John E Mc Murry http www cengage comchemistrymcmurry








- Slides: 88

John E. Mc. Murry http: //www. cengage. com/chemistry/mcmurry Chapter 14 Aldehydes and Ketones: Nucleophilic Additions Reactions Richard Morrison • University of Georgia, Athens

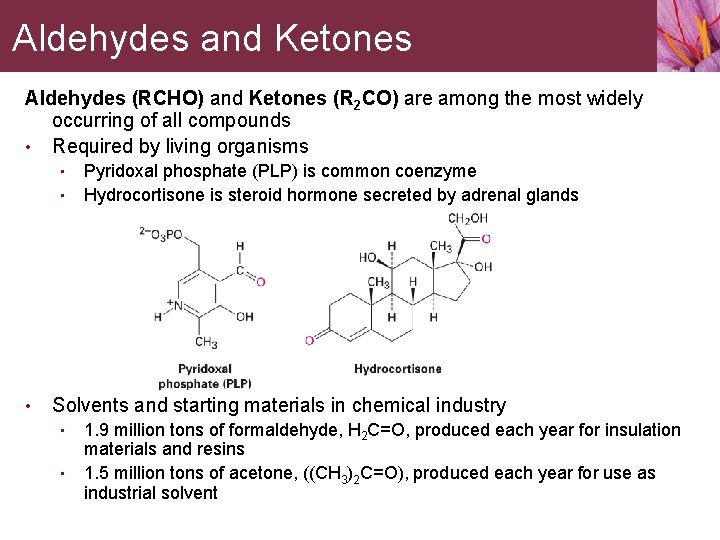
Aldehydes and Ketones Aldehydes (RCHO) and Ketones (R 2 CO) are among the most widely occurring of all compounds • Required by living organisms • • • Pyridoxal phosphate (PLP) is common coenzyme Hydrocortisone is steroid hormone secreted by adrenal glands Solvents and starting materials in chemical industry • • 1. 9 million tons of formaldehyde, H 2 C=O, produced each year for insulation materials and resins 1. 5 million tons of acetone, ((CH 3)2 C=O), produced each year for use as industrial solvent

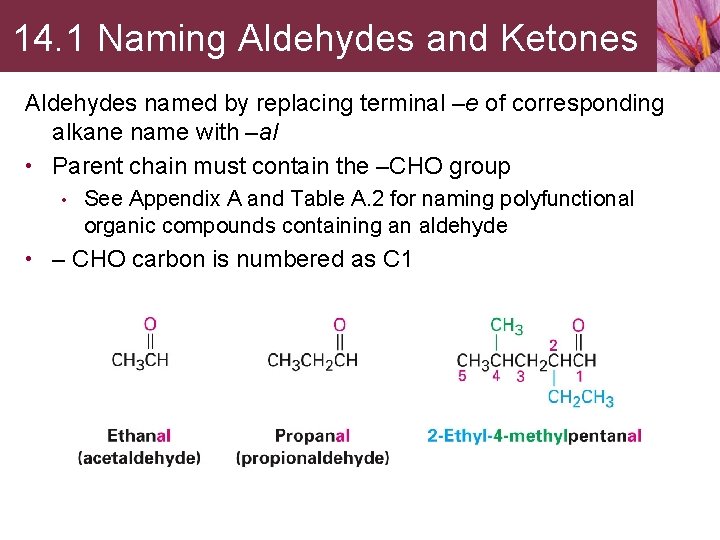
14. 1 Naming Aldehydes and Ketones Aldehydes named by replacing terminal –e of corresponding alkane name with –al • Parent chain must contain the –CHO group • See Appendix A and Table A. 2 for naming polyfunctional organic compounds containing an aldehyde • – CHO carbon is numbered as C 1

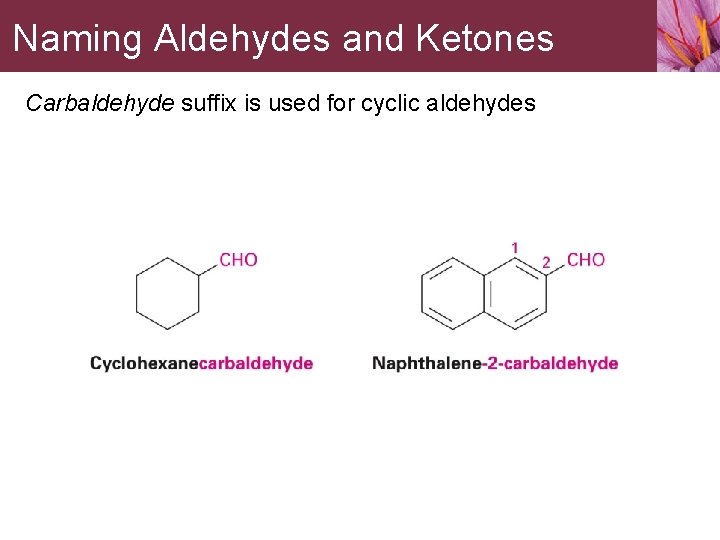
Naming Aldehydes and Ketones Carbaldehyde suffix is used for cyclic aldehydes

Naming Aldehydes and Ketones

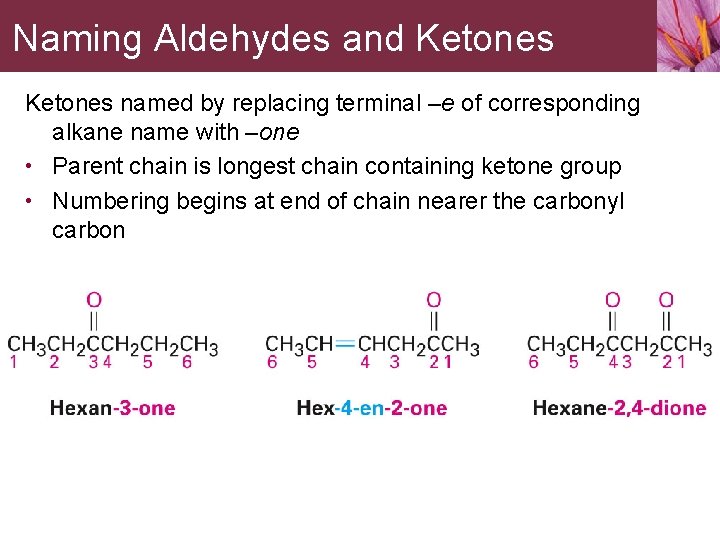
Naming Aldehydes and Ketones named by replacing terminal –e of corresponding alkane name with –one • Parent chain is longest chain containing ketone group • Numbering begins at end of chain nearer the carbonyl carbon

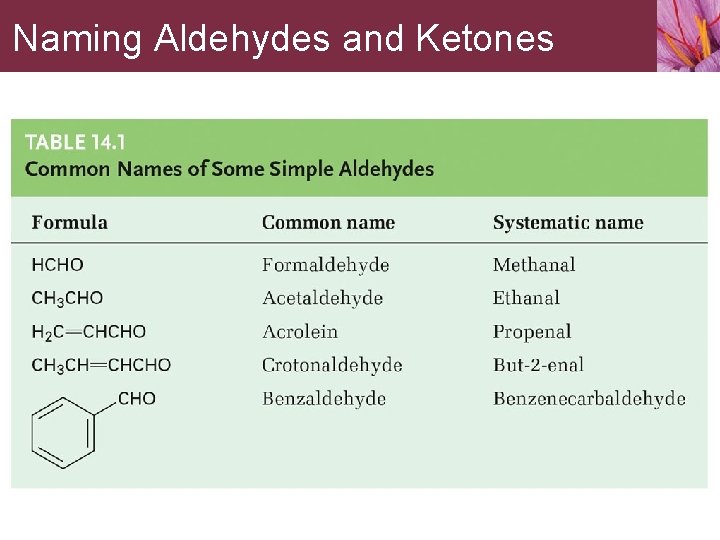
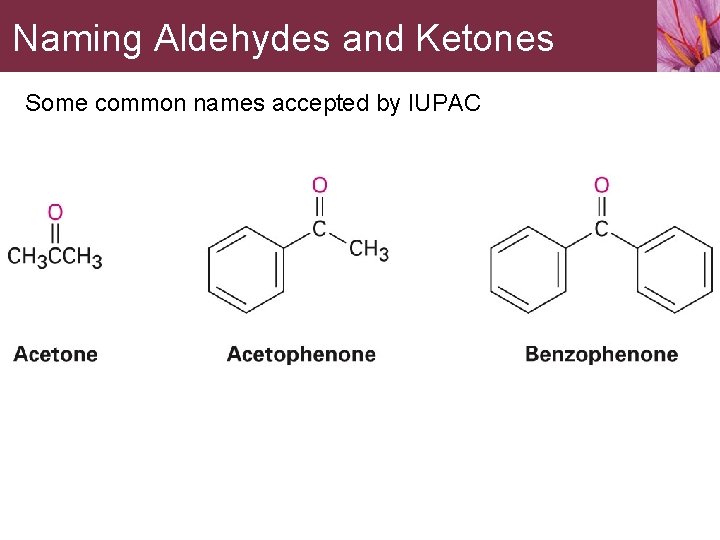
Naming Aldehydes and Ketones Some common names accepted by IUPAC

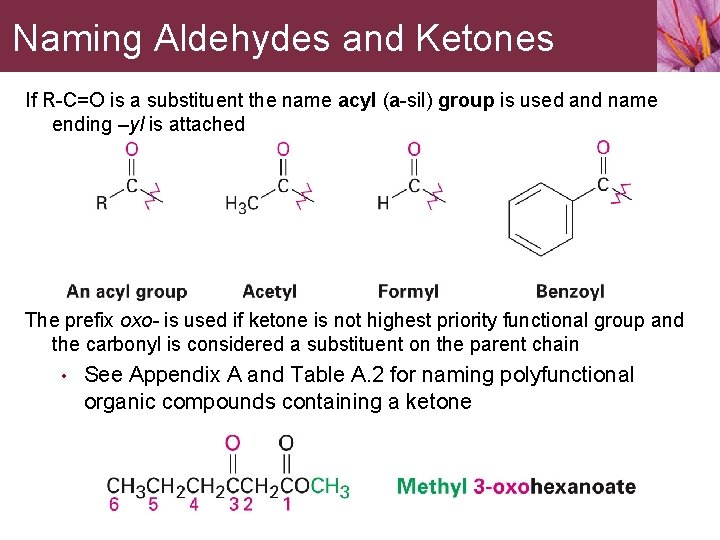
Naming Aldehydes and Ketones If R-C=O is a substituent the name acyl (a-sil) group is used and name ending –yl is attached The prefix oxo- is used if ketone is not highest priority functional group and the carbonyl is considered a substituent on the parent chain • See Appendix A and Table A. 2 for naming polyfunctional organic compounds containing a ketone

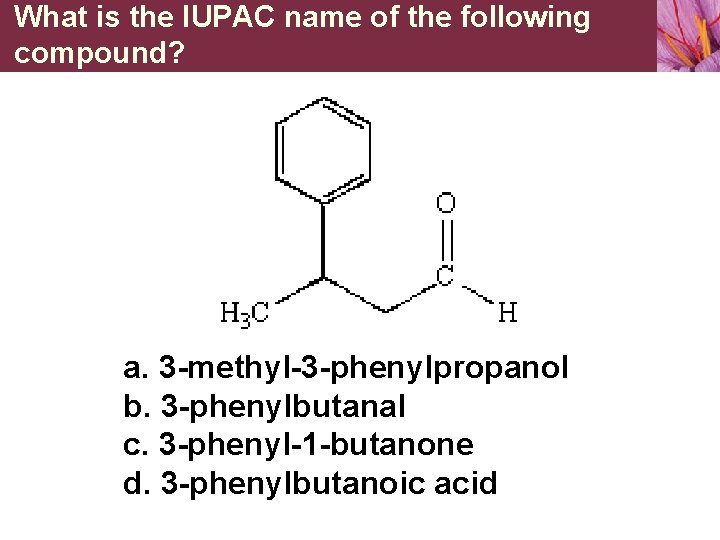
What is the IUPAC name of the following compound? a. 3 -methyl-3 -phenylpropanol b. 3 -phenylbutanal c. 3 -phenyl-1 -butanone d. 3 -phenylbutanoic acid

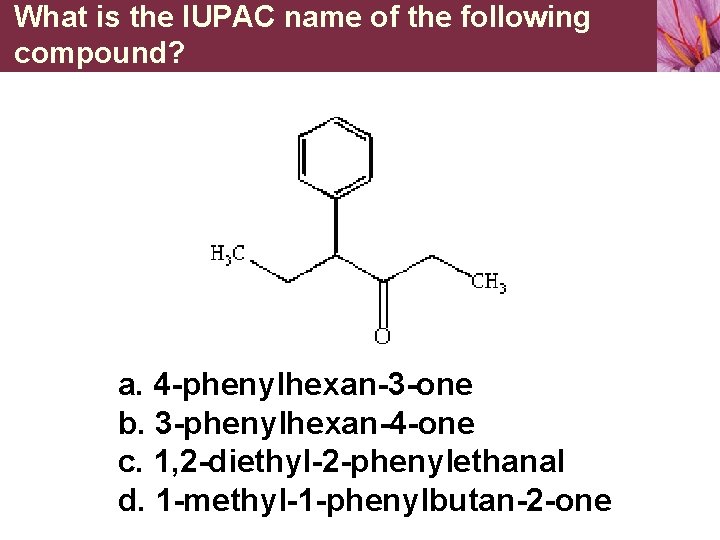
What is the IUPAC name of the following compound? a. 4 -phenylhexan-3 -one b. 3 -phenylhexan-4 -one c. 1, 2 -diethyl-2 -phenylethanal d. 1 -methyl-1 -phenylbutan-2 -one

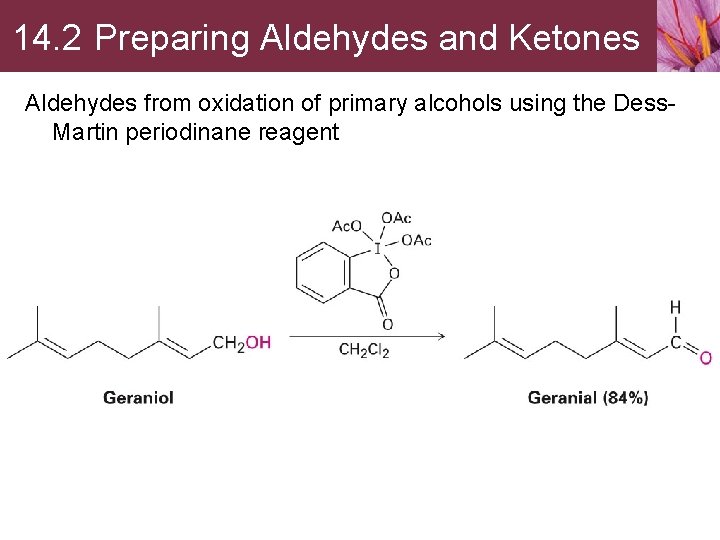
14. 2 Preparing Aldehydes and Ketones Aldehydes from oxidation of primary alcohols using the Dess. Martin periodinane reagent

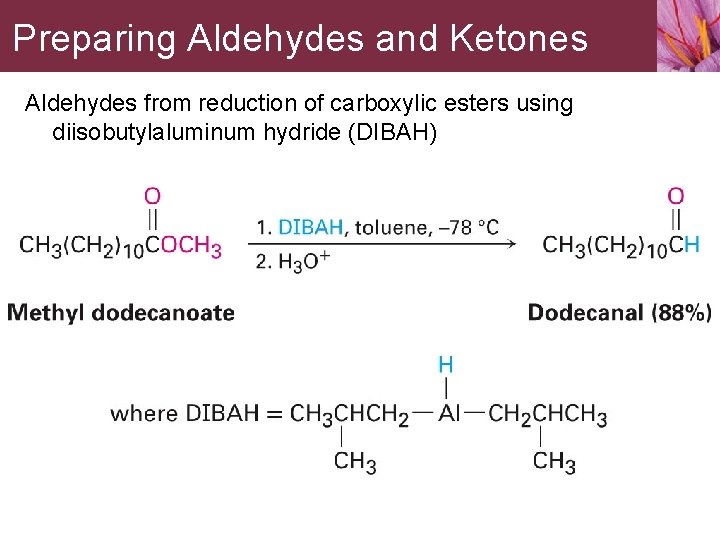
Preparing Aldehydes and Ketones Aldehydes from reduction of carboxylic esters using diisobutylaluminum hydride (DIBAH)

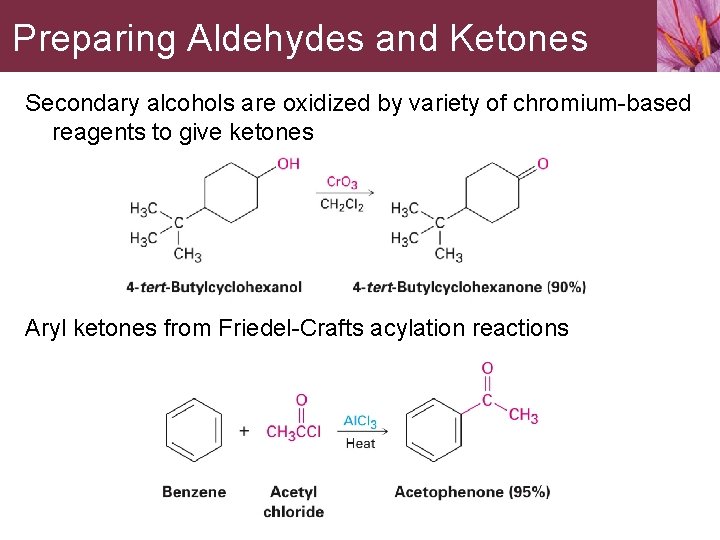
Preparing Aldehydes and Ketones Secondary alcohols are oxidized by variety of chromium-based reagents to give ketones Aryl ketones from Friedel-Crafts acylation reactions

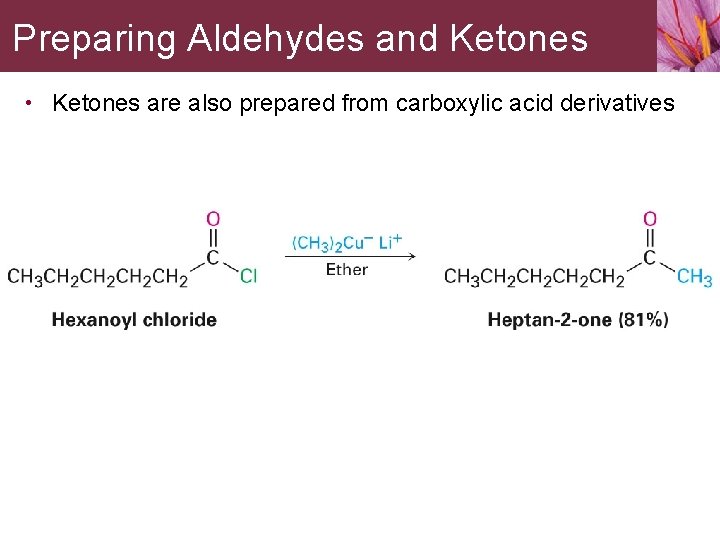
Preparing Aldehydes and Ketones • Ketones are also prepared from carboxylic acid derivatives

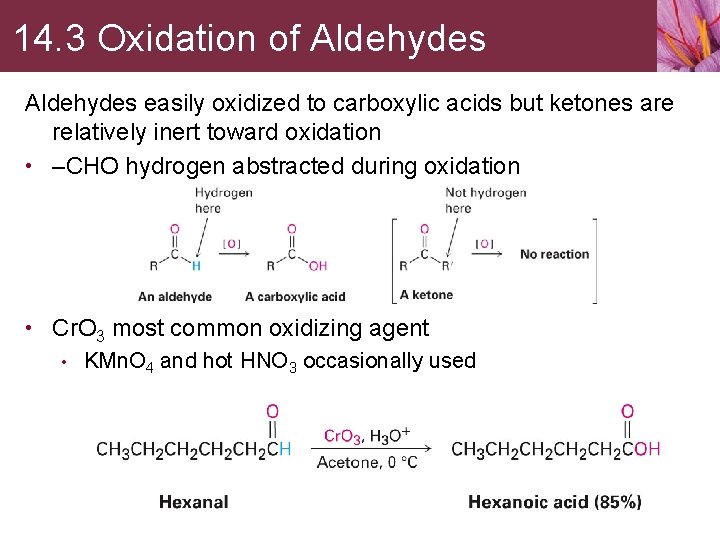
14. 3 Oxidation of Aldehydes easily oxidized to carboxylic acids but ketones are relatively inert toward oxidation • –CHO hydrogen abstracted during oxidation • Cr. O 3 most common oxidizing agent • KMn. O 4 and hot HNO 3 occasionally used

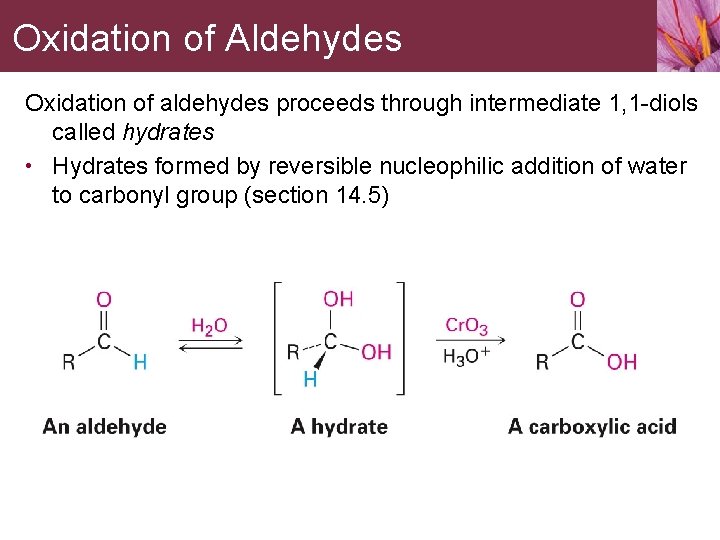
Oxidation of Aldehydes Oxidation of aldehydes proceeds through intermediate 1, 1 -diols called hydrates • Hydrates formed by reversible nucleophilic addition of water to carbonyl group (section 14. 5)

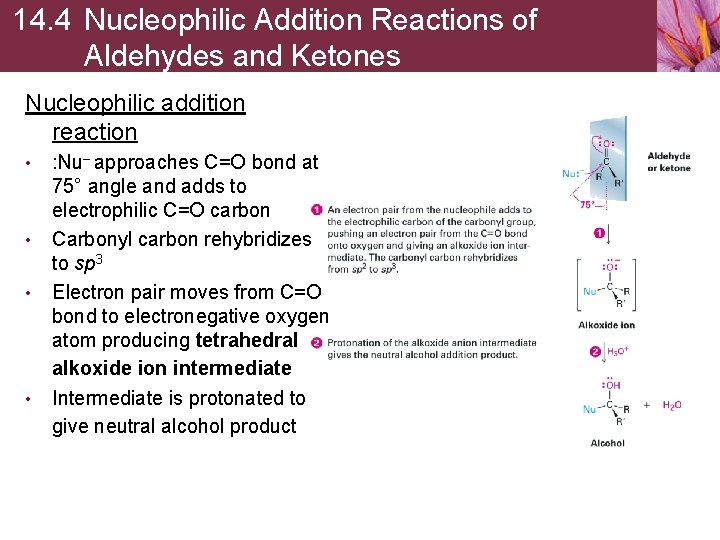
14. 4 Nucleophilic Addition Reactions of Aldehydes and Ketones Nucleophilic addition reaction • • : Nu– approaches C=O bond at 75° angle and adds to electrophilic C=O carbon Carbonyl carbon rehybridizes to sp 3 Electron pair moves from C=O bond to electronegative oxygen atom producing tetrahedral alkoxide ion intermediate Intermediate is protonated to give neutral alcohol product

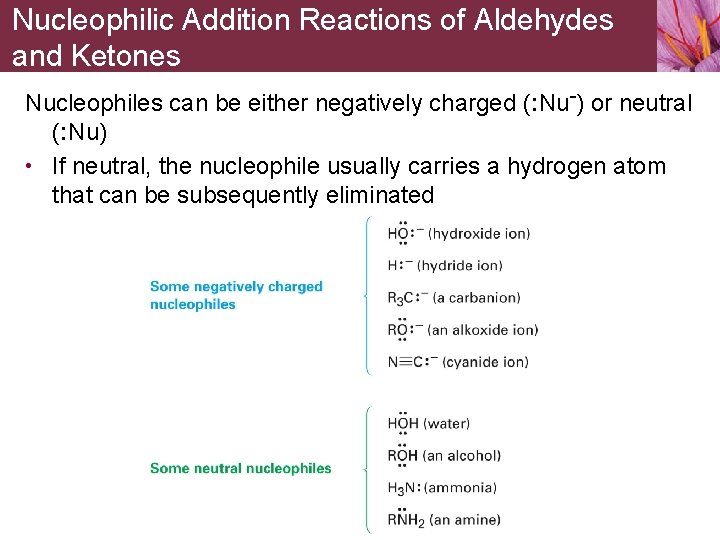
Nucleophilic Addition Reactions of Aldehydes and Ketones Nucleophiles can be either negatively charged (: Nu-) or neutral (: Nu) • If neutral, the nucleophile usually carries a hydrogen atom that can be subsequently eliminated

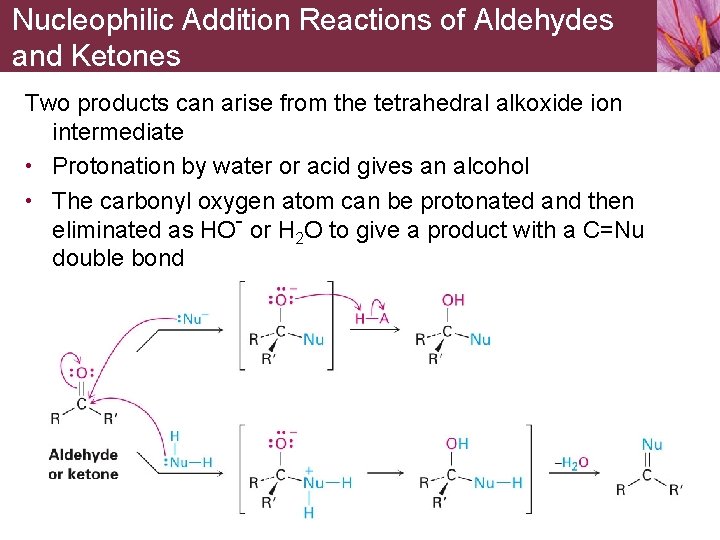
Nucleophilic Addition Reactions of Aldehydes and Ketones Two products can arise from the tetrahedral alkoxide ion intermediate • Protonation by water or acid gives an alcohol • The carbonyl oxygen atom can be protonated and then eliminated as HO- or H 2 O to give a product with a C=Nu double bond

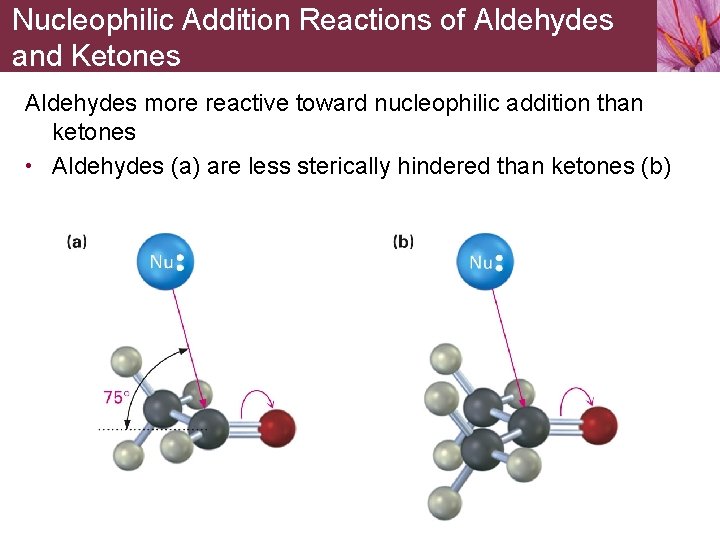
Nucleophilic Addition Reactions of Aldehydes and Ketones Aldehydes more reactive toward nucleophilic addition than ketones • Aldehydes (a) are less sterically hindered than ketones (b)

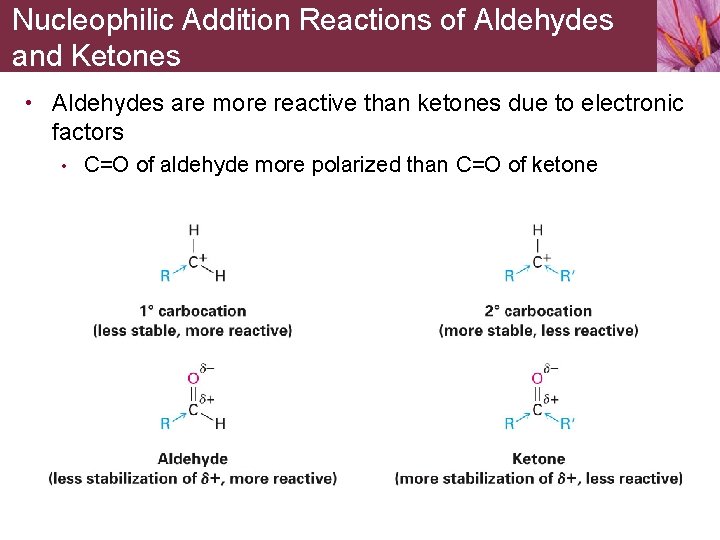
Nucleophilic Addition Reactions of Aldehydes and Ketones • Aldehydes are more reactive than ketones due to electronic factors • C=O of aldehyde more polarized than C=O of ketone

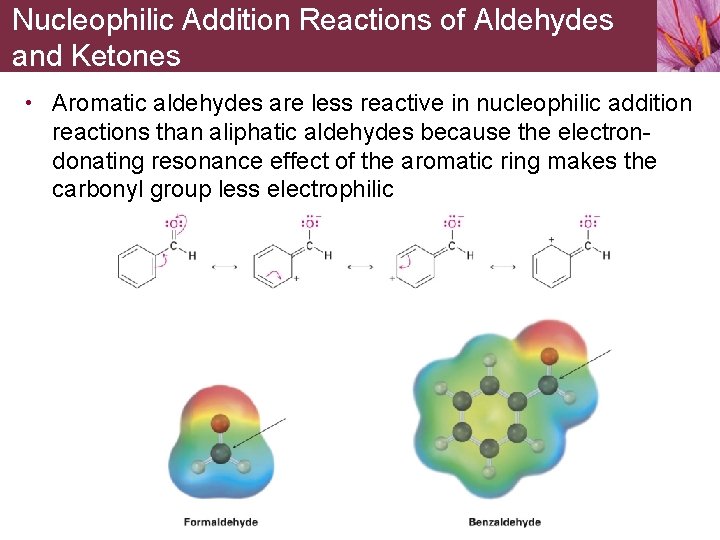
Nucleophilic Addition Reactions of Aldehydes and Ketones • Aromatic aldehydes are less reactive in nucleophilic addition reactions than aliphatic aldehydes because the electrondonating resonance effect of the aromatic ring makes the carbonyl group less electrophilic

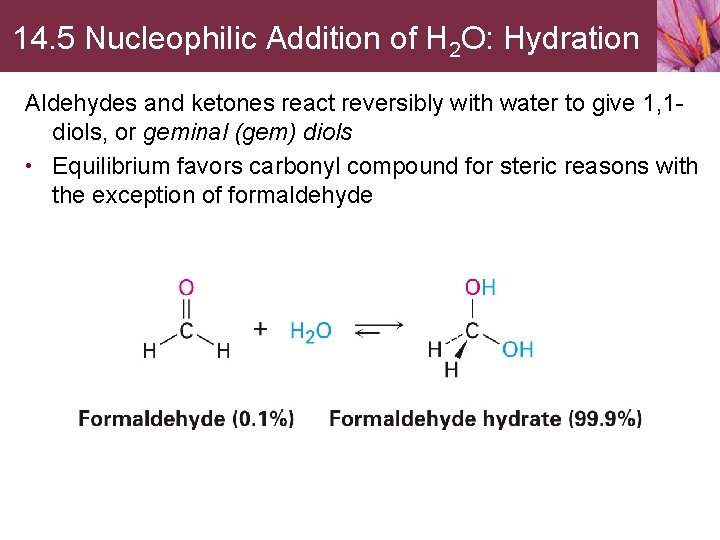
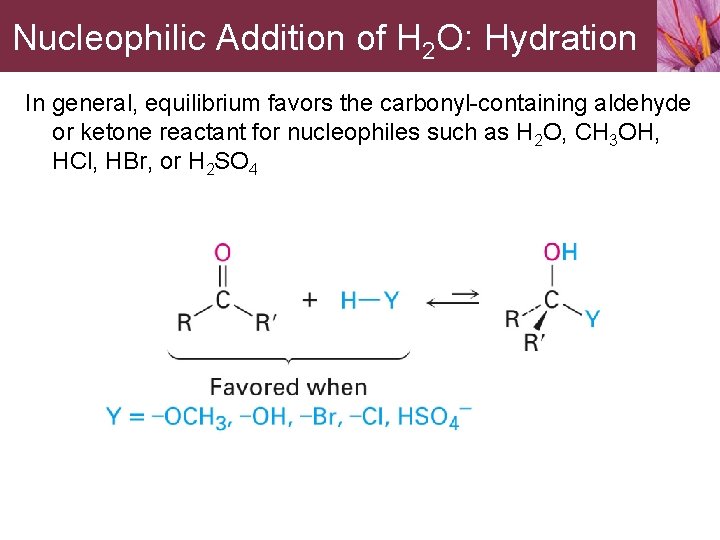
14. 5 Nucleophilic Addition of H 2 O: Hydration Aldehydes and ketones react reversibly with water to give 1, 1 diols, or geminal (gem) diols • Equilibrium favors carbonyl compound for steric reasons with the exception of formaldehyde

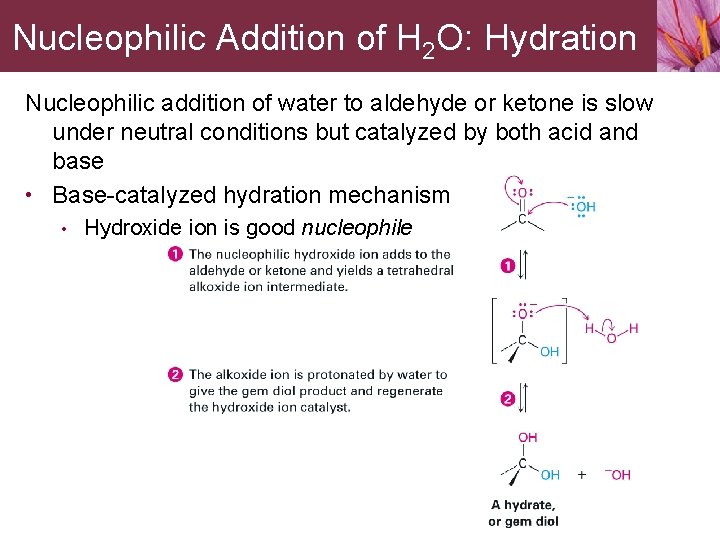
Nucleophilic Addition of H 2 O: Hydration Nucleophilic addition of water to aldehyde or ketone is slow under neutral conditions but catalyzed by both acid and base • Base-catalyzed hydration mechanism • Hydroxide ion is good nucleophile

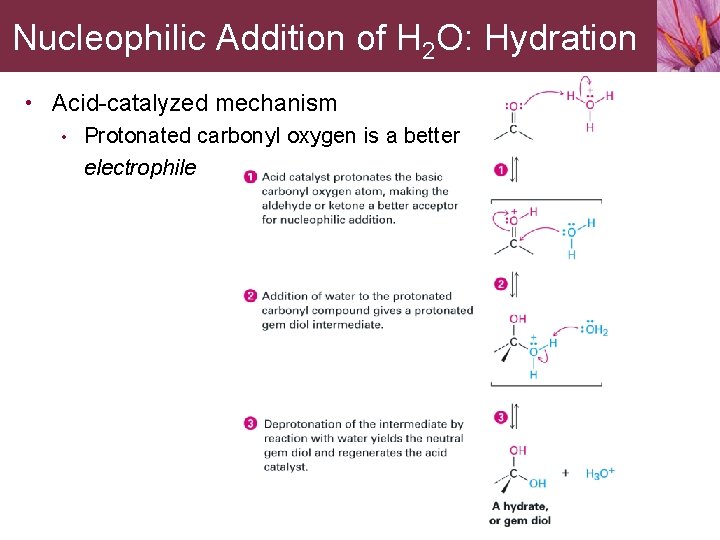
Nucleophilic Addition of H 2 O: Hydration • Acid-catalyzed mechanism • Protonated carbonyl oxygen is a better electrophile

Nucleophilic Addition of H 2 O: Hydration In general, equilibrium favors the carbonyl-containing aldehyde or ketone reactant for nucleophiles such as H 2 O, CH 3 OH, HCl, HBr, or H 2 SO 4

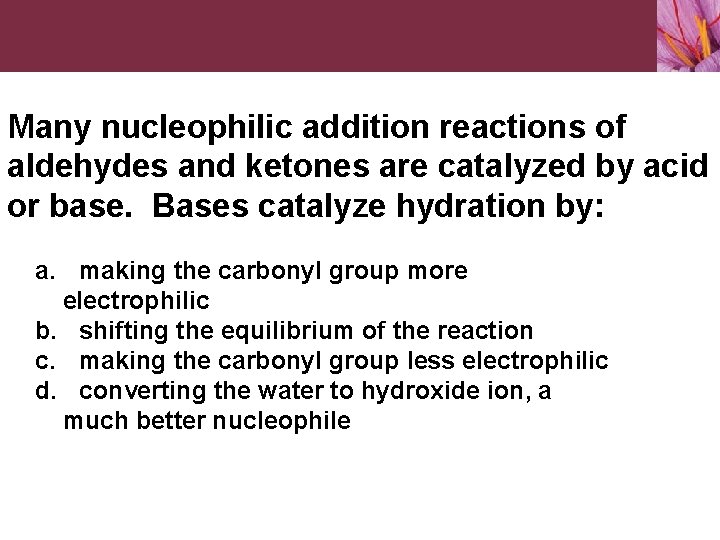
Many nucleophilic addition reactions of aldehydes and ketones are catalyzed by acid or base. Bases catalyze hydration by: a. making the carbonyl group more electrophilic b. shifting the equilibrium of the reaction c. making the carbonyl group less electrophilic d. converting the water to hydroxide ion, a much better nucleophile

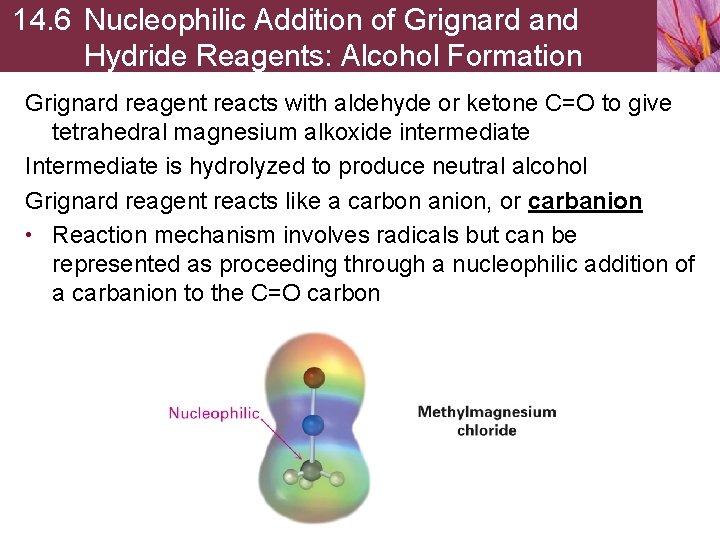
14. 6 Nucleophilic Addition of Grignard and Hydride Reagents: Alcohol Formation Grignard reagent reacts with aldehyde or ketone C=O to give tetrahedral magnesium alkoxide intermediate Intermediate is hydrolyzed to produce neutral alcohol Grignard reagent reacts like a carbon anion, or carbanion • Reaction mechanism involves radicals but can be represented as proceeding through a nucleophilic addition of a carbanion to the C=O carbon

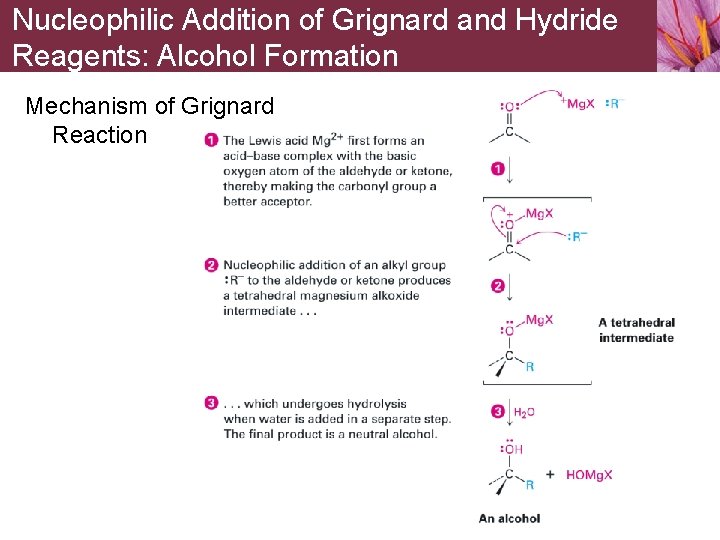
Nucleophilic Addition of Grignard and Hydride Reagents: Alcohol Formation Mechanism of Grignard Reaction

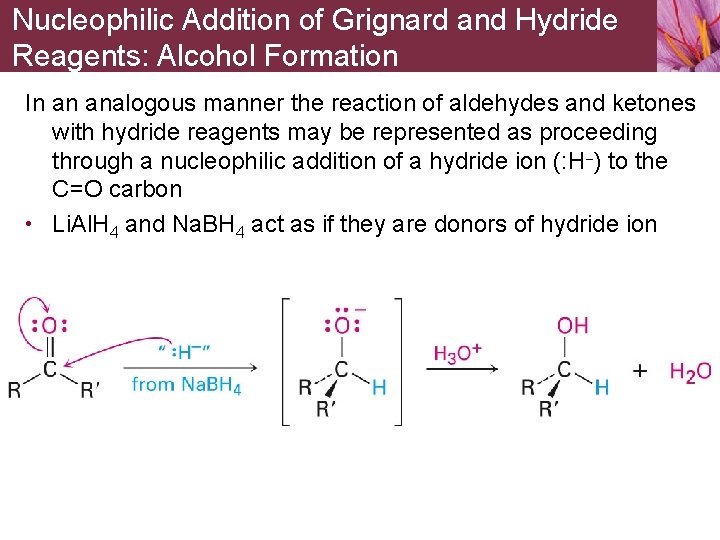
Nucleophilic Addition of Grignard and Hydride Reagents: Alcohol Formation In an analogous manner the reaction of aldehydes and ketones with hydride reagents may be represented as proceeding through a nucleophilic addition of a hydride ion (: H–) to the C=O carbon • Li. Al. H 4 and Na. BH 4 act as if they are donors of hydride ion

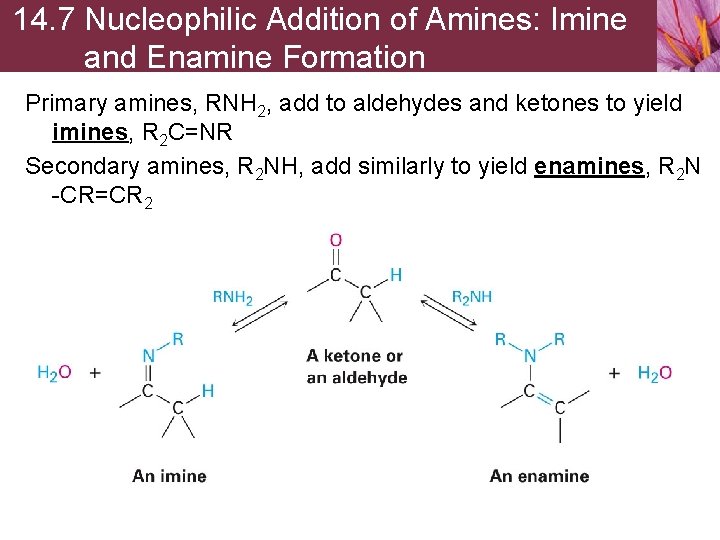
14. 7 Nucleophilic Addition of Amines: Imine and Enamine Formation Primary amines, RNH 2, add to aldehydes and ketones to yield imines, R 2 C=NR Secondary amines, R 2 NH, add similarly to yield enamines, R 2 N -CR=CR 2

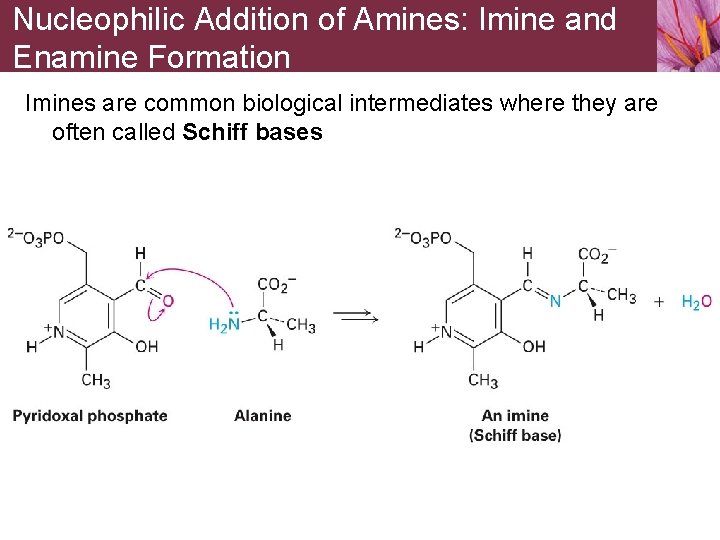
Nucleophilic Addition of Amines: Imine and Enamine Formation Imines are common biological intermediates where they are often called Schiff bases

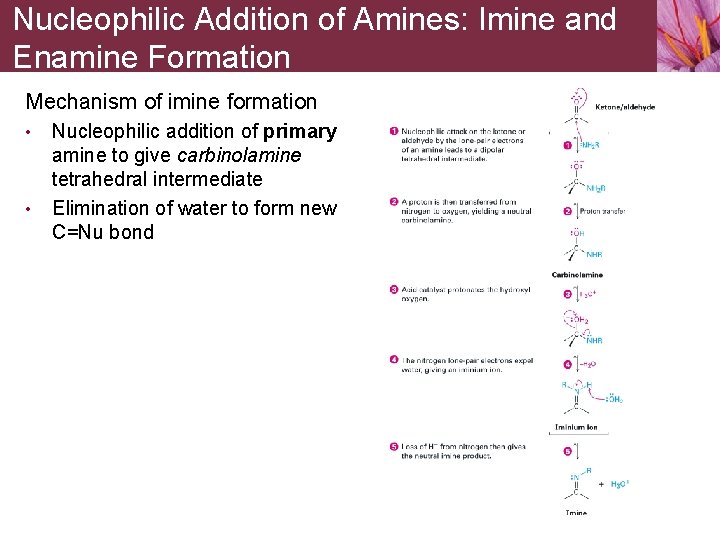
Nucleophilic Addition of Amines: Imine and Enamine Formation Mechanism of imine formation • • Nucleophilic addition of primary amine to give carbinolamine tetrahedral intermediate Elimination of water to form new C=Nu bond

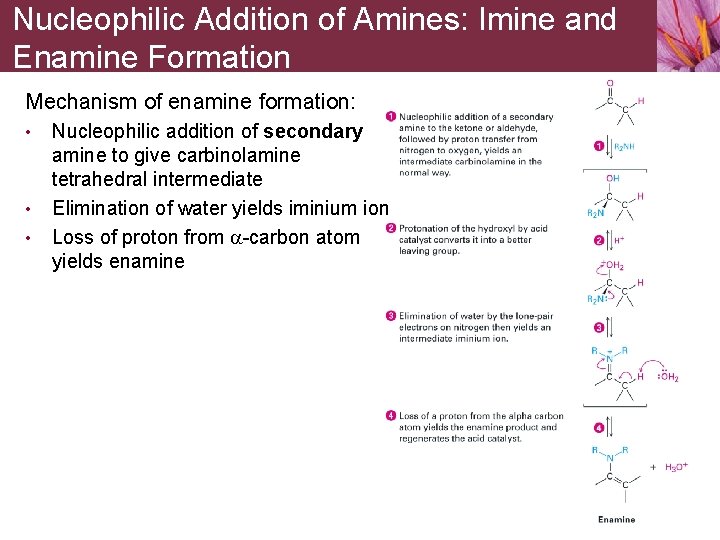
Nucleophilic Addition of Amines: Imine and Enamine Formation Mechanism of enamine formation: • • • Nucleophilic addition of secondary amine to give carbinolamine tetrahedral intermediate Elimination of water yields iminium ion Loss of proton from a-carbon atom yields enamine

Nucleophilic Addition of Amines: Imine and Enamine Formation Imine and enamine formations reach maximum rate around p. H = 4 to 5 • Slow at p. H > 5 because there is insufficient H+ present in solution to protonate intermediate carbinolamine –OH to yield the better leaving group –OH 2+ • Slow at p. H < 4 because the basic amine nucleophile is protonated and initial nucleophilic addition cannot occur

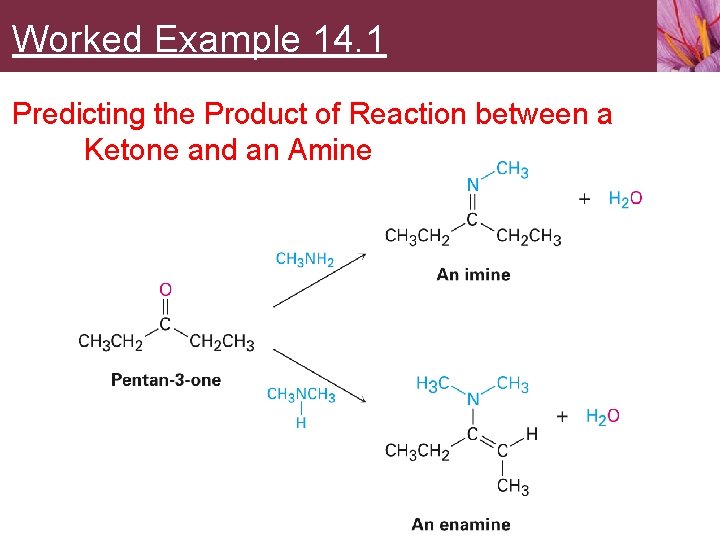
Worked Example 14. 1 Predicting the Product of Reaction between a Ketone and an Amine Show the products you would obtain by acid-catalyzed reaction of pentan-3 -one with methylamine, CH 3 NH 2, and with dimethylamine, (CH 3)2 NH

Worked Example 14. 1 Predicting the Product of Reaction between a Ketone and an Amine Strategy • An aldehyde or ketone reacts with a primary amine, RNH 2, to yield an imine, in which the carbonyl oxygen atom has been replaced by the =N-R group of the amine • Reaction of the same aldehyde or ketone with a secondary amine, R 2 NH, yields an enamine, in which the oxygen atom has been replaced by the –NR 2 group of the amine and the double bond has moved to a position between the former carbonyl carbon and the neighboring carbon

Worked Example 14. 1 Predicting the Product of Reaction between a Ketone and an Amine

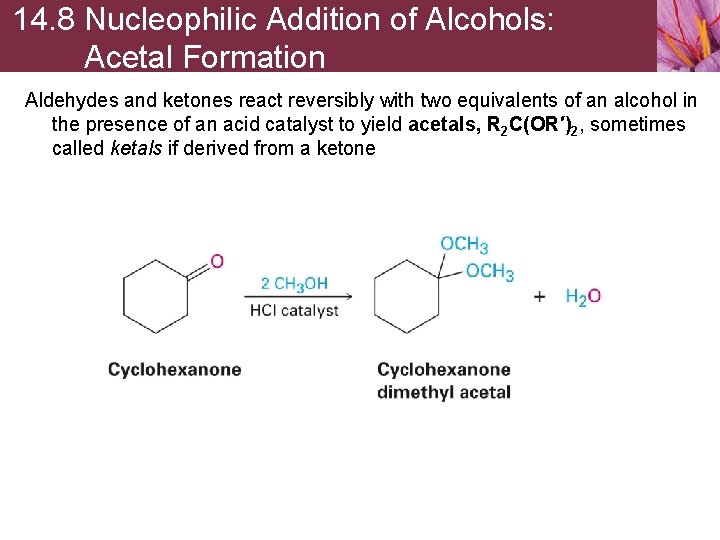
14. 8 Nucleophilic Addition of Alcohols: Acetal Formation Aldehydes and ketones react reversibly with two equivalents of an alcohol in the presence of an acid catalyst to yield acetals, R 2 C(OR′)2, sometimes called ketals if derived from a ketone

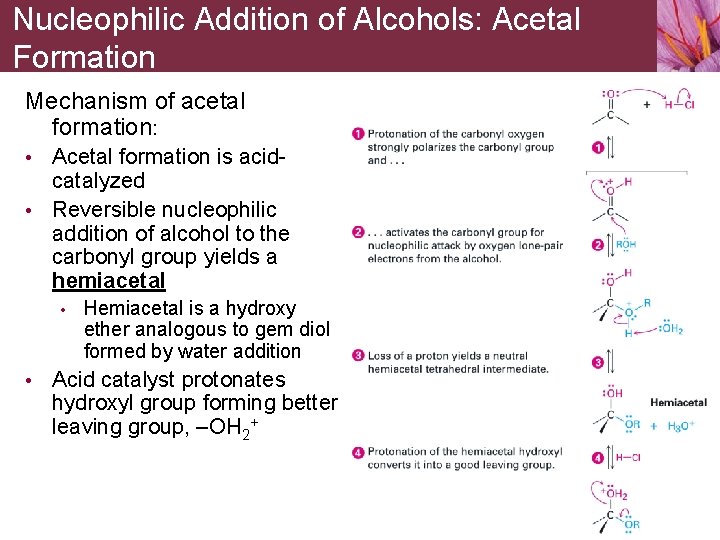
Nucleophilic Addition of Alcohols: Acetal Formation Mechanism of acetal formation: • Acetal formation is acid- catalyzed • Reversible nucleophilic addition of alcohol to the carbonyl group yields a hemiacetal • Hemiacetal is a hydroxy ether analogous to gem diol formed by water addition • Acid catalyst protonates hydroxyl group forming better leaving group, –OH 2+

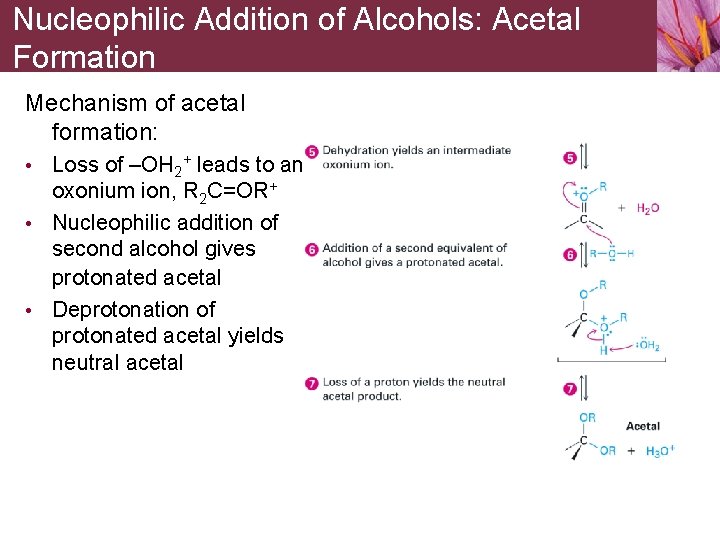
Nucleophilic Addition of Alcohols: Acetal Formation Mechanism of acetal formation: • Loss of –OH 2+ leads to an oxonium ion, R 2 C=OR+ • Nucleophilic addition of second alcohol gives protonated acetal • Deprotonation of protonated acetal yields neutral acetal

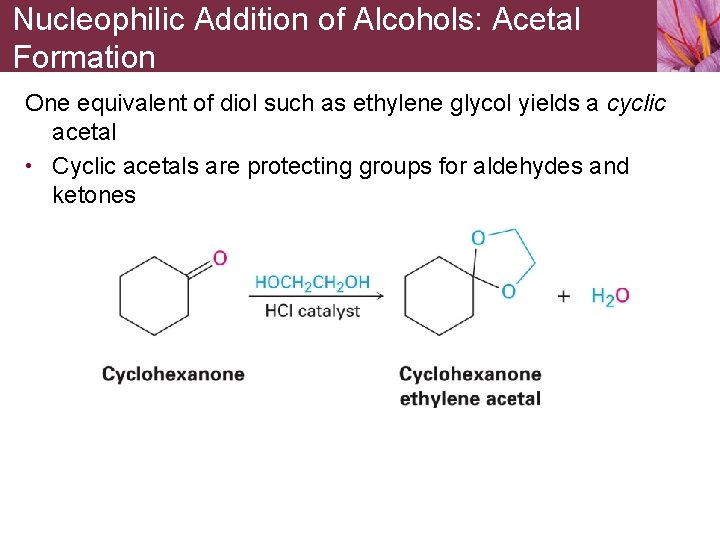
Nucleophilic Addition of Alcohols: Acetal Formation One equivalent of diol such as ethylene glycol yields a cyclic acetal • Cyclic acetals are protecting groups for aldehydes and ketones

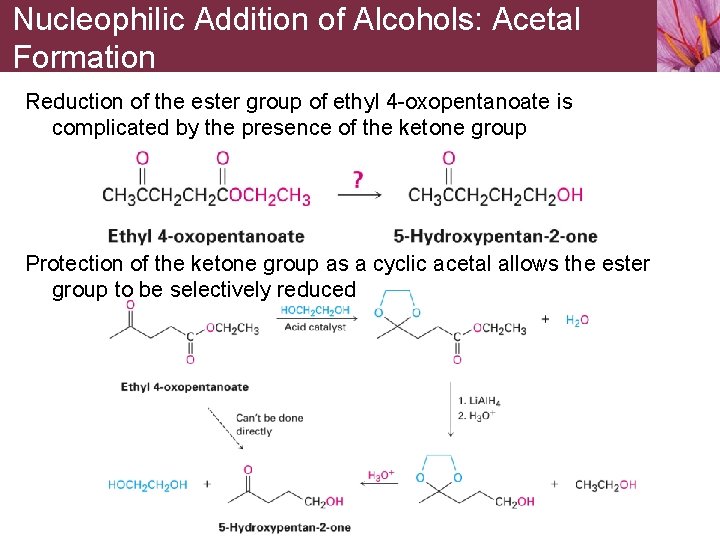
Nucleophilic Addition of Alcohols: Acetal Formation Reduction of the ester group of ethyl 4 -oxopentanoate is complicated by the presence of the ketone group Protection of the ketone group as a cyclic acetal allows the ester group to be selectively reduced

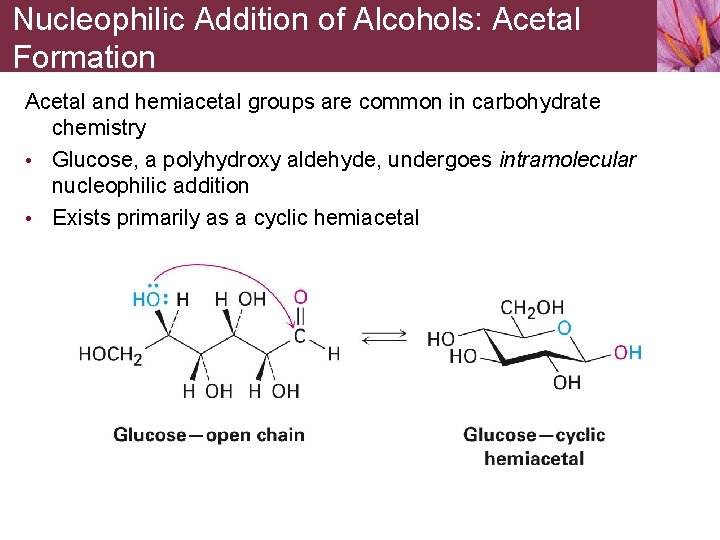
Nucleophilic Addition of Alcohols: Acetal Formation Acetal and hemiacetal groups are common in carbohydrate chemistry • Glucose, a polyhydroxy aldehyde, undergoes intramolecular nucleophilic addition • Exists primarily as a cyclic hemiacetal

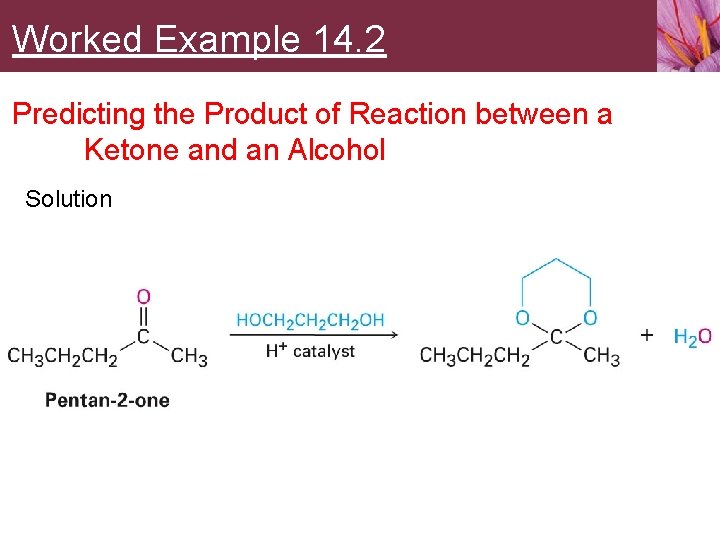
Worked Example 14. 2 Predicting the Product of Reaction between a Ketone and an Alcohol Show the structure of the acetal you would obtain by acid-catalyzed reaction of pentan-2 -one with propane-1, 3 -diol

Worked Example 14. 2 Predicting the Product of Reaction between a Ketone and an Alcohol Strategy • Acid-catalyzed reaction of an aldehyde or ketone with 2 equivalents of a monoalcohol or 1 equivalent of a diol yields an acetal, in which the carbonyl oxygen atom is replaced by two –OR groups from the alcohol

Worked Example 14. 2 Predicting the Product of Reaction between a Ketone and an Alcohol Solution

The substance formed on addition of water to an aldehyde or ketone is called a hydrate or a: a. b. c. d. vicinal diol geminal diol acetal ketal Ch. 14. 5

Many nucleophilic addition reactions of aldehydes and ketones are catalyzed by acid or base. Bases catalyze hydration by: Ch. 14. 5 a. making the carbonyl group more electrophilic b. shifting the equilibrium of the reaction c. making the carbonyl group less electrophilic d. converting the water to hydroxide ion, a much better nucleophile

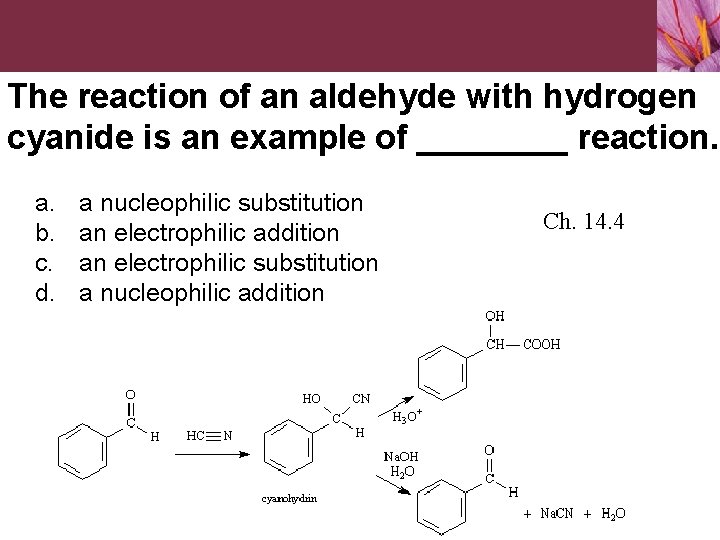
The reaction of an aldehyde with hydrogen cyanide is an example of ____ reaction. a. b. c. d. a nucleophilic substitution an electrophilic addition an electrophilic substitution a nucleophilic addition Ch. 14. 4

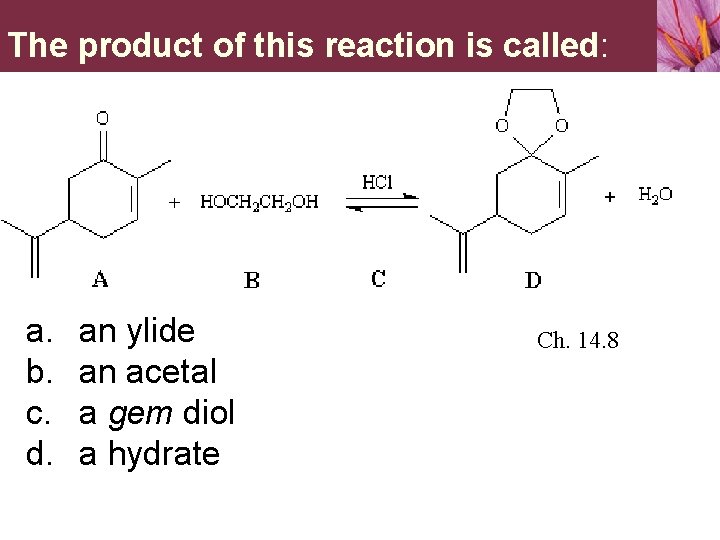
The product of this reaction is called: a. b. c. d. an ylide an acetal a gem diol a hydrate Ch. 14. 8

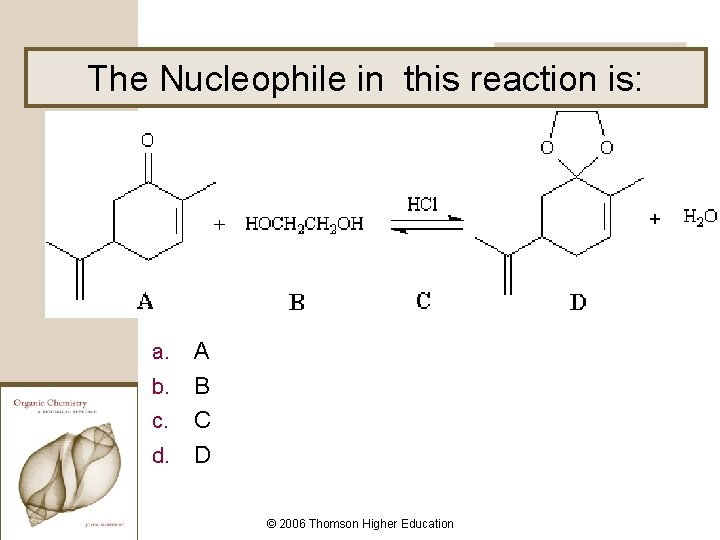
The Nucleophile in this reaction is: A b. B c. C d. D a. © 2006 Thomson Higher Education

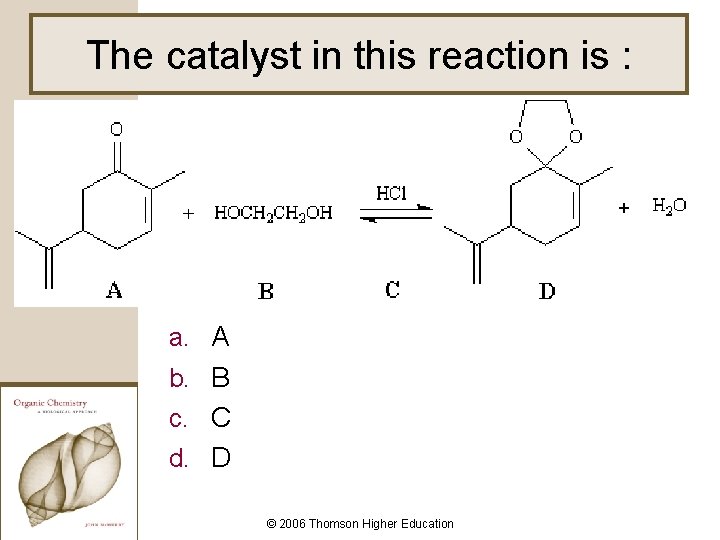
The catalyst in this reaction is : a. A b. B c. C d. D © 2006 Thomson Higher Education

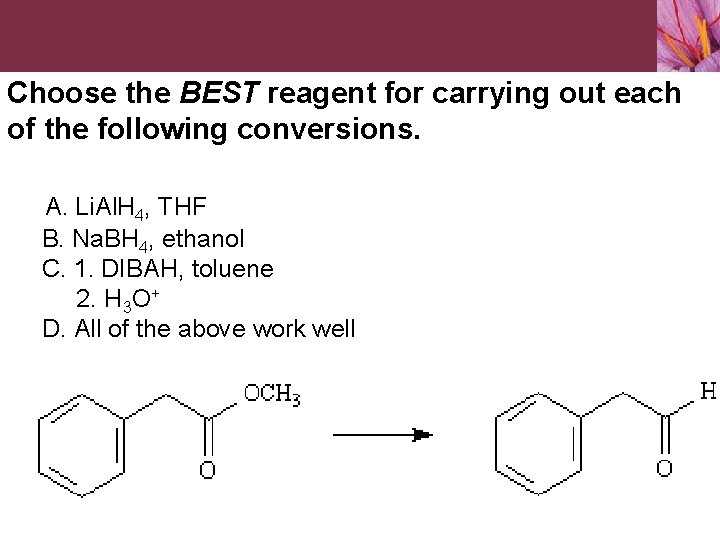
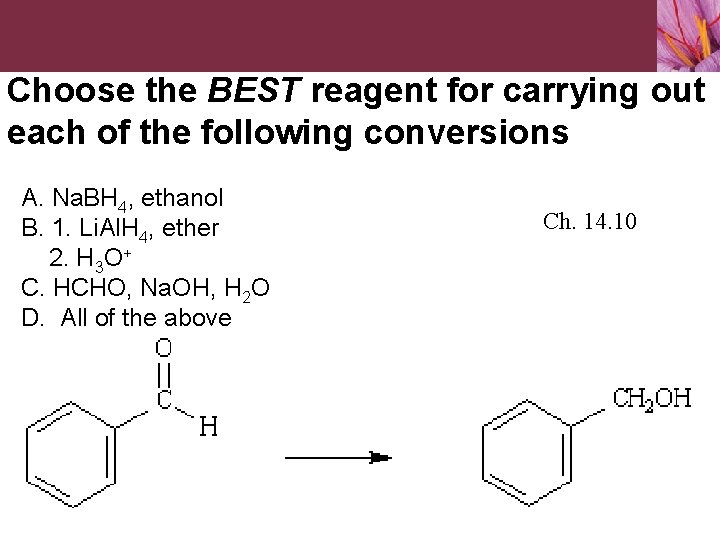
Choose the BEST reagent for carrying out each of the following conversions. A. Li. Al. H 4, THF B. Na. BH 4, ethanol C. 1. DIBAH, toluene 2. H 3 O+ D. All of the above work well

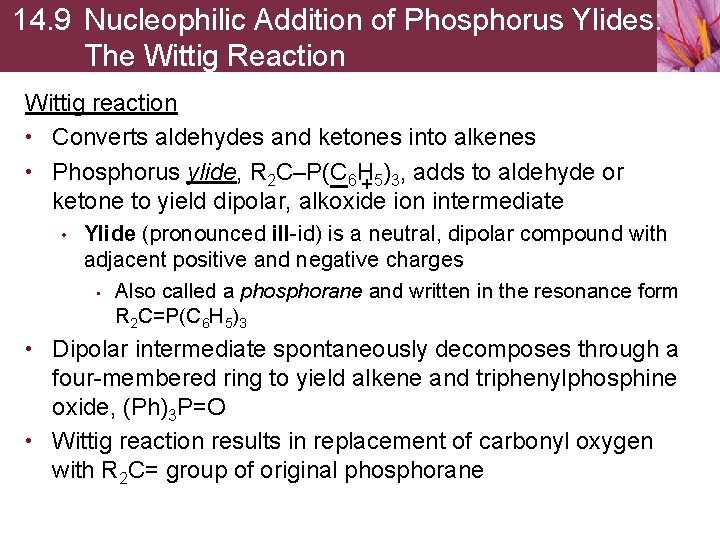
14. 9 Nucleophilic Addition of Phosphorus Ylides: The Wittig Reaction Wittig reaction • Converts aldehydes and ketones into alkenes • Phosphorus ylide, R 2 C–P(C 6 H ) , adds to aldehyde or +5 3 ketone to yield dipolar, alkoxide ion intermediate • Ylide (pronounced ill-id) is a neutral, dipolar compound with adjacent positive and negative charges • Also called a phosphorane and written in the resonance form R 2 C=P(C 6 H 5)3 • Dipolar intermediate spontaneously decomposes through a four-membered ring to yield alkene and triphenylphosphine oxide, (Ph)3 P=O • Wittig reaction results in replacement of carbonyl oxygen with R 2 C= group of original phosphorane

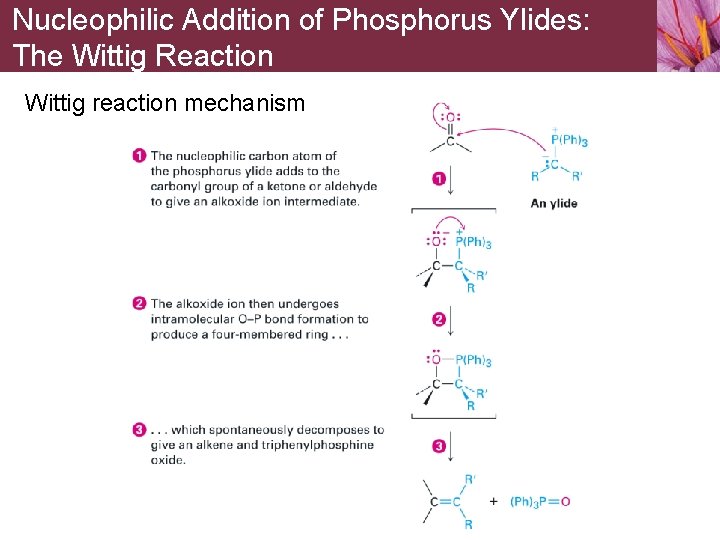
Nucleophilic Addition of Phosphorus Ylides: The Wittig Reaction Wittig reaction mechanism

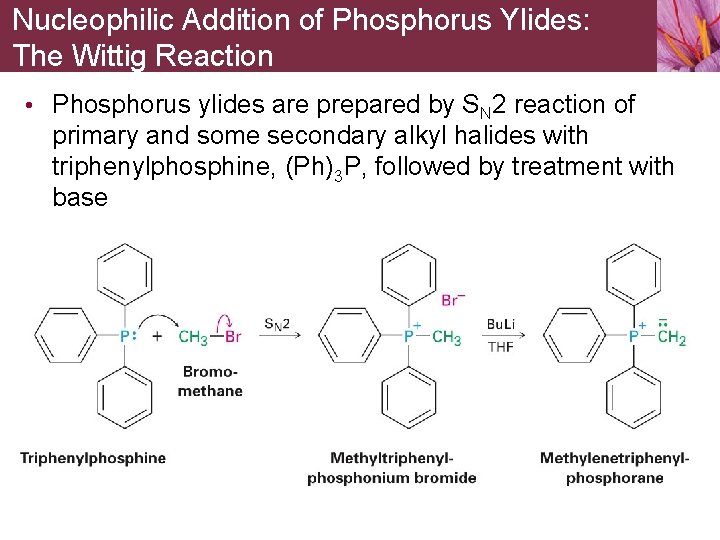
Nucleophilic Addition of Phosphorus Ylides: The Wittig Reaction • Phosphorus ylides are prepared by SN 2 reaction of primary and some secondary alkyl halides with triphenylphosphine, (Ph)3 P, followed by treatment with base

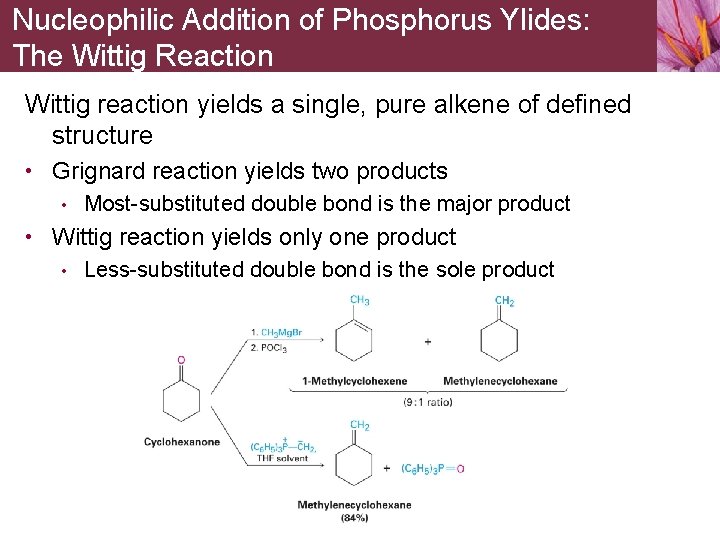
Nucleophilic Addition of Phosphorus Ylides: The Wittig Reaction Wittig reaction yields a single, pure alkene of defined structure • Grignard reaction yields two products • Most-substituted double bond is the major product • Wittig reaction yields only one product • Less-substituted double bond is the sole product

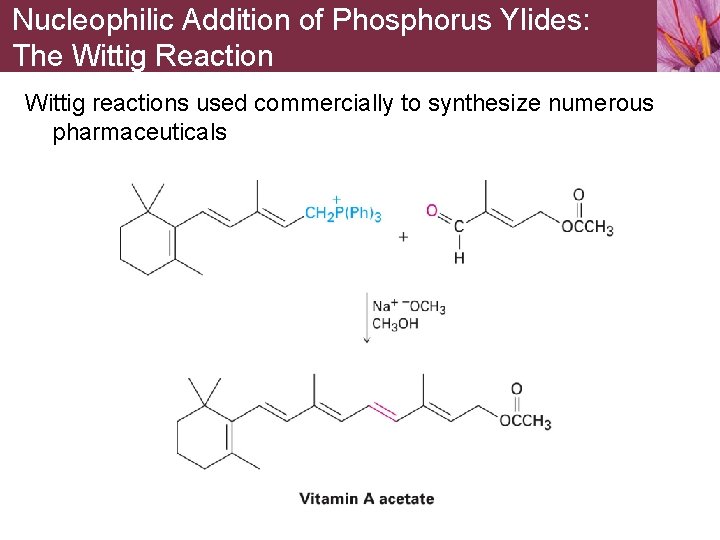
Nucleophilic Addition of Phosphorus Ylides: The Wittig Reaction Wittig reactions used commercially to synthesize numerous pharmaceuticals

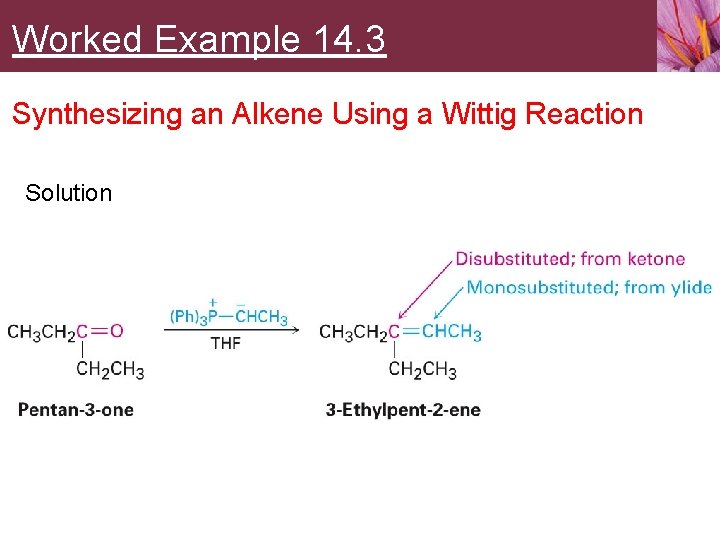
Worked Example 14. 3 Synthesizing an Alkene Using a Wittig Reaction What carbonyl compound and what phosphorus ylide might you use to prepare 3 -ethylpent-2 -ene?

Worked Example 14. 3 Synthesizing an Alkene Using a Wittig Reaction Strategy • An aldehyde or ketone reacts with a phosphorus ylide to yield an alkene in which the oxygen atom of the carbonyl reactant is replaced by the =CR 2 of the ylide • Preparation of the phosphorus ylide itself usually involves SN 2 reaction of a primary alkyl halide with triphenylphosphine, so the ylide is typically primary, RCH=P(Ph)3 • Disubstituted alkene carbon in product comes from carbonyl reactant, while the monosubstituted alkene carbon comes from the ylide

Worked Example 14. 3 Synthesizing an Alkene Using a Wittig Reaction Solution

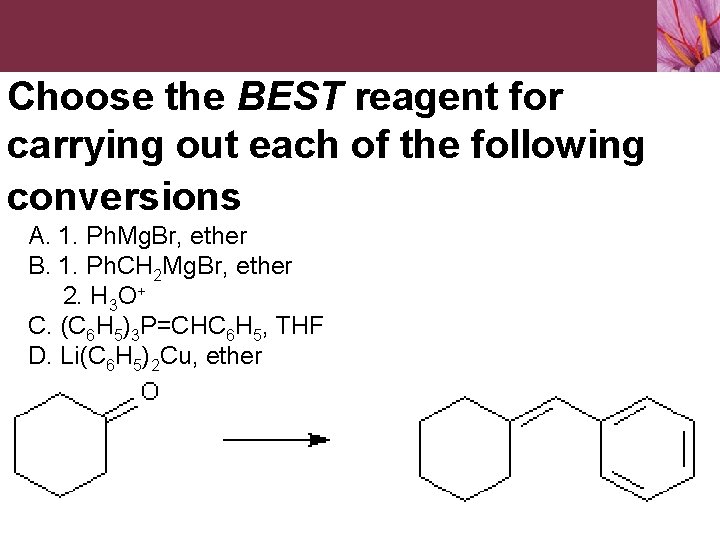
Choose the BEST reagent for carrying out each of the following conversions A. 1. Ph. Mg. Br, ether B. 1. Ph. CH 2 Mg. Br, ether 2. H 3 O+ C. (C 6 H 5)3 P=CHC 6 H 5, THF D. Li(C 6 H 5)2 Cu, ether

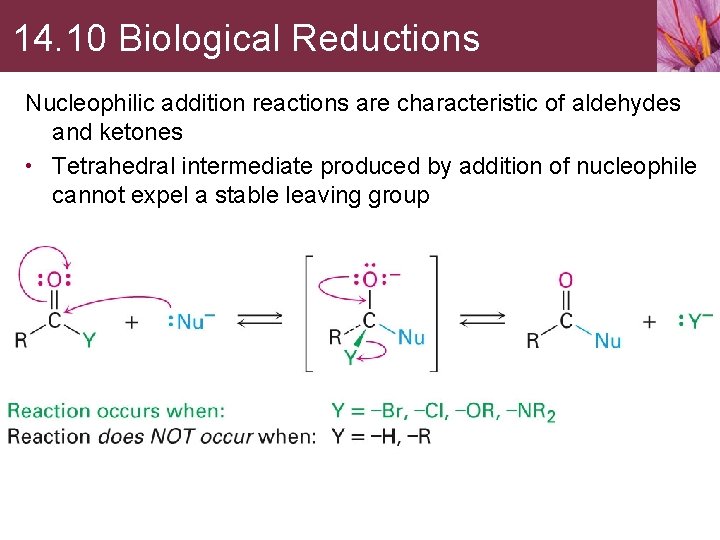
14. 10 Biological Reductions Nucleophilic addition reactions are characteristic of aldehydes and ketones • Tetrahedral intermediate produced by addition of nucleophile cannot expel a stable leaving group

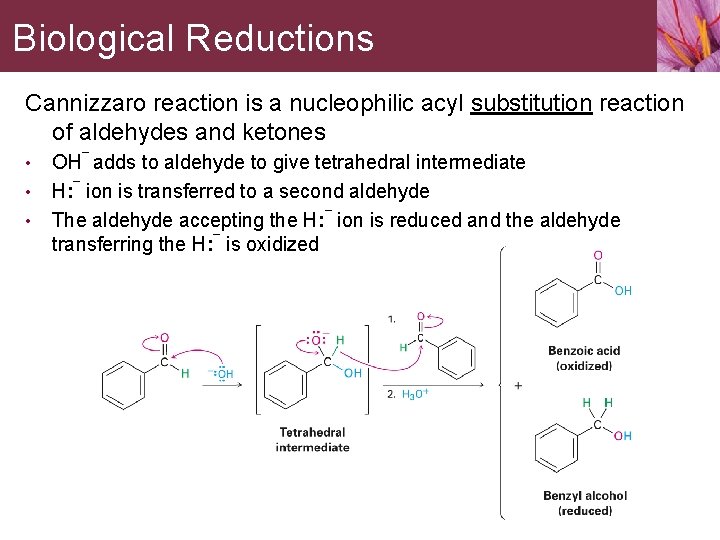
Biological Reductions Cannizzaro reaction is a nucleophilic acyl substitution reaction of aldehydes and ketones • • • OH¯ adds to aldehyde to give tetrahedral intermediate H: ¯ ion is transferred to a second aldehyde The aldehyde accepting the H: ¯ ion is reduced and the aldehyde transferring the H: ¯ is oxidized

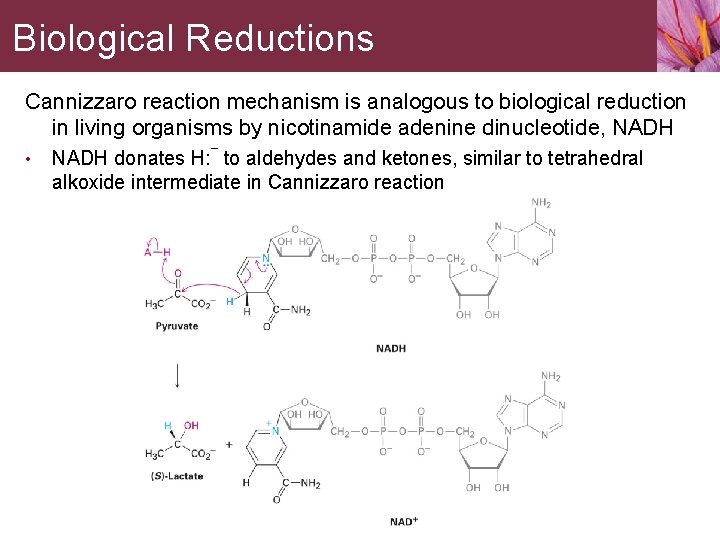
Biological Reductions Cannizzaro reaction mechanism is analogous to biological reduction in living organisms by nicotinamide adenine dinucleotide, NADH • NADH donates H: ¯ to aldehydes and ketones, similar to tetrahedral alkoxide intermediate in Cannizzaro reaction

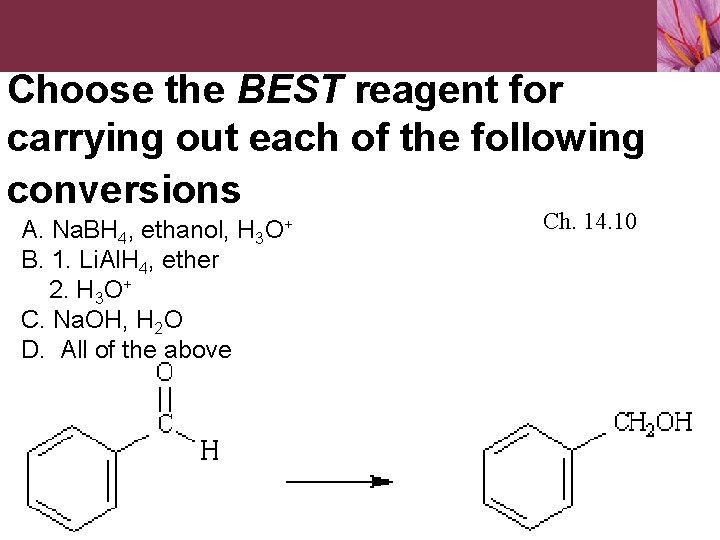
Choose the BEST reagent for carrying out each of the following conversions A. Na. BH 4, ethanol, H 3 O+ B. 1. Li. Al. H 4, ether 2. H 3 O+ C. Na. OH, H 2 O D. All of the above Ch. 14. 10

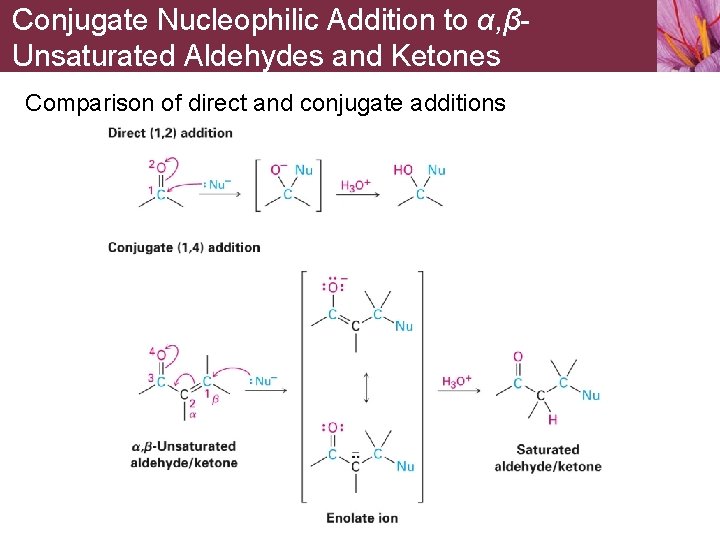
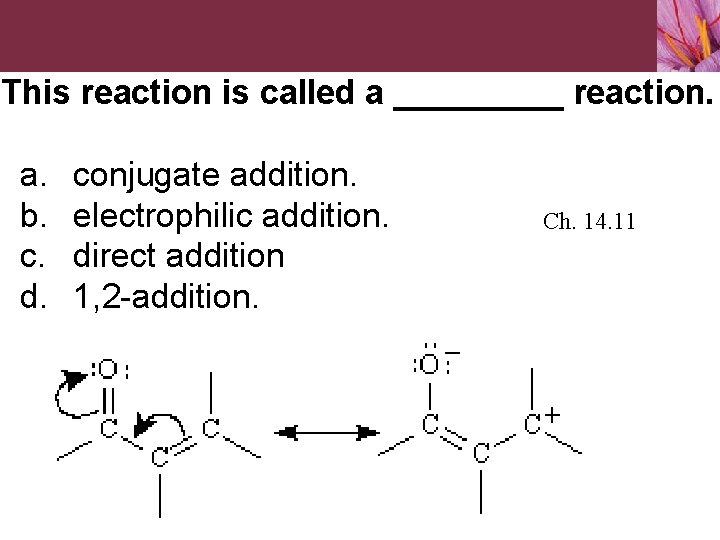
14. 11 Conjugate Nucleophilic Addition to α, βUnsaturated Aldehydes and Ketones Nucleophiles can add directly to carbonyl group of aldehydes and ketones, called 1, 2 -addition Nucleophiles can also add to conjugated C=C bond adjacent to carbonyl group of aldehydes and ketones, called conjugate addition or 1, 4 - addition • Carbon atom adjacent to carbonyl carbon is the a carbon atom and the next one is the b carbon atom • Initial product of conjugate addition is a resonance-stabilized enolate ion

Conjugate Nucleophilic Addition to α, βUnsaturated Aldehydes and Ketones Comparison of direct and conjugate additions

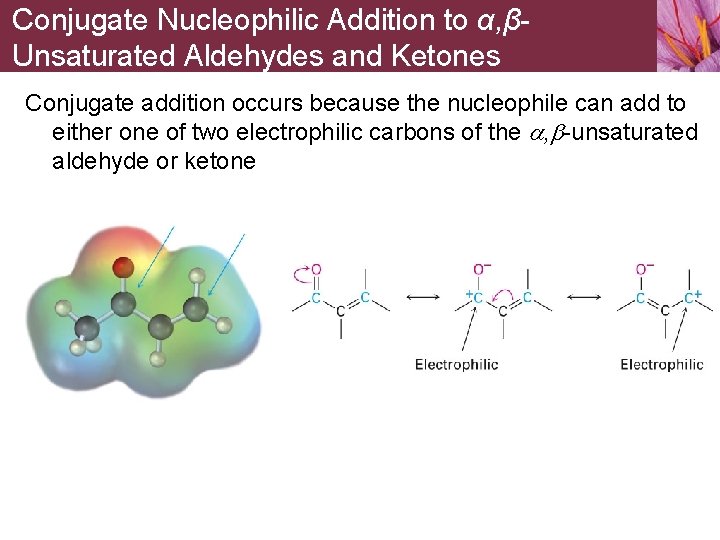
Conjugate Nucleophilic Addition to α, βUnsaturated Aldehydes and Ketones Conjugate addition occurs because the nucleophile can add to either one of two electrophilic carbons of the a, b-unsaturated aldehyde or ketone

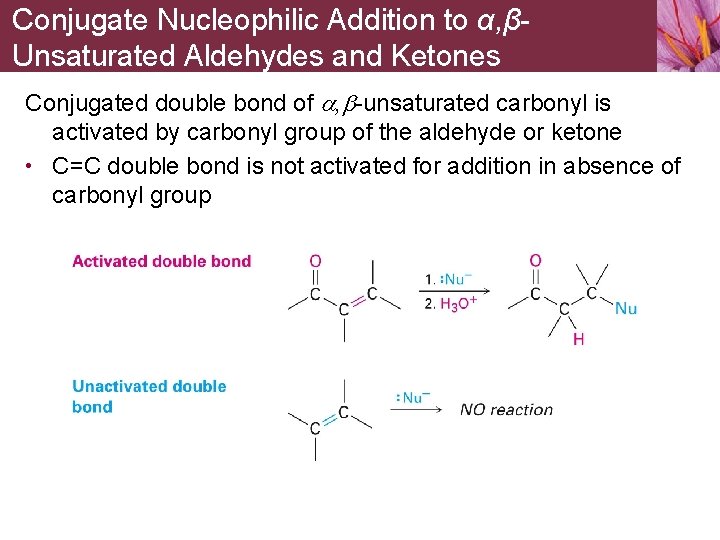
Conjugate Nucleophilic Addition to α, βUnsaturated Aldehydes and Ketones Conjugated double bond of a, b-unsaturated carbonyl is activated by carbonyl group of the aldehyde or ketone • C=C double bond is not activated for addition in absence of carbonyl group

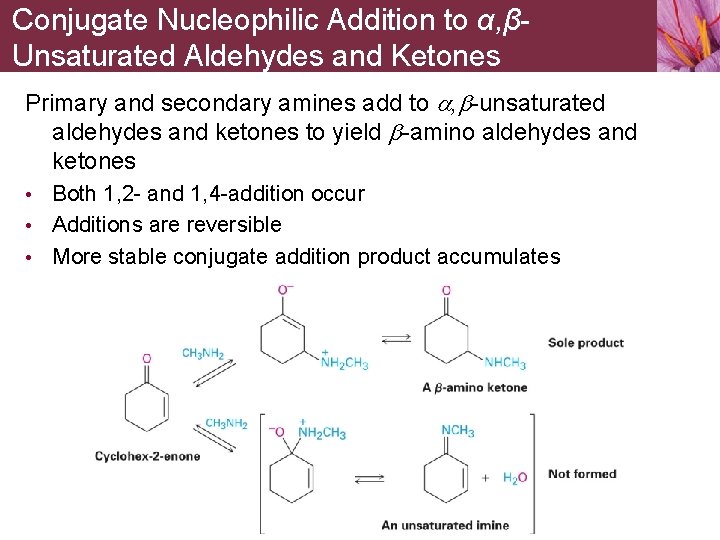
Conjugate Nucleophilic Addition to α, βUnsaturated Aldehydes and Ketones Primary and secondary amines add to a, b-unsaturated aldehydes and ketones to yield b-amino aldehydes and ketones • Both 1, 2 - and 1, 4 -addition occur • Additions are reversible • More stable conjugate addition product accumulates

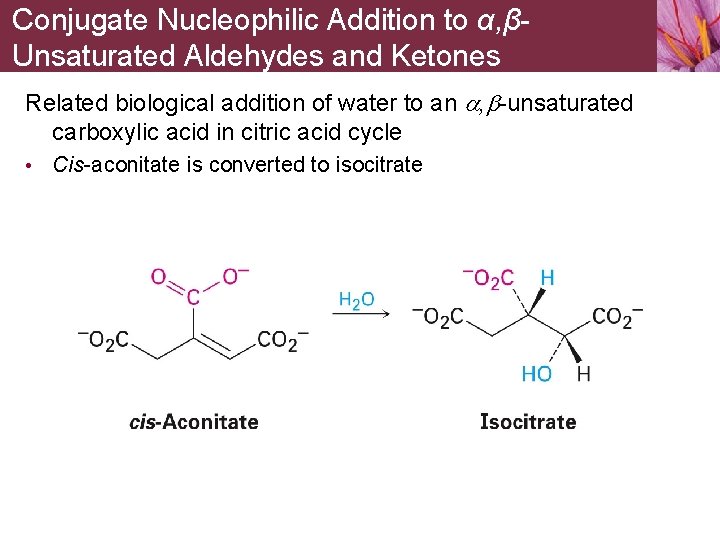
Conjugate Nucleophilic Addition to α, βUnsaturated Aldehydes and Ketones Related biological addition of water to an a, b-unsaturated carboxylic acid in citric acid cycle • Cis-aconitate is converted to isocitrate

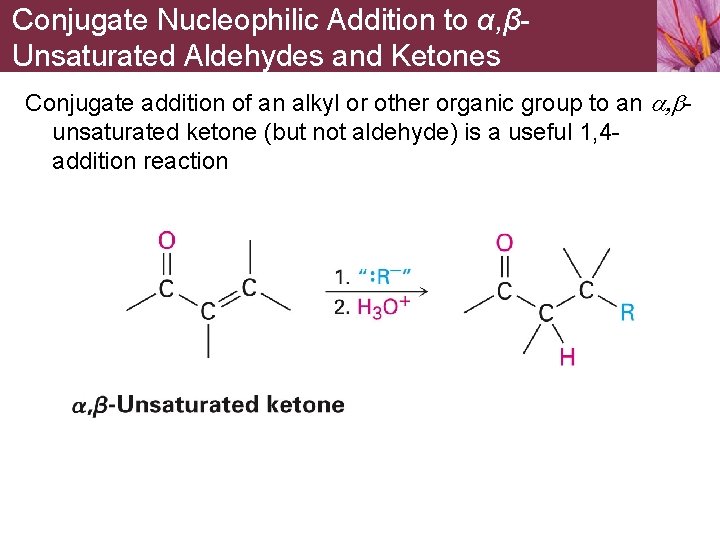
Conjugate Nucleophilic Addition to α, βUnsaturated Aldehydes and Ketones Conjugate addition of an alkyl or other organic group to an a, bunsaturated ketone (but not aldehyde) is a useful 1, 4 addition reaction

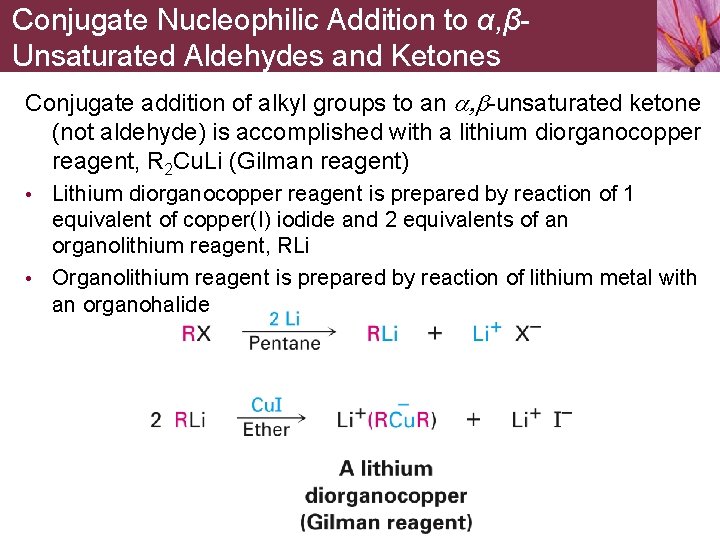
Conjugate Nucleophilic Addition to α, βUnsaturated Aldehydes and Ketones Conjugate addition of alkyl groups to an a, b-unsaturated ketone (not aldehyde) is accomplished with a lithium diorganocopper reagent, R 2 Cu. Li (Gilman reagent) • Lithium diorganocopper reagent is prepared by reaction of 1 equivalent of copper(I) iodide and 2 equivalents of an organolithium reagent, RLi • Organolithium reagent is prepared by reaction of lithium metal with an organohalide

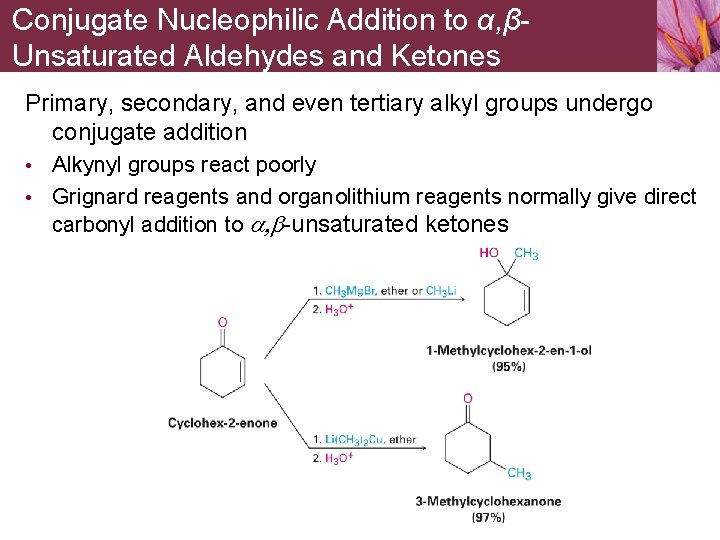
Conjugate Nucleophilic Addition to α, βUnsaturated Aldehydes and Ketones Primary, secondary, and even tertiary alkyl groups undergo conjugate addition • Alkynyl groups react poorly • Grignard reagents and organolithium reagents normally give direct carbonyl addition to a, b-unsaturated ketones

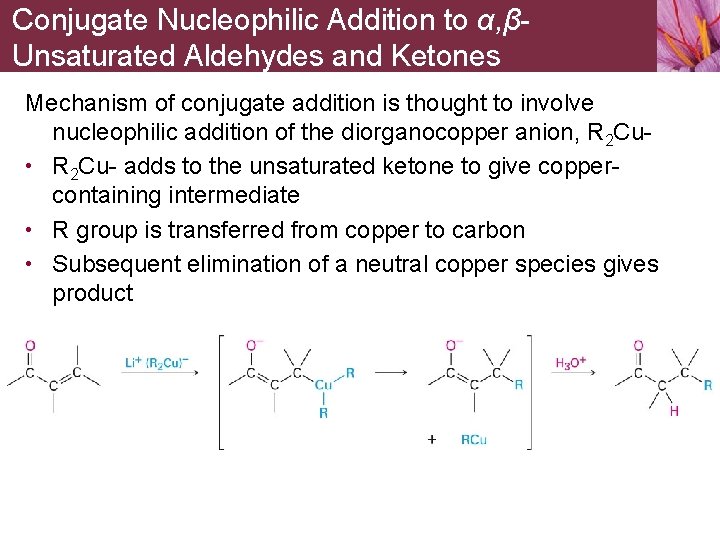
Conjugate Nucleophilic Addition to α, βUnsaturated Aldehydes and Ketones Mechanism of conjugate addition is thought to involve nucleophilic addition of the diorganocopper anion, R 2 Cu • R 2 Cu- adds to the unsaturated ketone to give coppercontaining intermediate • R group is transferred from copper to carbon • Subsequent elimination of a neutral copper species gives product

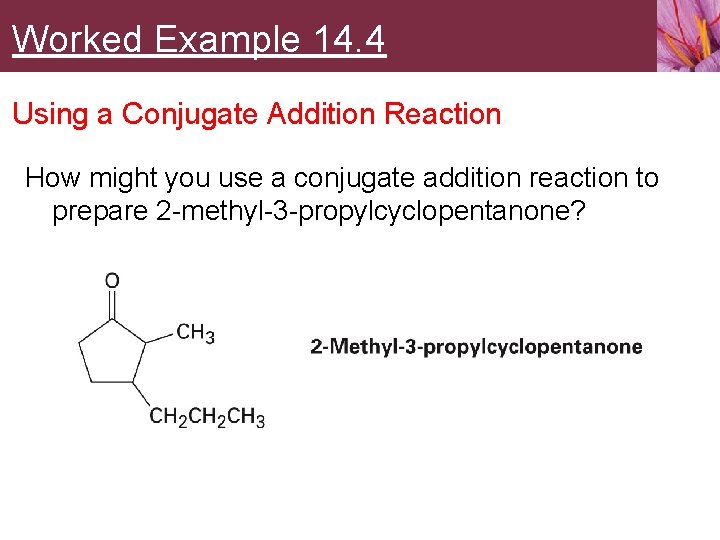
Worked Example 14. 4 Using a Conjugate Addition Reaction How might you use a conjugate addition reaction to prepare 2 -methyl-3 -propylcyclopentanone?

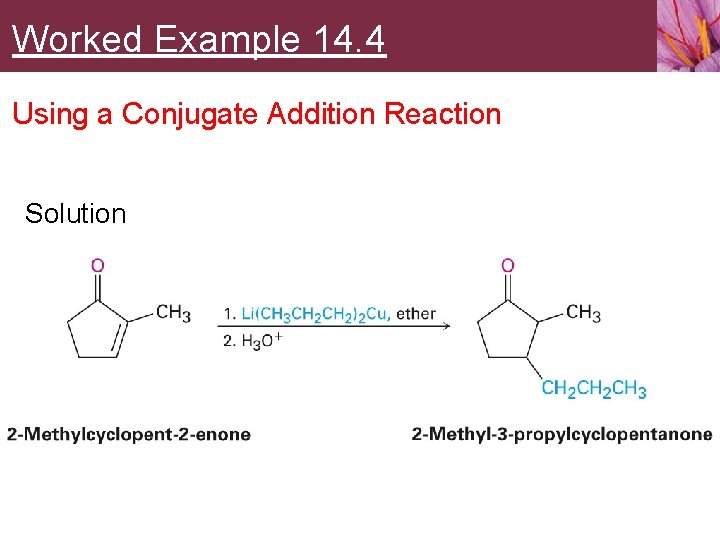
Worked Example 14. 4 Using a Conjugate Addition Reaction Strategy • • A ketone with a substituent group in its b position might be prepared by a conjugate addition of that group to an a, b-unsaturated ketone Propyl substituent on the b carbon might be prepared from 2 -methylcyclopenten-2 -one by reaction with lithium dipropylcopper

Worked Example 14. 4 Using a Conjugate Addition Reaction Solution

This reaction is called a _____ reaction. a. b. c. d. conjugate addition. electrophilic addition. direct addition 1, 2 -addition. Ch. 14. 11

Choose the BEST reagent for carrying out each of the following conversions A. Na. BH 4, ethanol B. 1. Li. Al. H 4, ether 2. H 3 O+ C. HCHO, Na. OH, H 2 O D. All of the above Ch. 14. 10

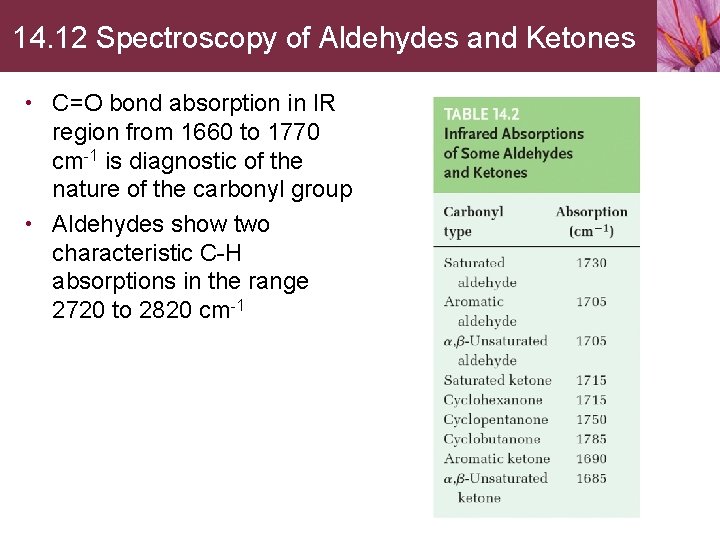
14. 12 Spectroscopy of Aldehydes and Ketones • C=O bond absorption in IR region from 1660 to 1770 cm-1 is diagnostic of the nature of the carbonyl group • Aldehydes show two characteristic C-H absorptions in the range 2720 to 2820 cm-1

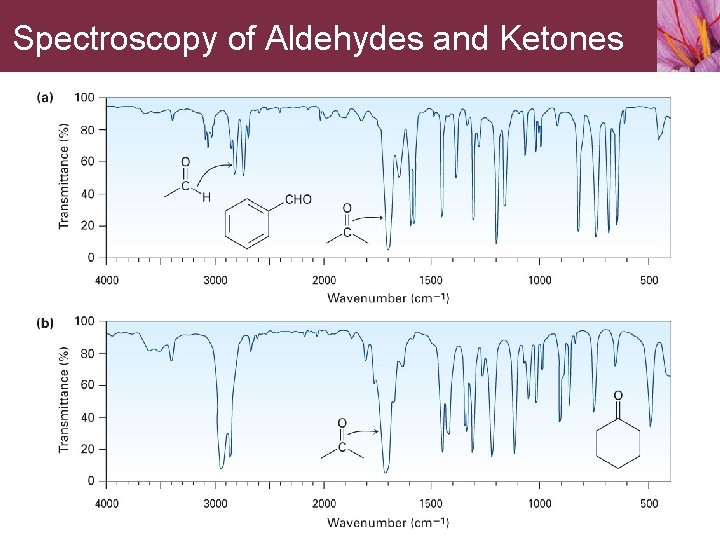
Spectroscopy of Aldehydes and Ketones

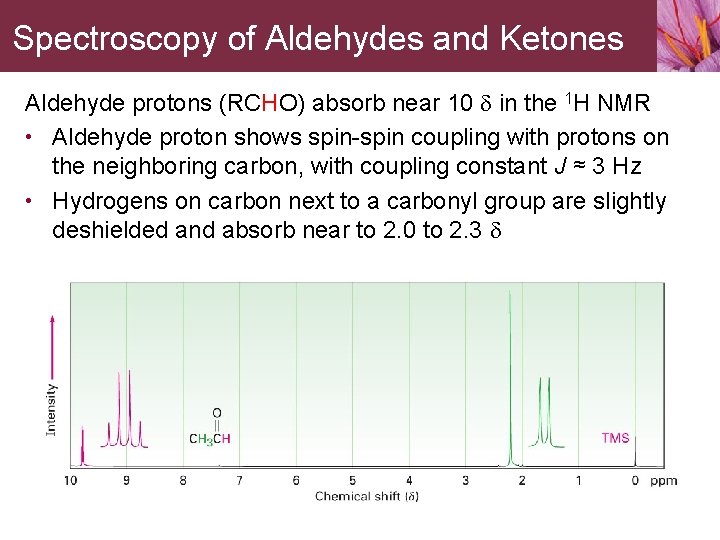
Spectroscopy of Aldehydes and Ketones Aldehyde protons (RCHO) absorb near 10 d in the 1 H NMR • Aldehyde proton shows spin-spin coupling with protons on the neighboring carbon, with coupling constant J ≈ 3 Hz • Hydrogens on carbon next to a carbonyl group are slightly deshielded and absorb near to 2. 0 to 2. 3 d

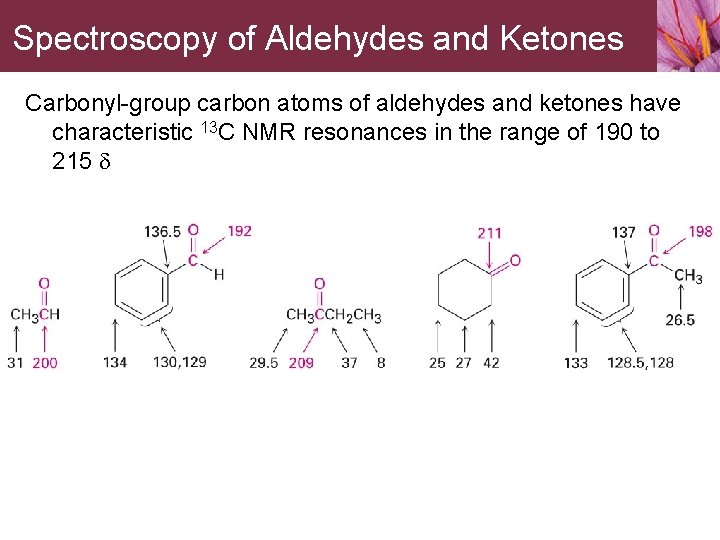
Spectroscopy of Aldehydes and Ketones Carbonyl-group carbon atoms of aldehydes and ketones have characteristic 13 C NMR resonances in the range of 190 to 215 d

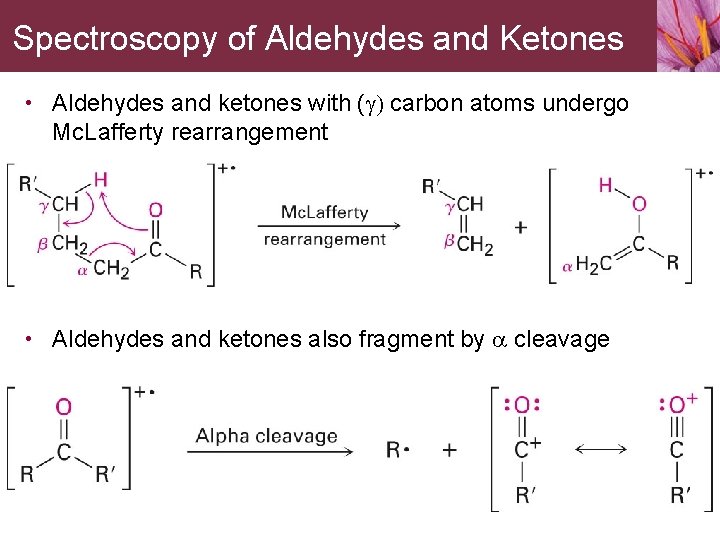
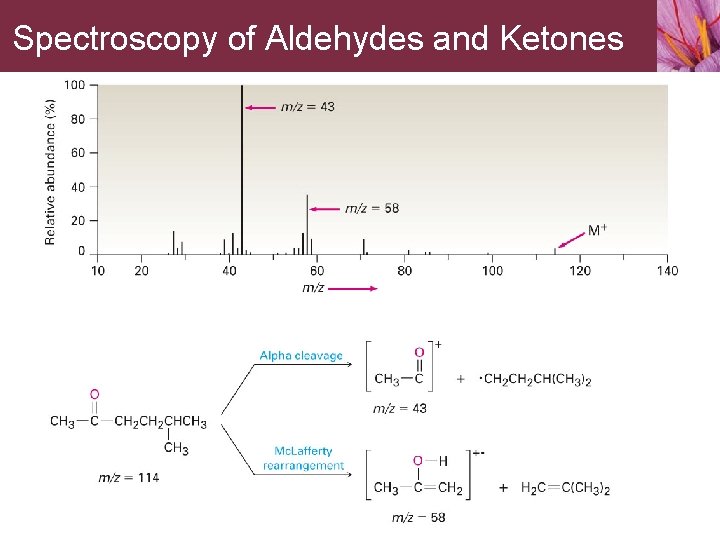
Spectroscopy of Aldehydes and Ketones • Aldehydes and ketones with (g) carbon atoms undergo Mc. Lafferty rearrangement • Aldehydes and ketones also fragment by a cleavage

Spectroscopy of Aldehydes and Ketones