on – Overall charge – Distribution of that charge over the protein surface • They displace mobile counter ions bound to the resin • Mobile counter ions: When the packing material is suspended in buffer containing Na. Cl, the charged groups become loosely associated with Na+ and Clions of the opposite charge. These loosely bound ions are called mobile counter-ions.

• Net charge on protein will depend on – Composition of amino acids in the protein – p. H of the buffering solution. • Charge distribution will depend on – How the charges are distributed on the folded protein • • Isoelectric point ( p. I) - The p. H at which a particular protein is overall electrically neutral ie. the number of positive charges is equal to the number of negative charges. < p. I - A protein has more positively charged amino acids and therefore an overall positive charge. It will bind to cation exchangers > p. I - A a protein has more negatively charged amino acids and an overall negative charge. It will bind to anion exchangers At its p. I, a protein will not bind to either a cationic or an anionic exchanger.

Column materials - Polystyrene • • • Ion-exchangers made by co-polymerisation of styrene with divinyl benzene. Polystyrene itself is a linear polymer. Divinyl benzene, is a cross-linker Resins with low degree of cross-linking are more permeable to high molecular weight compounds, but they are less rigid and swell more when placed in buffer Sulphonation of cross-linked polystyrene results in sulphonated polystyrene resin such as Dowex 50 - strong acidic exchanger Basic exchangers are prepared by reacting cross-linked polystyrene with chlormethyl ether and then by the chlorogroup with tertiary amines – CH 2 N+ (CH 3)3 Cl- groups are ionized.

Modified cellulose and Sepharose • Modified cellulose is an alternative to polystyrene based exchangers • Cellulose is a high molecular weight i. e. carboxymethyl cellulose (CM-cellulose) where the – CH 2 OH group is converted to –CH 2 OCH 2 COOH and DEAE-cellulose [CH 2 OCH 2 N(CH 2 CH 3)2] • Commercially available in gel and bead form • Sepharose type is derived from cross-linked agarose • Sephadex and Sepharose are used for the separation of high molecular weight proteins and nucleic acids

Total exchange capacity • Defined as number of milliequivalents of exchangeable ions available either per gram of dried exchanger or per unit volume of hydrated resins – Bio Rad AG 1 -X 4 = 1. 2 meq cm-3 – DEAE-Sephadex A-25 = 0. 5 meq cm-3 – CM-Sepharose CL-6 B = 0. 2 meq cm-3 • Polystyrene exchangers are obtainable in a number of mesh size. All exchangers are supplied with counter ions i. e Na+ or Cl-.

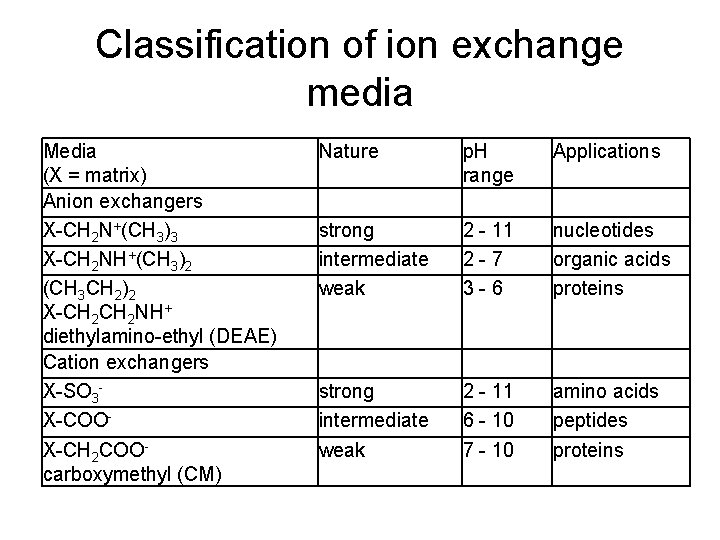
Classification of ion exchange media Media (X = matrix) Anion exchangers X-CH 2 N+(CH 3)3 X-CH 2 NH+(CH 3)2 (CH 3 CH 2)2 X-CH 2 NH+ diethylamino-ethyl (DEAE) Cation exchangers X-SO 3 - X-COO- X-CH 2 COOcarboxymethyl (CM) Nature Applications strong intermediate weak p. H range 2 - 11 2 - 7 3 - 6 strong intermediate weak 2 - 11 6 - 10 7 - 10 amino acids peptides proteins nucleotides organic acids proteins

Buffer • The p. H of the buffer used should be one p. H unit above or below isoionic point of the compounds. • Cationic buffers are tris, pyridine and alkyl amines and they are used with anion exchangers. • Anionic buffers are acetate, barbiturate and phosphate and they are used with cationic exchangers.

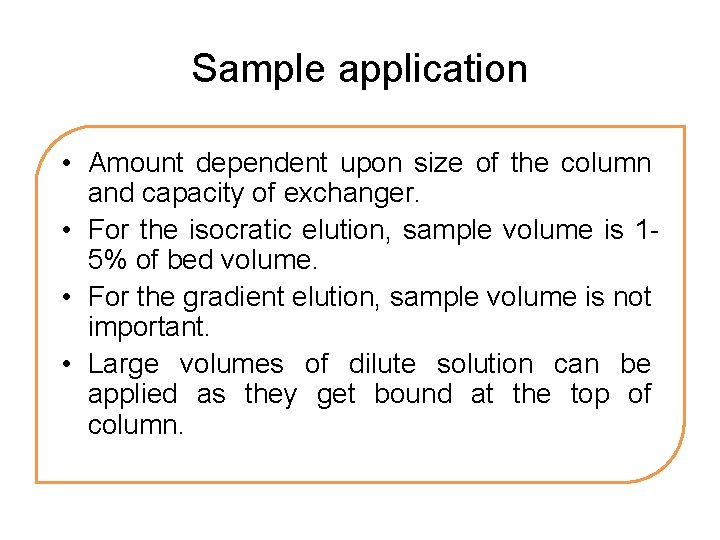
Sample application • Amount dependent upon size of the column and capacity of exchanger. • For the isocratic elution, sample volume is 15% of bed volume. • For the gradient elution, sample volume is not important. • Large volumes of dilute solution can be applied as they get bound at the top of column.

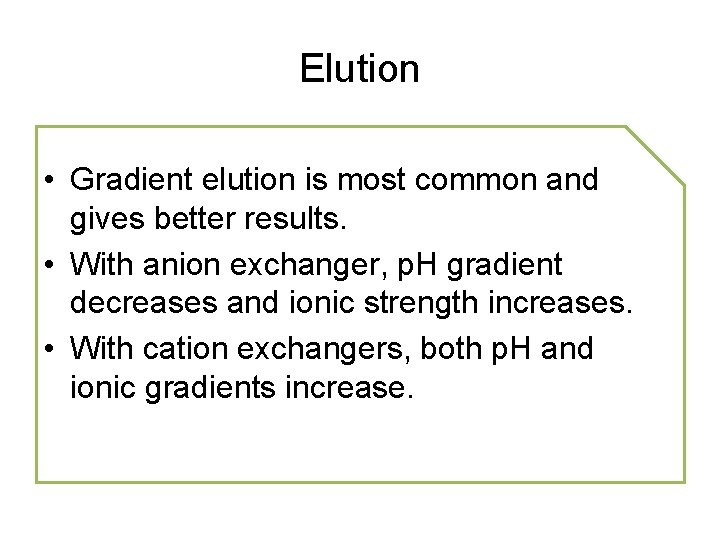
Elution • Gradient elution is most common and gives better results. • With anion exchanger, p. H gradient decreases and ionic strength increases. • With cation exchangers, both p. H and ionic gradients increase.

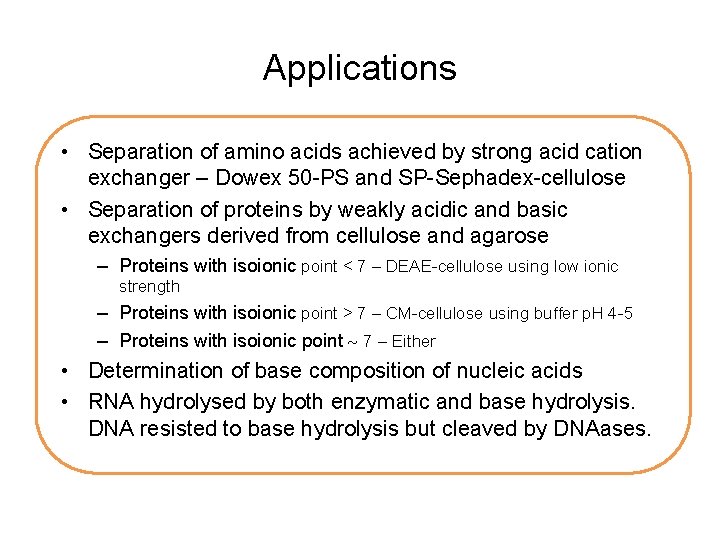
Applications • Separation of amino acids achieved by strong acid cation exchanger – Dowex 50 -PS and SP-Sephadex-cellulose • Separation of proteins by weakly acidic and basic exchangers derived from cellulose and agarose – Proteins with isoionic point < 7 – DEAE-cellulose using low ionic strength – Proteins with isoionic point > 7 – CM-cellulose using buffer p. H 4 -5 – Proteins with isoionic point 7 – Either • Determination of base composition of nucleic acids • RNA hydrolysed by both enzymatic and base hydrolysis. DNA resisted to base hydrolysis but cleaved by DNAases.

• Separation of amino acids achieved by strong acid cation exchanger – Dowex 50 -PS and SPSephadex-cellulose – Introduction of sample at p. H 1 -2 to ensure complete binding – Gradient elution by increasing p. H and ionic concentration – Acidic amino acids, aspartic and glutamic acids elute first followed by neutral amino acids, glycine and valine. Basic amino acids retain their net negative charge up to p. H values 6 -11 and elute last.
 ü-ion
ü-ion Chromatography plate theory
Chromatography plate theory Difference between affinity and ion exchange chromatography
Difference between affinity and ion exchange chromatography Peptides and proteins
Peptides and proteins Factors affecting ion exchange chromatography
Factors affecting ion exchange chromatography Counter ion
Counter ion Mobile phase in adsorption chromatography
Mobile phase in adsorption chromatography Cation exchange chromatography elution order
Cation exchange chromatography elution order Gfp purification flow chart
Gfp purification flow chart Ion chromatography
Ion chromatography Ejemplo de fuerza ion ion
Ejemplo de fuerza ion ion Fuerzas dipolo dipolo ejemplos
Fuerzas dipolo dipolo ejemplos Ejemplo de fuerza ion ion
Ejemplo de fuerza ion ion Induced dipole induced dipole interaction
Induced dipole induced dipole interaction Elution order
Elution order Ion exchange reaction examples
Ion exchange reaction examples Francois de dardel
Francois de dardel