John E Mc Murry http www cengage comchemistrymcmurry




- Slides: 42

John E. Mc. Murry http: //www. cengage. com/chemistry/mcmurry Chapter 15 Carboxylic Acids and Nitriles Richard Morrison • University of Georgia, Athens

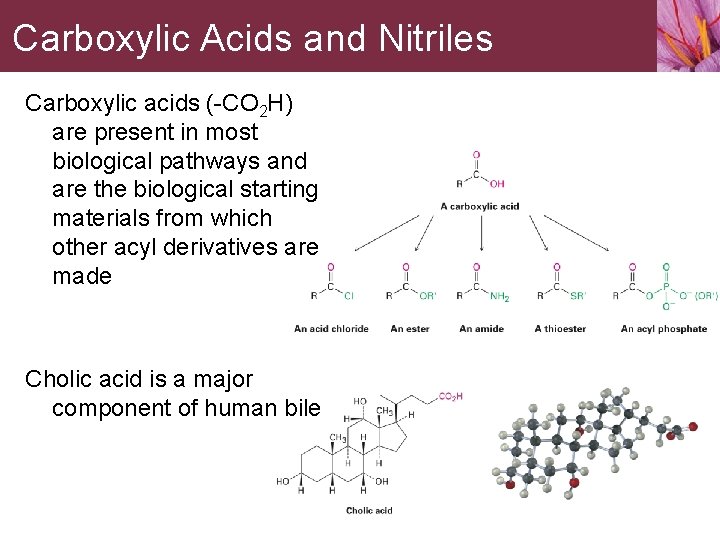
Carboxylic Acids and Nitriles Carboxylic acids (-CO 2 H) are present in most biological pathways and are the biological starting materials from which other acyl derivatives are made Cholic acid is a major component of human bile

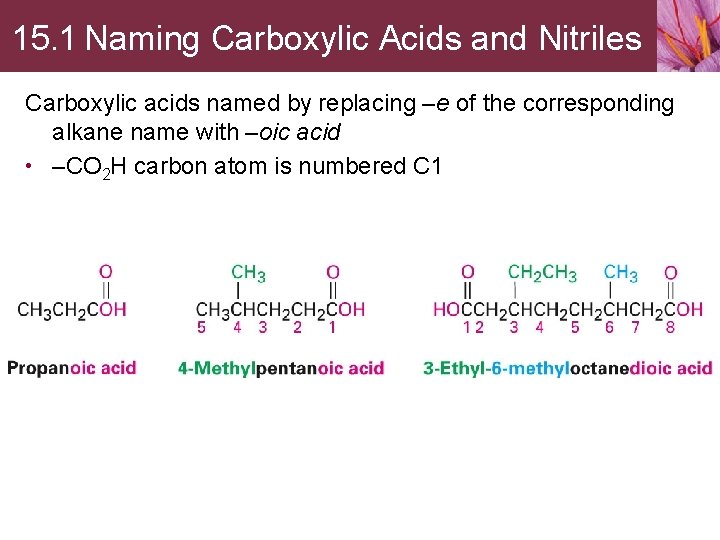
15. 1 Naming Carboxylic Acids and Nitriles Carboxylic acids named by replacing –e of the corresponding alkane name with –oic acid • –CO 2 H carbon atom is numbered C 1

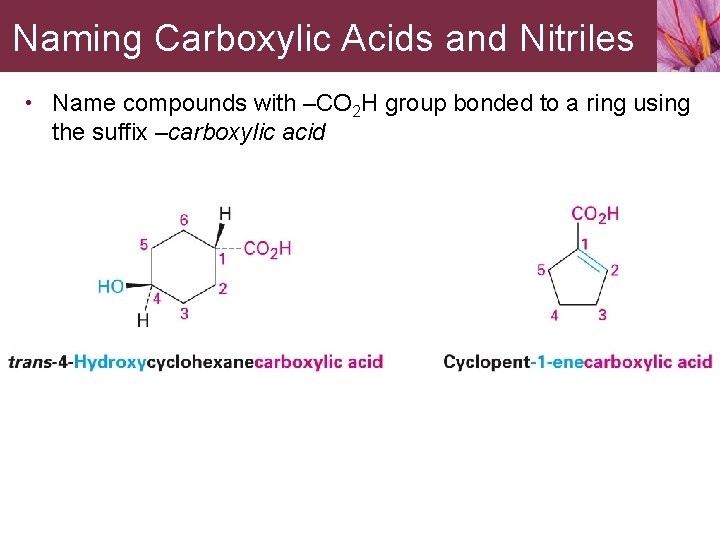
Naming Carboxylic Acids and Nitriles • Name compounds with –CO 2 H group bonded to a ring using the suffix –carboxylic acid

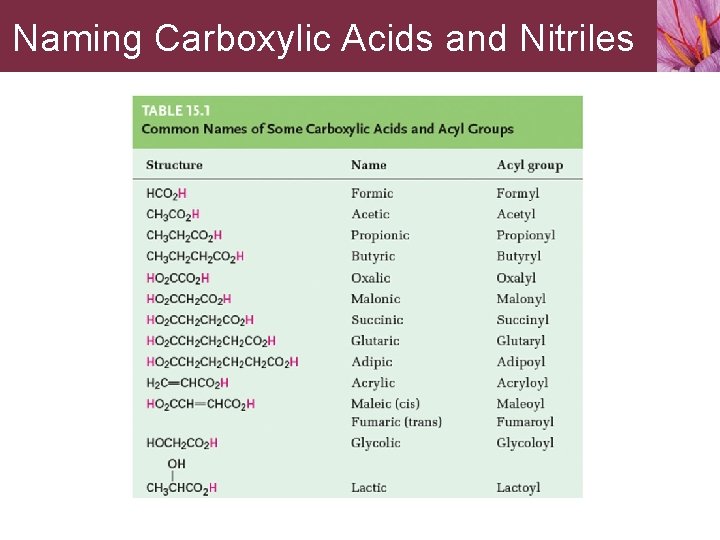
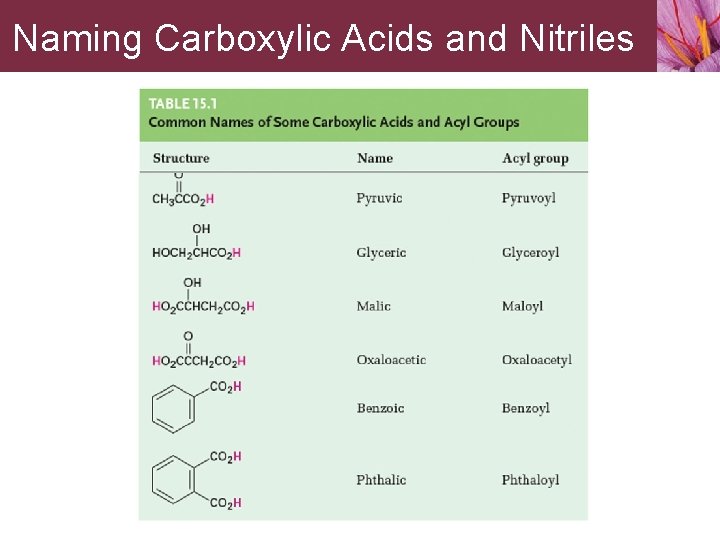
Naming Carboxylic Acids and Nitriles

Naming Carboxylic Acids and Nitriles

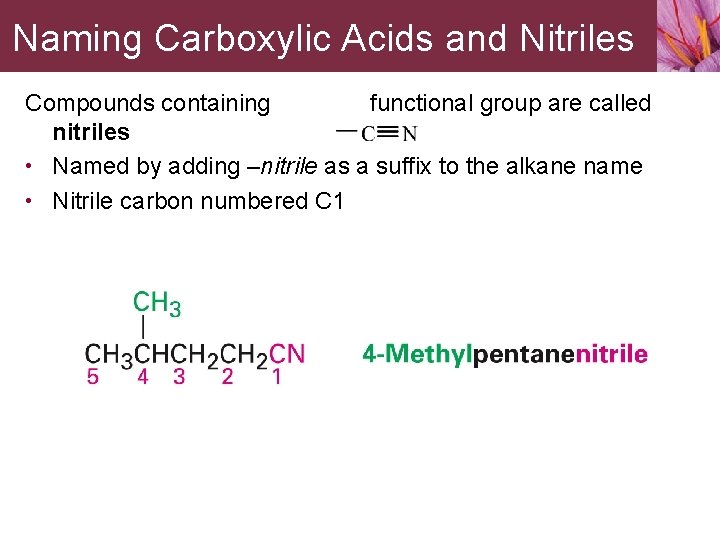
Naming Carboxylic Acids and Nitriles Compounds containing functional group are called nitriles • Named by adding –nitrile as a suffix to the alkane name • Nitrile carbon numbered C 1

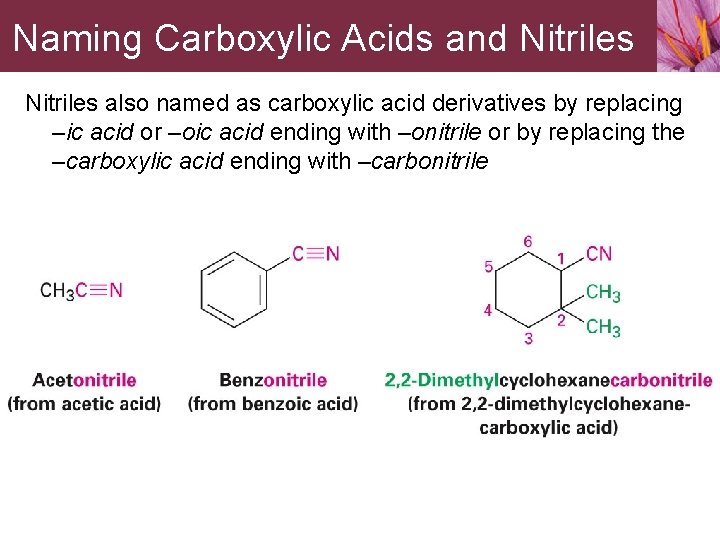
Naming Carboxylic Acids and Nitriles also named as carboxylic acid derivatives by replacing –ic acid or –oic acid ending with –onitrile or by replacing the –carboxylic acid ending with –carbonitrile

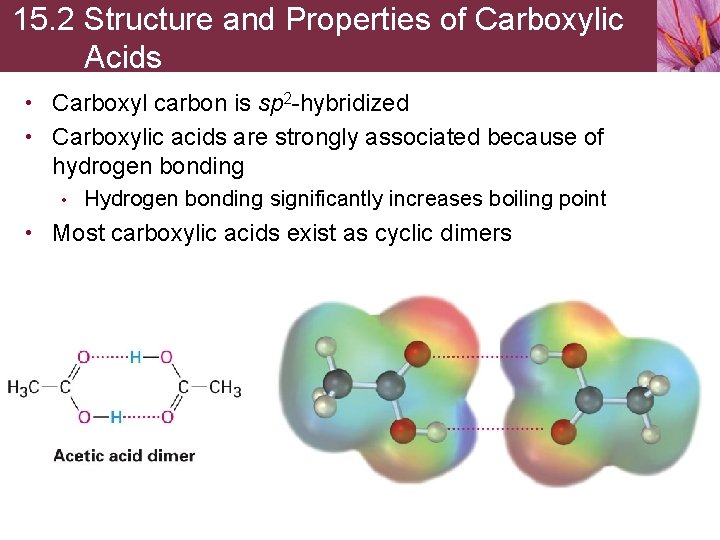
15. 2 Structure and Properties of Carboxylic Acids • Carboxyl carbon is sp 2 -hybridized • Carboxylic acids are strongly associated because of hydrogen bonding • Hydrogen bonding significantly increases boiling point • Most carboxylic acids exist as cyclic dimers

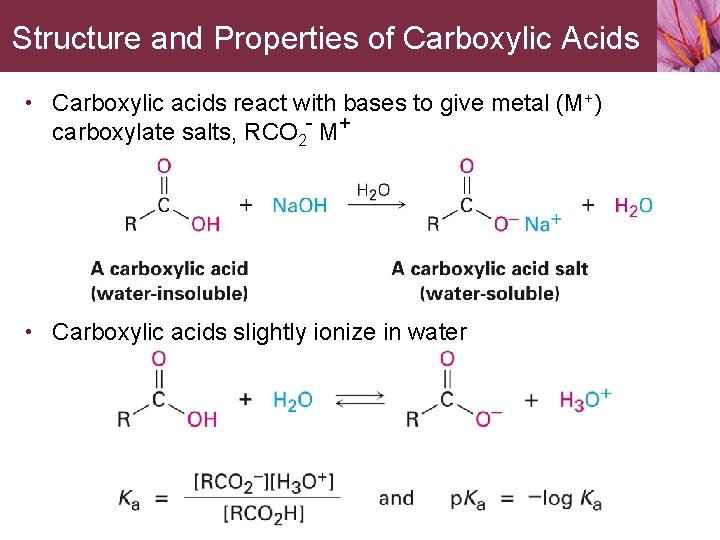
Structure and Properties of Carboxylic Acids • Carboxylic acids react with bases to give metal (M+) carboxylate salts, RCO - M+ 2 • Carboxylic acids slightly ionize in water

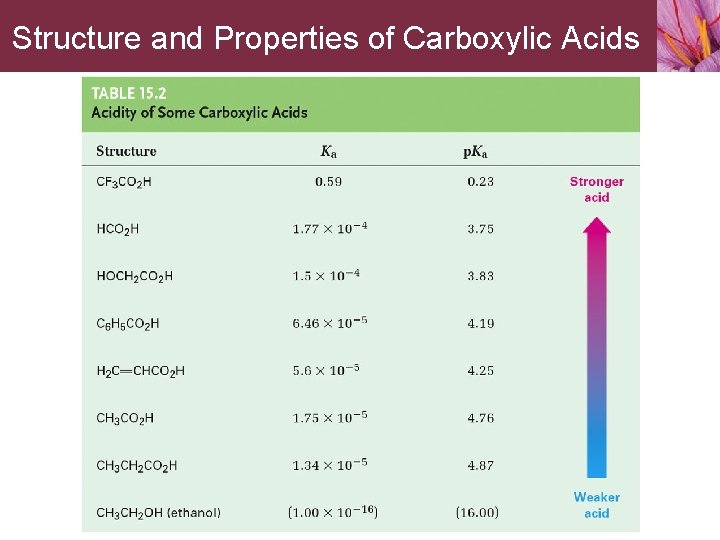
Structure and Properties of Carboxylic Acids

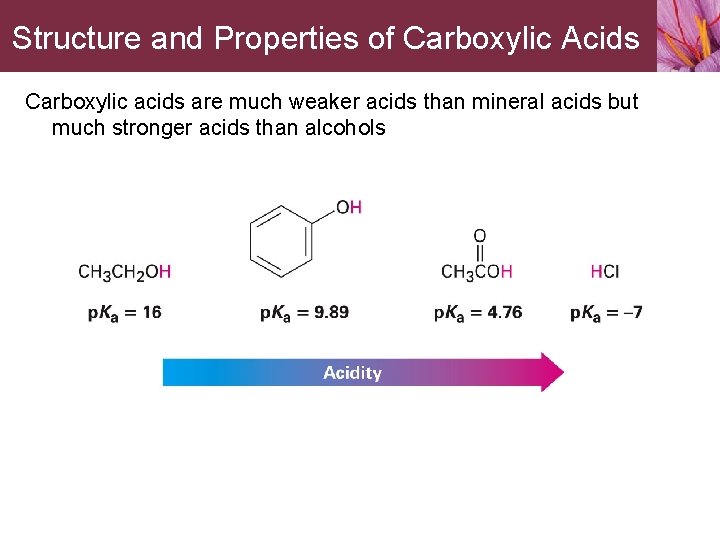
Structure and Properties of Carboxylic Acids Carboxylic acids are much weaker acids than mineral acids but much stronger acids than alcohols

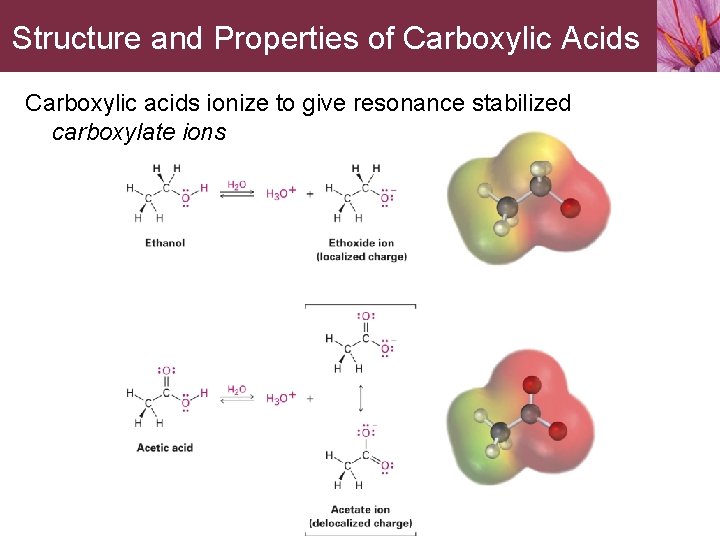
Structure and Properties of Carboxylic Acids Carboxylic acids ionize to give resonance stabilized carboxylate ions

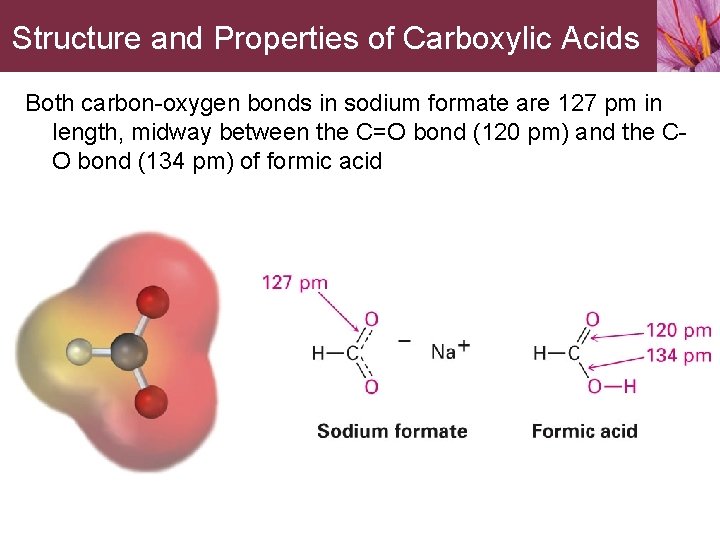
Structure and Properties of Carboxylic Acids Both carbon-oxygen bonds in sodium formate are 127 pm in length, midway between the C=O bond (120 pm) and the CO bond (134 pm) of formic acid

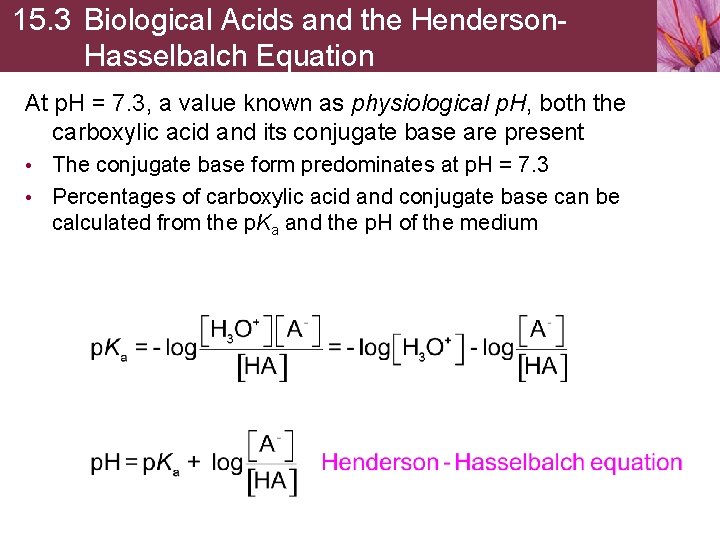
15. 3 Biological Acids and the Henderson. Hasselbalch Equation At p. H = 7. 3, a value known as physiological p. H, both the carboxylic acid and its conjugate base are present • The conjugate base form predominates at p. H = 7. 3 • Percentages of carboxylic acid and conjugate base can be calculated from the p. Ka and the p. H of the medium

Biological Acids and the Henderson-Hasselbalch Equation The percent dissociation of a 0. 0010 M solution of acetic acid (p. Ka = 4. 76) can be calculated from the Henderson-Hasselbalch equation

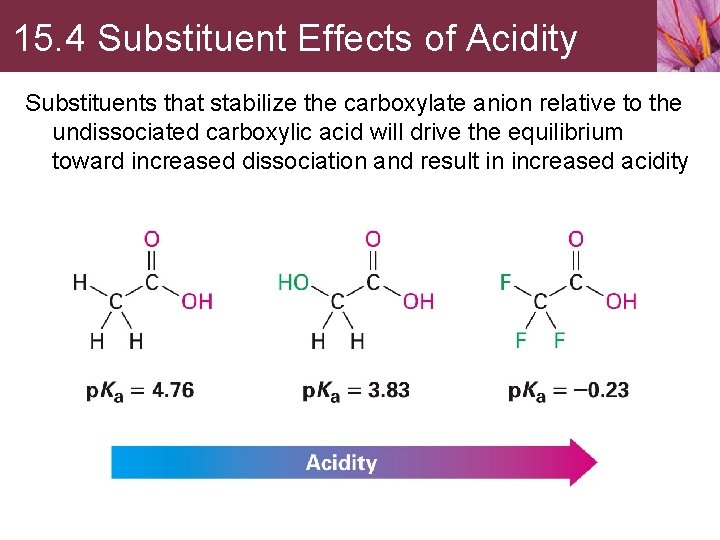
15. 4 Substituent Effects of Acidity Substituents that stabilize the carboxylate anion relative to the undissociated carboxylic acid will drive the equilibrium toward increased dissociation and result in increased acidity

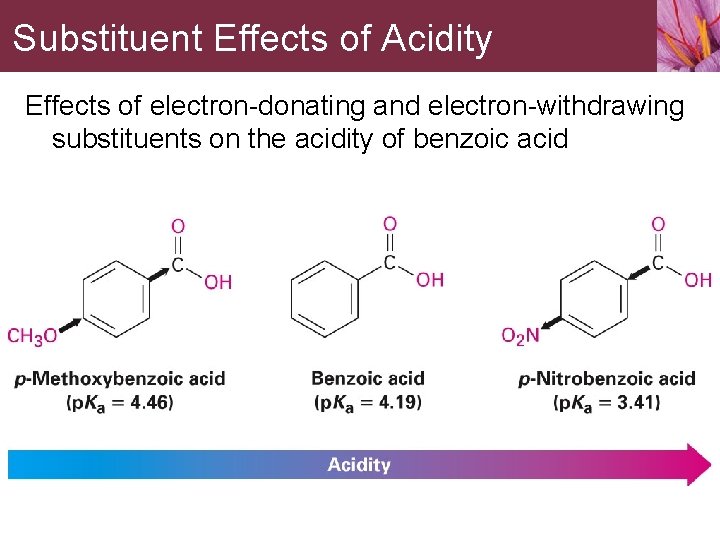
Substituent Effects of Acidity Effects of electron-donating and electron-withdrawing substituents on the acidity of benzoic acid

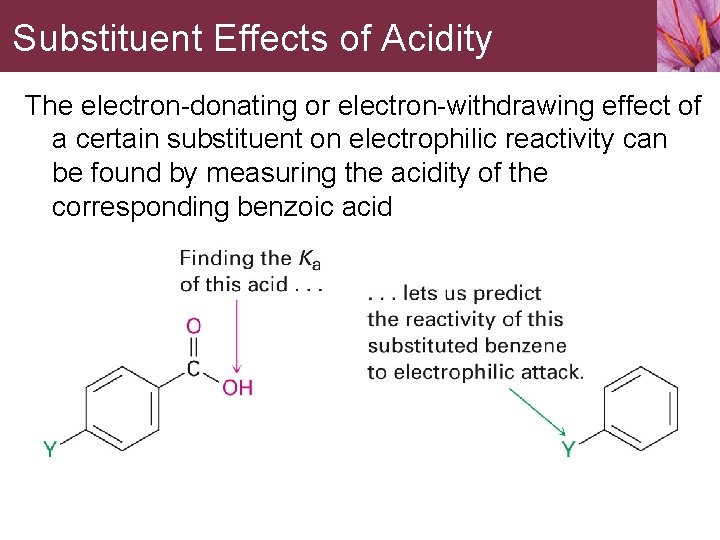
Substituent Effects of Acidity The electron-donating or electron-withdrawing effect of a certain substituent on electrophilic reactivity can be found by measuring the acidity of the corresponding benzoic acid

Worked Example 15. 1 Predicting the Effect of a Substituent on the Reactivity of an Aromatic Ring toward Electrophilic Substitution The p. Ka of p-(trifluoromethyl)benzoic acid is 3. 6. Is the trifluoromethyl substituent an activating or deactivating group in electrophilic aromatic substitution?

Worked Example 15. 1 Predicting the Effect of a Substituent on the Reactivity of an Aromatic Ring toward Electrophilic Substitution Strategy • Decide whether p-(trifluoromethyl)benzoic acid is stronger or weaker than benzoic acid. A substituent that strengthens the acid is a deactivating group because it withdraws electrons, and a substituent that weakens the acid is an activating group because it donates electrons.

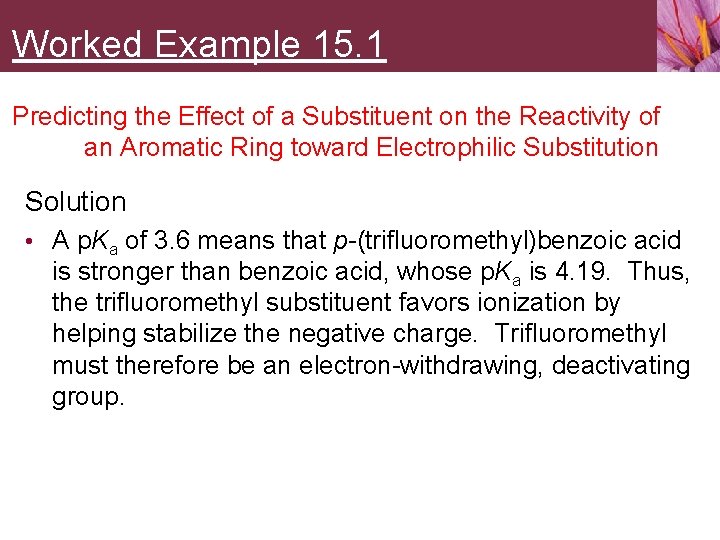
Worked Example 15. 1 Predicting the Effect of a Substituent on the Reactivity of an Aromatic Ring toward Electrophilic Substitution Solution • A p. Ka of 3. 6 means that p-(trifluoromethyl)benzoic acid is stronger than benzoic acid, whose p. Ka is 4. 19. Thus, the trifluoromethyl substituent favors ionization by helping stabilize the negative charge. Trifluoromethyl must therefore be an electron-withdrawing, deactivating group.

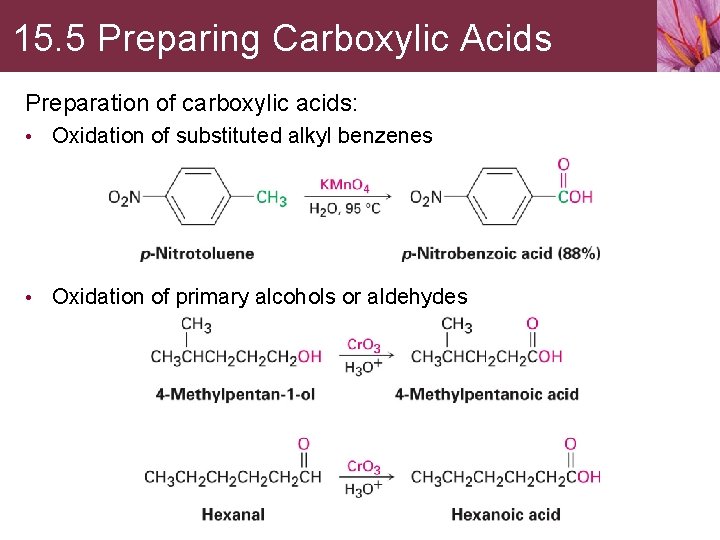
15. 5 Preparing Carboxylic Acids Preparation of carboxylic acids: • Oxidation of substituted alkyl benzenes • Oxidation of primary alcohols or aldehydes

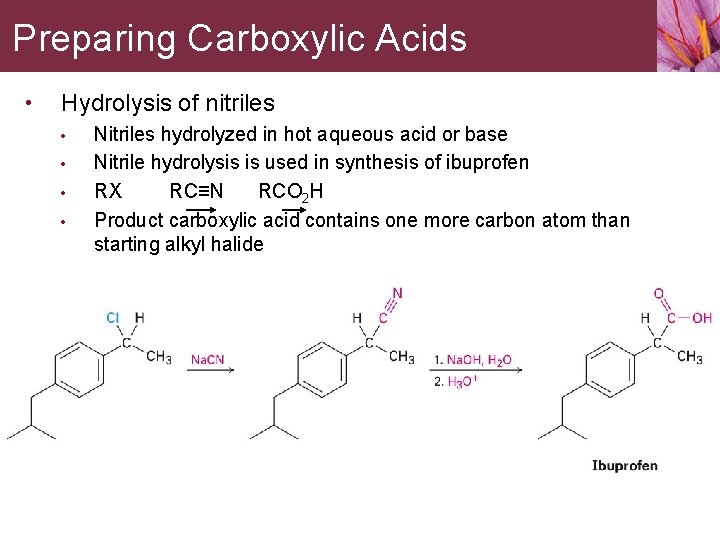
Preparing Carboxylic Acids • Hydrolysis of nitriles • • Nitriles hydrolyzed in hot aqueous acid or base Nitrile hydrolysis is used in synthesis of ibuprofen RX RC≡N RCO 2 H Product carboxylic acid contains one more carbon atom than starting alkyl halide

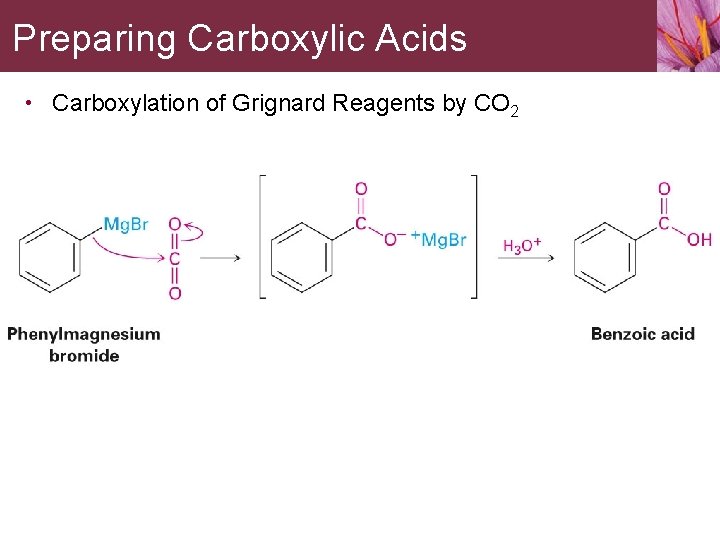
Preparing Carboxylic Acids • Carboxylation of Grignard Reagents by CO 2

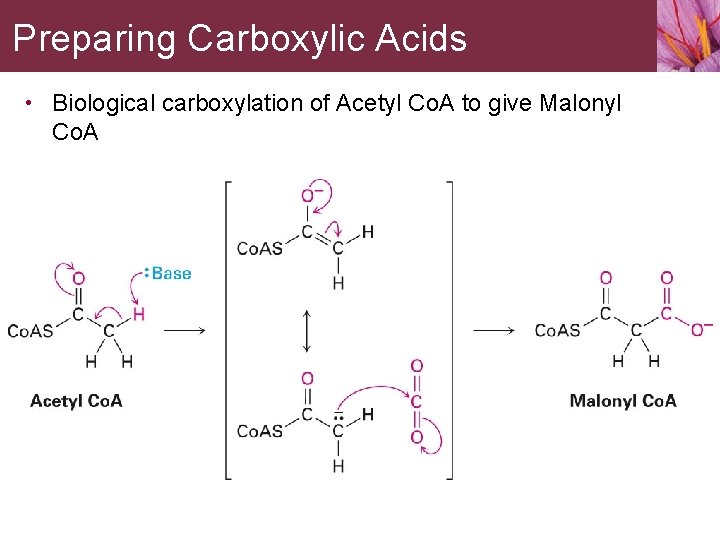
Preparing Carboxylic Acids • Biological carboxylation of Acetyl Co. A to give Malonyl Co. A

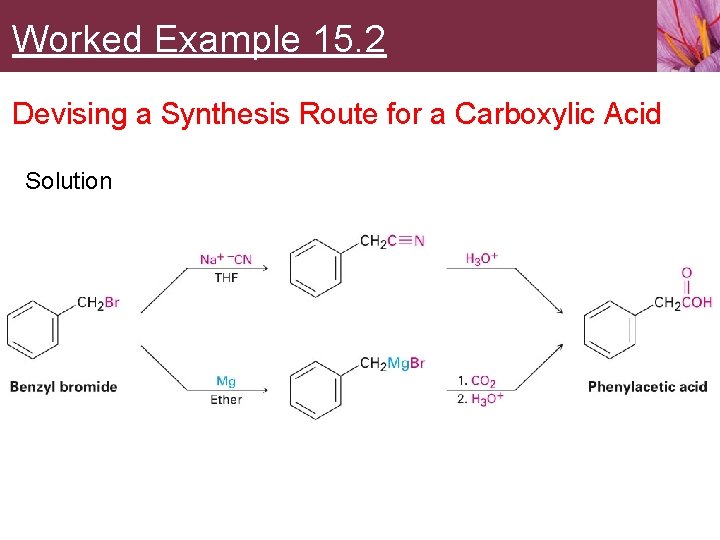
Worked Example 15. 2 Devising a Synthesis Route for a Carboxylic Acid How would you prepare phenylacetic acid (Ph. CH 2 CO 2 H) from benzyl bromide (Ph. CH 2 Br)?

Worked Example 15. 2 Devising a Synthesis Route for a Carboxylic Acid Strategy We’ve seen two methods for preparing carboxylic acids from alkyl halides: 1. 2. Cyanide ion displacement followed by hydrolysis, and Formation of a Grignard reagent followed by carboxylation. The first method involves an SN 2 reaction and is therefore limited to use with primary and some secondary alkyl halides. The second method involves formation of a Grignard reagent and is therefore limited to use with organic halides that have no acidic hydrogens or reactive functional groups. In the present instance, either method would work well.

Worked Example 15. 2 Devising a Synthesis Route for a Carboxylic Acid Solution

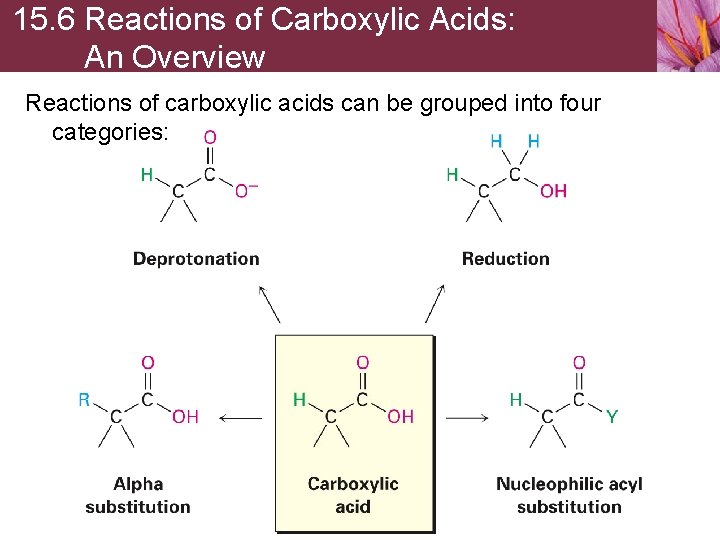
15. 6 Reactions of Carboxylic Acids: An Overview Reactions of carboxylic acids can be grouped into four categories:

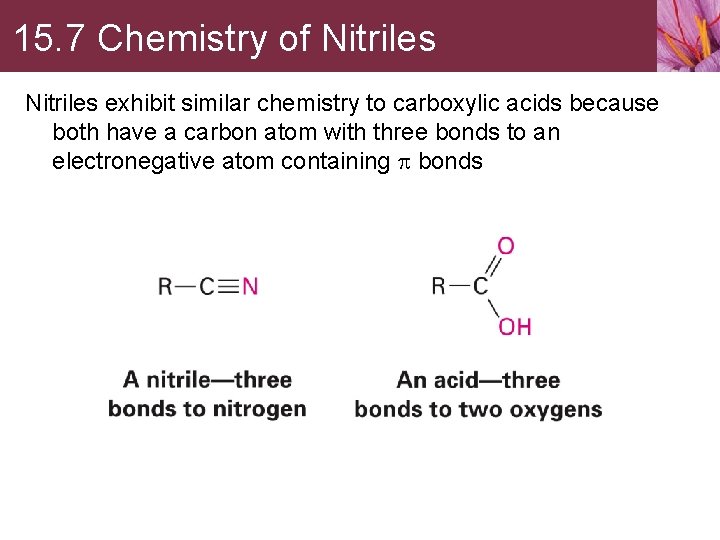
15. 7 Chemistry of Nitriles exhibit similar chemistry to carboxylic acids because both have a carbon atom with three bonds to an electronegative atom containing p bonds

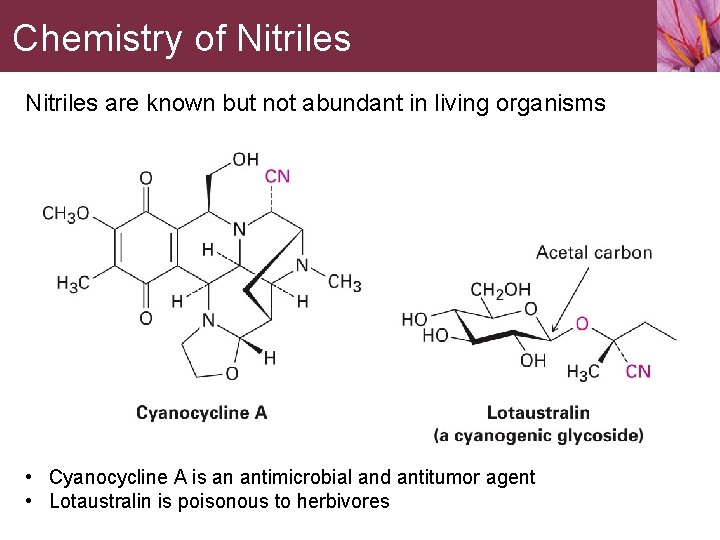
Chemistry of Nitriles are known but not abundant in living organisms • Cyanocycline A is an antimicrobial and antitumor agent • Lotaustralin is poisonous to herbivores

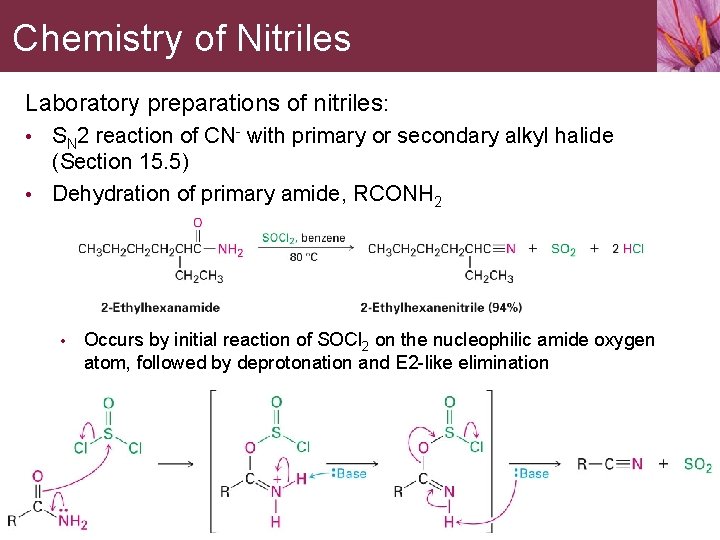
Chemistry of Nitriles Laboratory preparations of nitriles: • SN 2 reaction of CN- with primary or secondary alkyl halide (Section 15. 5) • Dehydration of primary amide, RCONH 2 • Occurs by initial reaction of SOCl 2 on the nucleophilic amide oxygen atom, followed by deprotonation and E 2 -like elimination

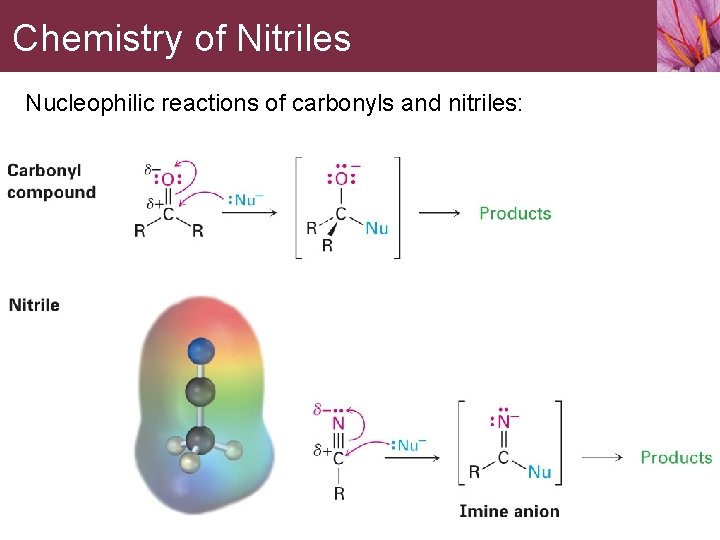
Chemistry of Nitriles Nucleophilic reactions of carbonyls and nitriles:

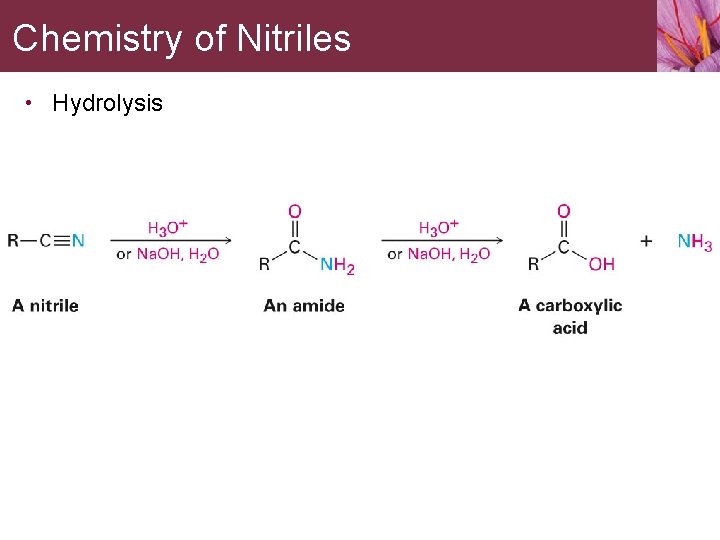
Chemistry of Nitriles • Hydrolysis

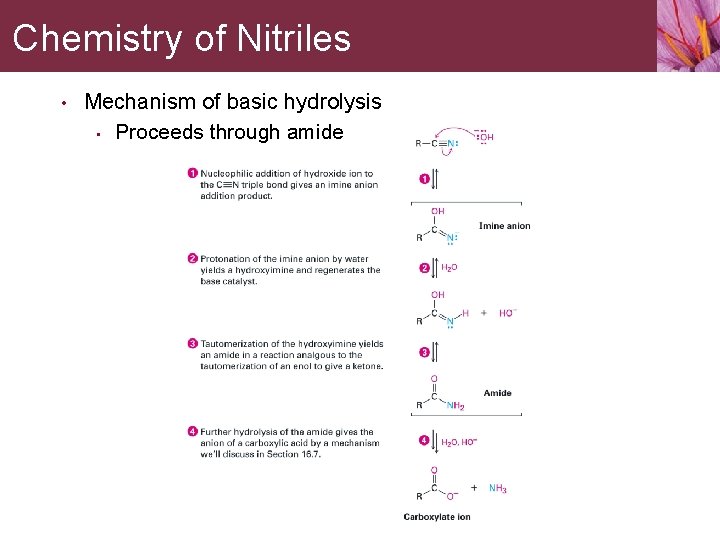
Chemistry of Nitriles • Mechanism of basic hydrolysis • Proceeds through amide

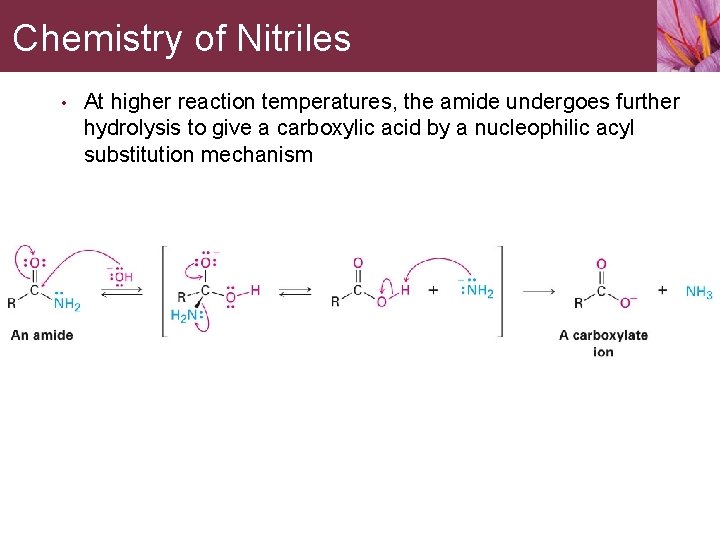
Chemistry of Nitriles • At higher reaction temperatures, the amide undergoes further hydrolysis to give a carboxylic acid by a nucleophilic acyl substitution mechanism

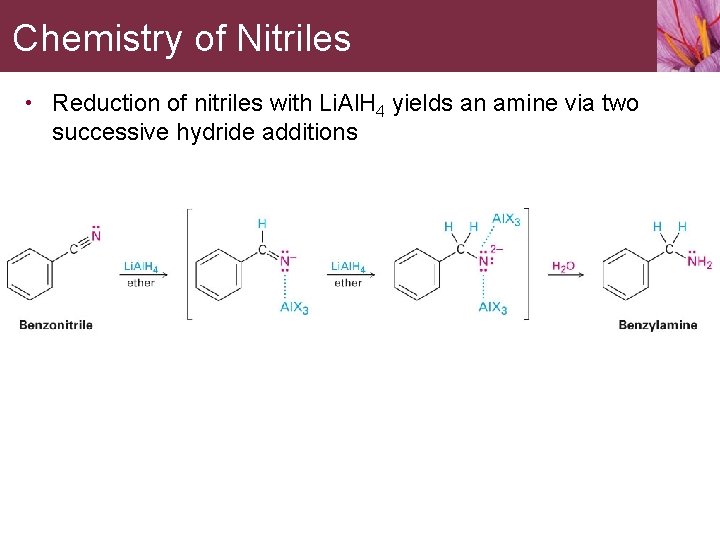
Chemistry of Nitriles • Reduction of nitriles with Li. Al. H 4 yields an amine via two successive hydride additions

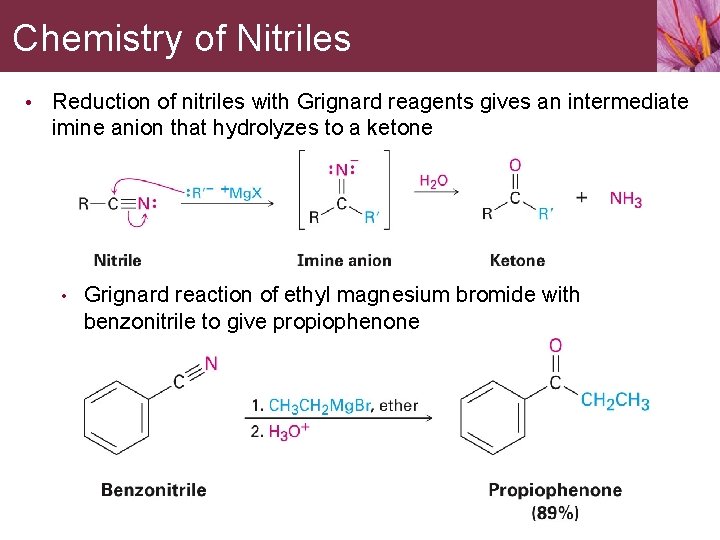
Chemistry of Nitriles • Reduction of nitriles with Grignard reagents gives an intermediate imine anion that hydrolyzes to a ketone • Grignard reaction of ethyl magnesium bromide with benzonitrile to give propiophenone

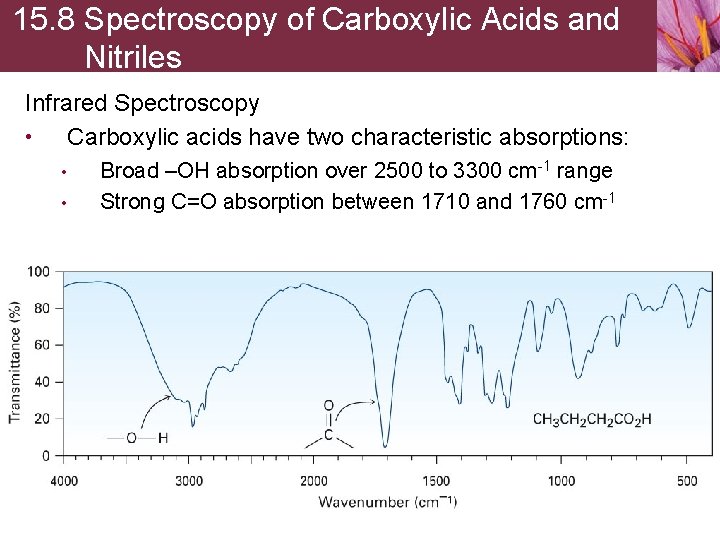
15. 8 Spectroscopy of Carboxylic Acids and Nitriles Infrared Spectroscopy • Carboxylic acids have two characteristic absorptions: • • Broad –OH absorption over 2500 to 3300 cm-1 range Strong C=O absorption between 1710 and 1760 cm-1

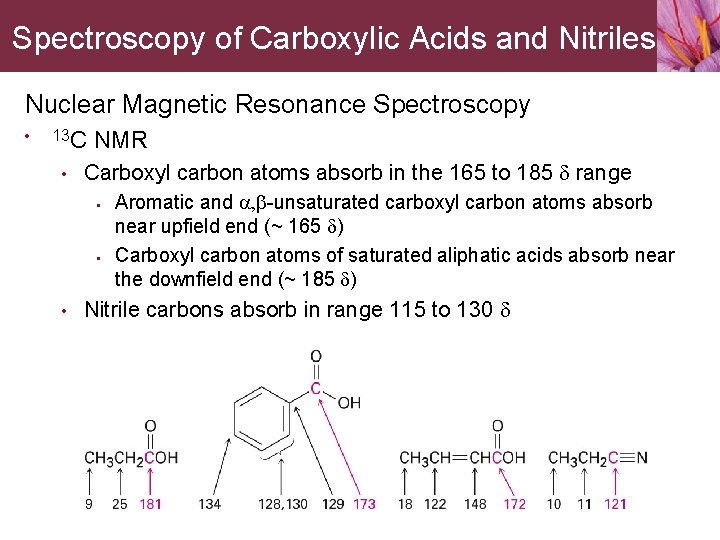
Spectroscopy of Carboxylic Acids and Nitriles Nuclear Magnetic Resonance Spectroscopy • 13 C • NMR Carboxyl carbon atoms absorb in the 165 to 185 d range • • • Aromatic and a, b-unsaturated carboxyl carbon atoms absorb near upfield end (~ 165 d) Carboxyl carbon atoms of saturated aliphatic acids absorb near the downfield end (~ 185 d) Nitrile carbons absorb in range 115 to 130 d

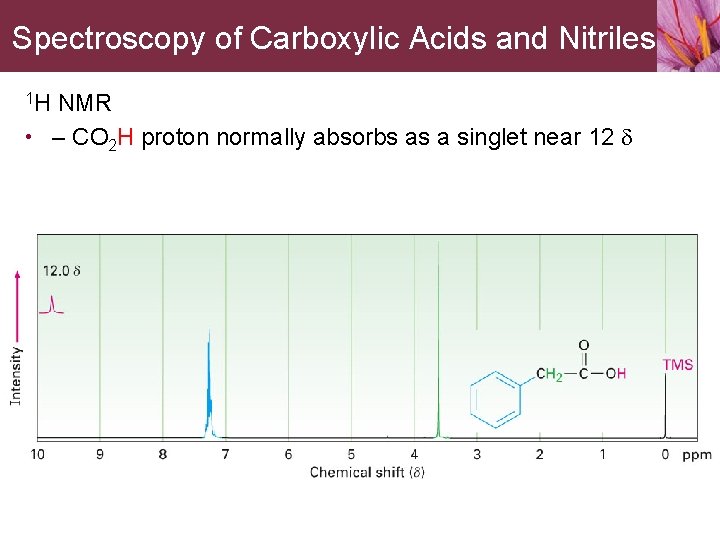
Spectroscopy of Carboxylic Acids and Nitriles 1 H NMR • – CO 2 H proton normally absorbs as a singlet near 12 d