Types of Liquid Chromatography I Ion Exchange Chromatography



- Slides: 20

Types of Liquid Chromatography I. Ion Exchange Chromatography A. Factors influencing retention B. Suppressed ion exchange II. Partitioning Chromatography A. Normal phase/ reverse phase III. Size Exclusion Chromatography IV. Supercritical Fluid Chromatography/ SFE V. Capillary Electrophoresis

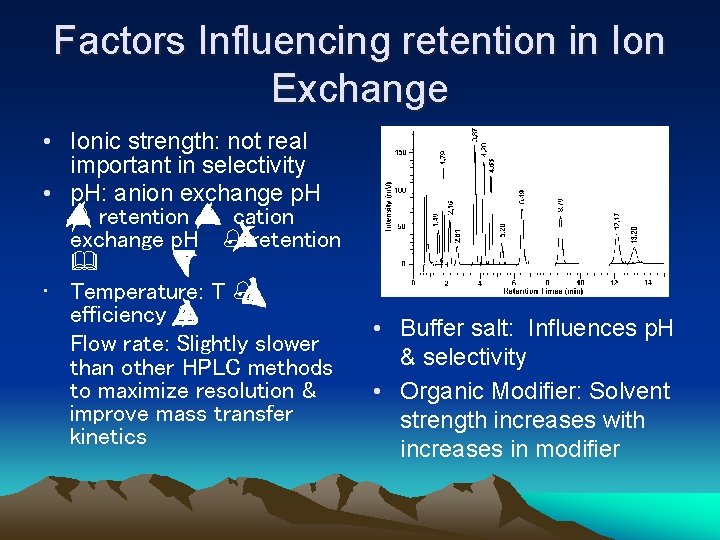
Factors Influencing retention in Ion Exchange • Ionic strength: not real important in selectivity • p. H: anion exchange p. H % retention % cation exchange p. H % retention & • Temperature: T % efficiency % Flow rate: Slightly slower than other HPLC methods to maximize resolution & improve mass transfer kinetics • Buffer salt: Influences p. H & selectivity • Organic Modifier: Solvent strength increases with increases in modifier

Suppressed Ion Chromatography

Partitioning Chromatography • Analyte interacts with mobile and stationary phase, differential interaction leads to selectivity • Interactions that are important – Proton accepting ability * most important – Dipole interaction – Proton Donor * most important – e- pair donating ability – Van der Waals dispersion forces

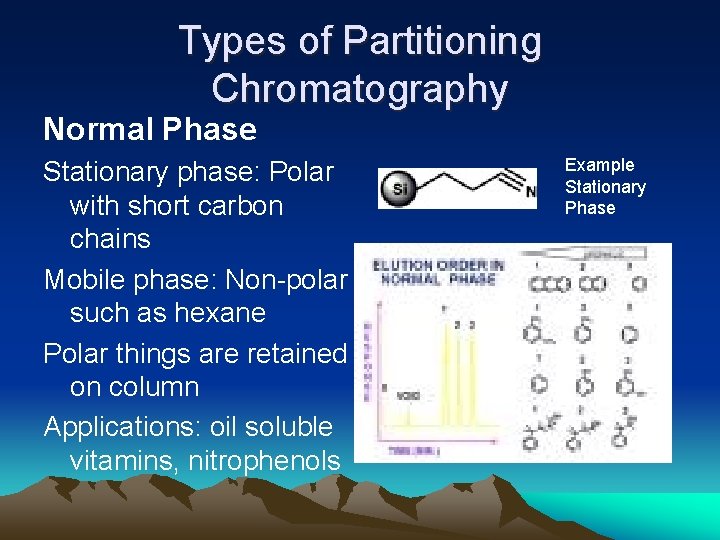
Types of Partitioning Chromatography Normal Phase Stationary phase: Polar with short carbon chains Mobile phase: Non-polar such as hexane Polar things are retained on column Applications: oil soluble vitamins, nitrophenols Example Stationary Phase

Types of Partitioning Chromatography REVERSE PHASE More common Stationary Phase: Hydrophobic C 18 or C 8 Mobile Phase: Polar usually aqueous Polar substance elute first

Solvophobic Theory • Water has a lot of intermolecular interactions in the liquid phase • Solute dissolved in water disrupts those intermolecular interactions • Solute is forced out of aqueous phase not because of favorable interactions between analyte and stationary phase but because of unfavorable interactions between solute and water when solute is dissolved in aqueous phase hence: SOLVOPHOBIC THEORY • Polar functional groups such as –OH would increase the favorability of interaction and thus decrease retention (in mobile phase longer) • Polar things elute non-polar things elute

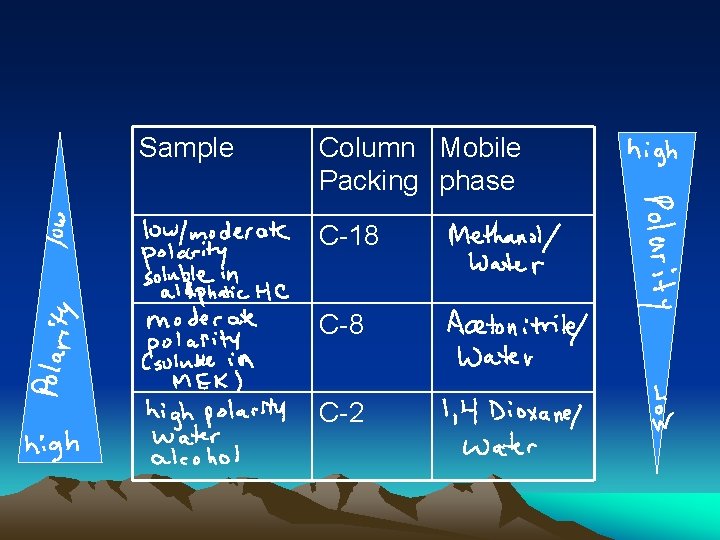
Sample Column Mobile Packing phase C-18 C-2

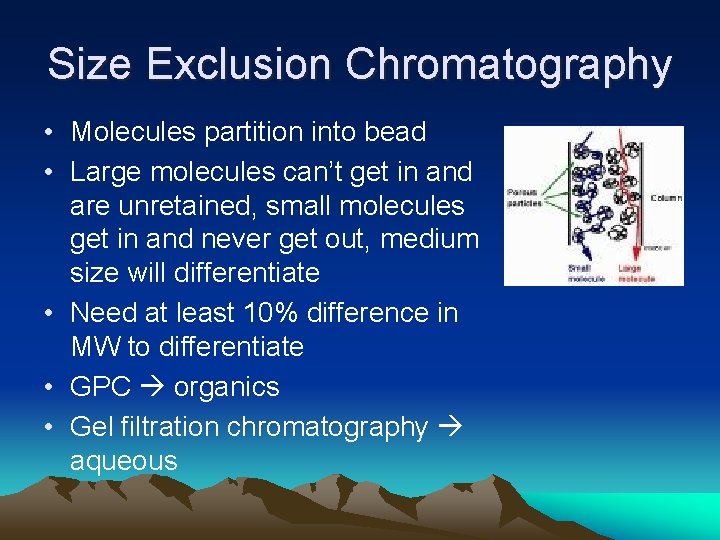
Size Exclusion Chromatography • Molecules partition into bead • Large molecules can’t get in and are unretained, small molecules get in and never get out, medium size will differentiate • Need at least 10% difference in MW to differentiate • GPC organics • Gel filtration chromatography aqueous

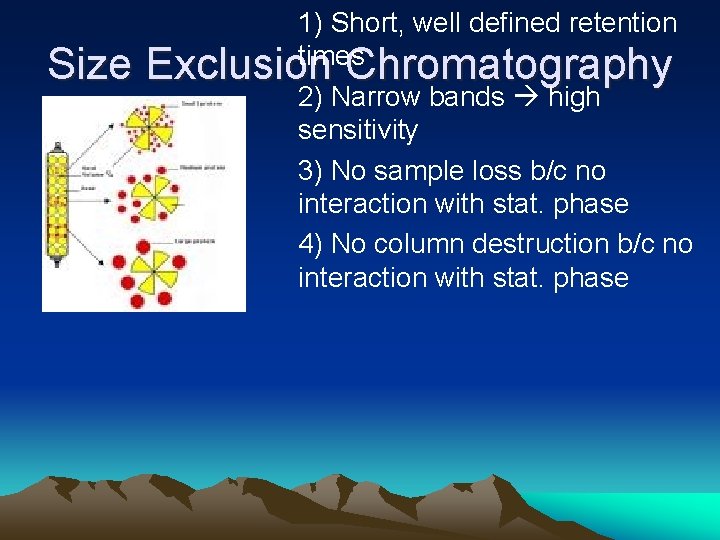
Size 1) Short, well defined retention times Exclusion Chromatography 2) Narrow bands high sensitivity 3) No sample loss b/c no interaction with stat. phase 4) No column destruction b/c no interaction with stat. phase

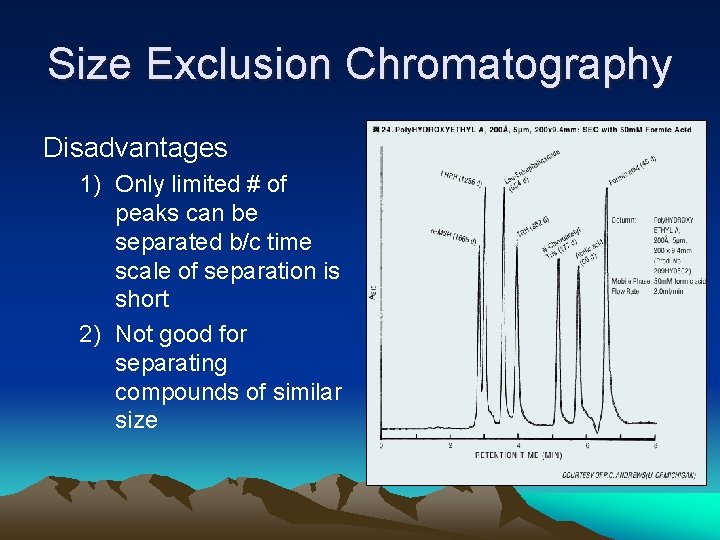
Size Exclusion Chromatography Disadvantages 1) Only limited # of peaks can be separated b/c time scale of separation is short 2) Not good for separating compounds of similar size

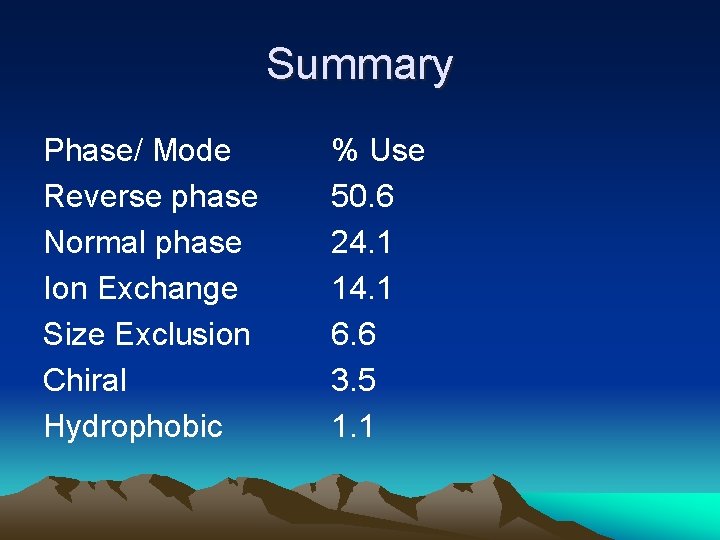
Summary Phase/ Mode Reverse phase Normal phase Ion Exchange Size Exclusion Chiral Hydrophobic % Use 50. 6 24. 1 14. 1 6. 6 3. 5 1. 1

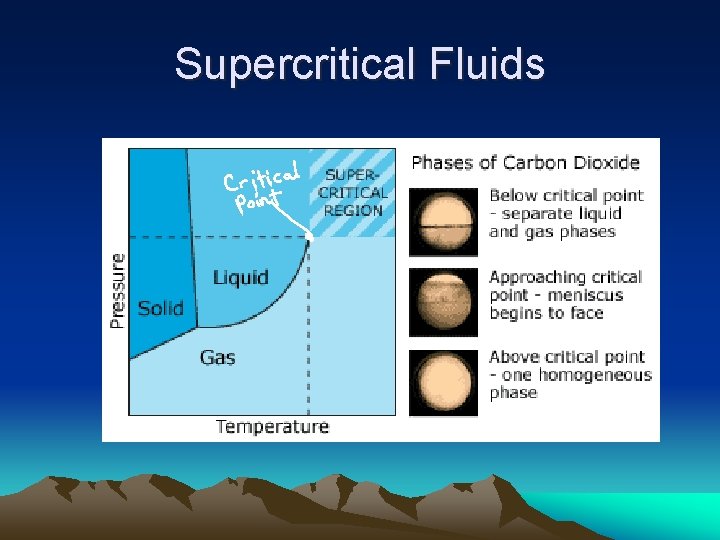
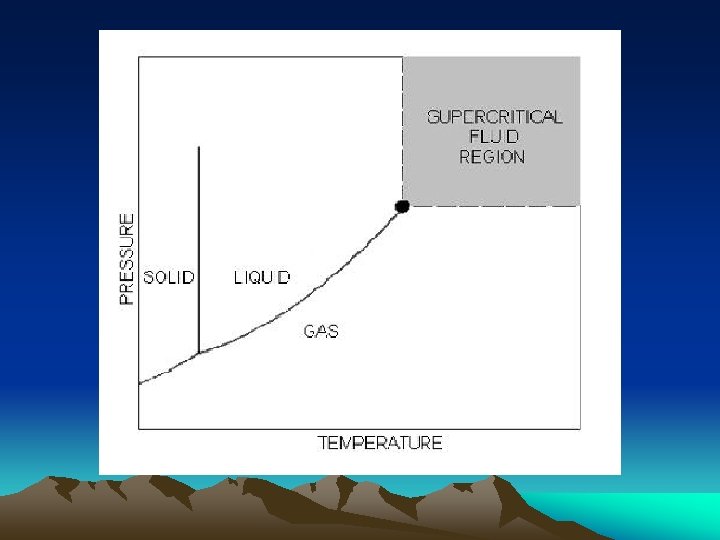
Supercritical Fluids


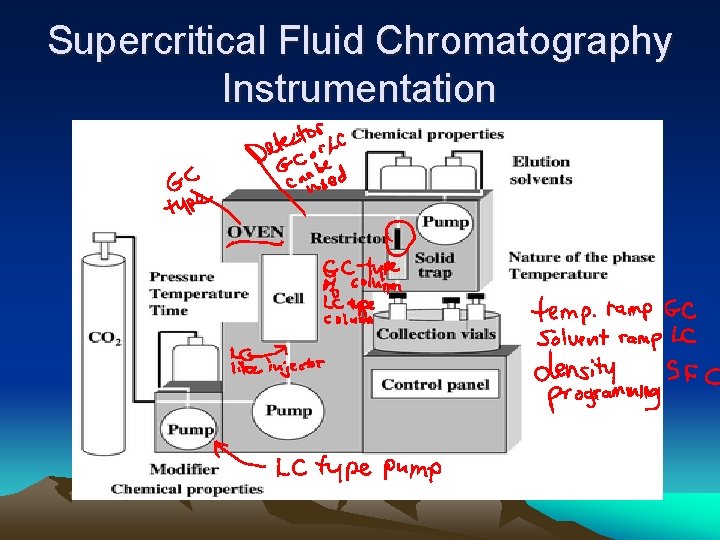
Supercritical Fluid Chromatography Instrumentation

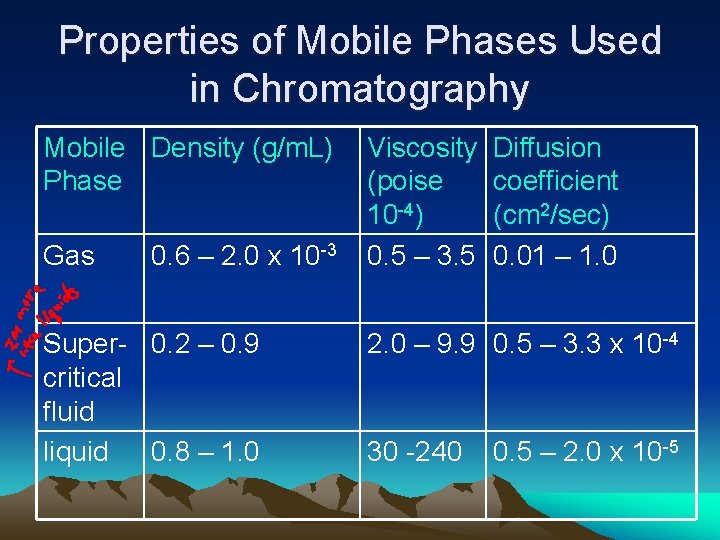
Properties of Mobile Phases Used in Chromatography Mobile Density (g/m. L) Phase Gas 0. 6 – 2. 0 x 10 -3 Super- 0. 2 – 0. 9 critical fluid liquid 0. 8 – 1. 0 Viscosity (poise 10 -4) 0. 5 – 3. 5 Diffusion coefficient (cm 2/sec) 0. 01 – 1. 0 2. 0 – 9. 9 0. 5 – 3. 3 x 10 -4 30 -240 0. 5 – 2. 0 x 10 -5

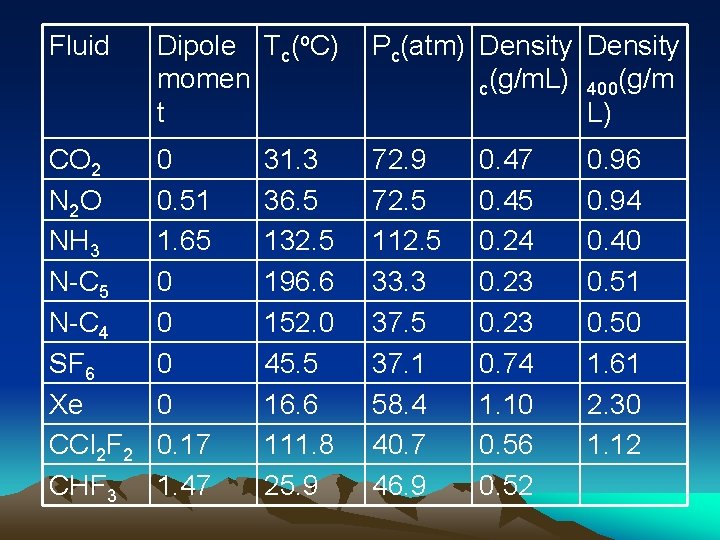
Fluid Dipole Tc(o. C) momen t Pc(atm) Density c(g/m. L) 400(g/m L) CO 2 N 2 O NH 3 N-C 5 N-C 4 SF 6 Xe CCl 2 F 2 CHF 3 0 0. 51 1. 65 0 0 0. 17 1. 47 72. 9 72. 5 112. 5 33. 3 37. 5 37. 1 58. 4 40. 7 46. 9 31. 3 36. 5 132. 5 196. 6 152. 0 45. 5 16. 6 111. 8 25. 9 0. 47 0. 45 0. 24 0. 23 0. 74 1. 10 0. 56 0. 52 0. 96 0. 94 0. 40 0. 51 0. 50 1. 61 2. 30 1. 12

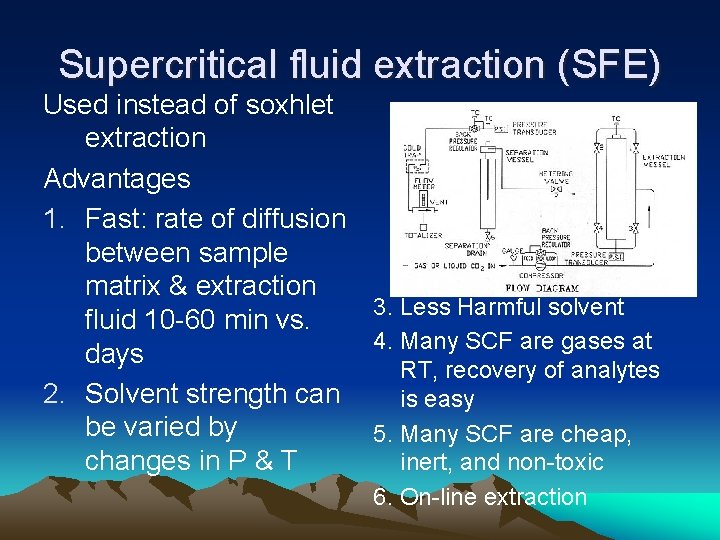
Supercritical fluid extraction (SFE) Used instead of soxhlet extraction Advantages 1. Fast: rate of diffusion between sample matrix & extraction 3. Less Harmful solvent fluid 10 -60 min vs. 4. Many SCF are gases at days RT, recovery of analytes 2. Solvent strength can is easy be varied by 5. Many SCF are cheap, changes in P & T inert, and non-toxic 6. On-line extraction

Supercritical fluid extraction • Disadvantages 1. Method development is more complex 2. Limited # of mobile phases 3. Capital equipment & CO 2 expensive 4. Requires more operator time to do 1 at time

• Insert Hawthorne paper
 Protein chromatography
Protein chromatography Counter ion
Counter ion Slidetodoc.com
Slidetodoc.com Ion exchange chromatography
Ion exchange chromatography Difference between affinity and ion exchange chromatography
Difference between affinity and ion exchange chromatography Introduction chromatography
Introduction chromatography Gfp purification flow chart
Gfp purification flow chart Principle hplc
Principle hplc Factors affecting ion exchange chromatography
Factors affecting ion exchange chromatography Chromatography introduction
Chromatography introduction C6h12 fuerza intermolecular
C6h12 fuerza intermolecular Qumica
Qumica Que es fuerzas intramoleculares
Que es fuerzas intramoleculares Intermolecular forces vapor pressure
Intermolecular forces vapor pressure Ion chromatography
Ion chromatography Hplc introduction
Hplc introduction Advantages of flame ionization detector
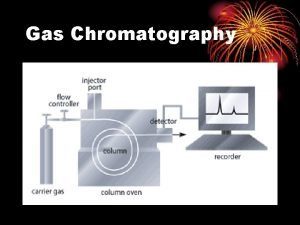
Advantages of flame ionization detector Gas liquid chromatography
Gas liquid chromatography Fast protein liquid chromatography principle
Fast protein liquid chromatography principle Liquid chromatography definition
Liquid chromatography definition High performance liquid chromatography hplc machine
High performance liquid chromatography hplc machine