Islamic University in Madinah Department of Chemistry Nuclear







- Slides: 52

Islamic University in Madinah Department of Chemistry Nuclear Magnetic Resonance Spectroscopy Part-3 Prepared By Dr. Khalid Ahmad Shadid 13 -1

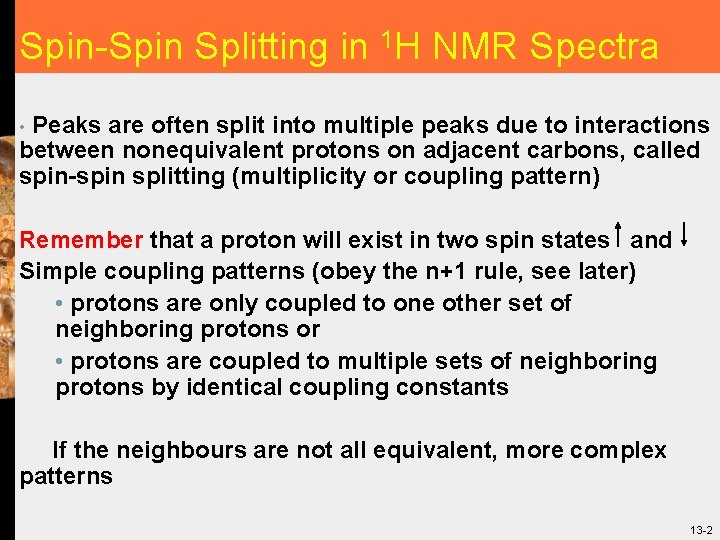
Spin-Spin Splitting in 1 H NMR Spectra Peaks are often split into multiple peaks due to interactions between nonequivalent protons on adjacent carbons, called spin-spin splitting (multiplicity or coupling pattern) • Remember that a proton will exist in two spin states and Simple coupling patterns (obey the n+1 rule, see later) • protons are only coupled to one other set of neighboring protons or • protons are coupled to multiple sets of neighboring protons by identical coupling constants If the neighbours are not all equivalent, more complex patterns 13 -2

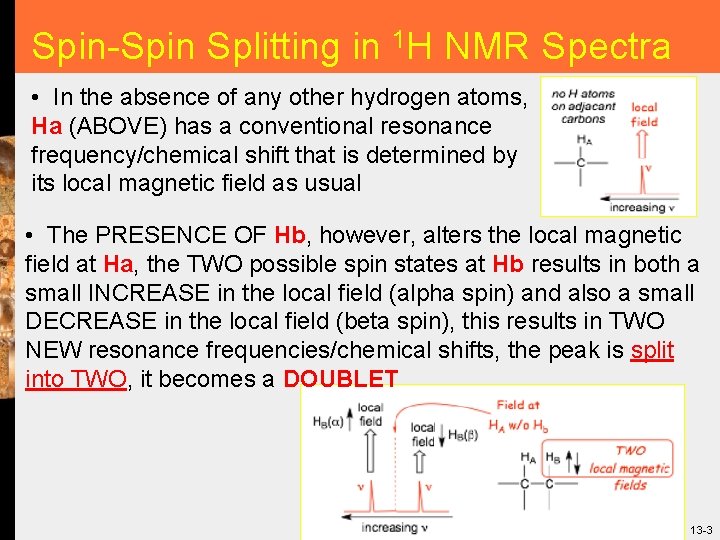
Spin-Spin Splitting in 1 H NMR Spectra • In the absence of any other hydrogen atoms, Ha (ABOVE) has a conventional resonance frequency/chemical shift that is determined by its local magnetic field as usual • The PRESENCE OF Hb, however, alters the local magnetic field at Ha, the TWO possible spin states at Hb results in both a small INCREASE in the local field (alpha spin) and also a small DECREASE in the local field (beta spin), this results in TWO NEW resonance frequencies/chemical shifts, the peak is split into TWO, it becomes a DOUBLET 13 -3

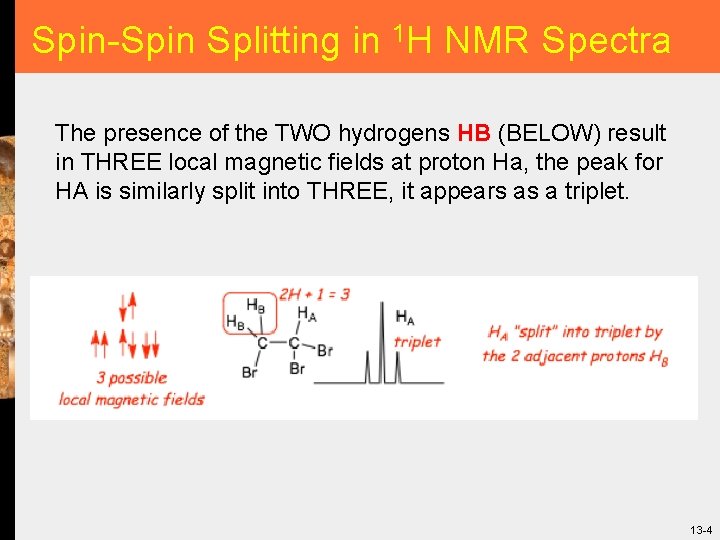
Spin-Spin Splitting in 1 H NMR Spectra The presence of the TWO hydrogens HB (BELOW) result in THREE local magnetic fields at proton Ha, the peak for HA is similarly split into THREE, it appears as a triplet. 13 -4

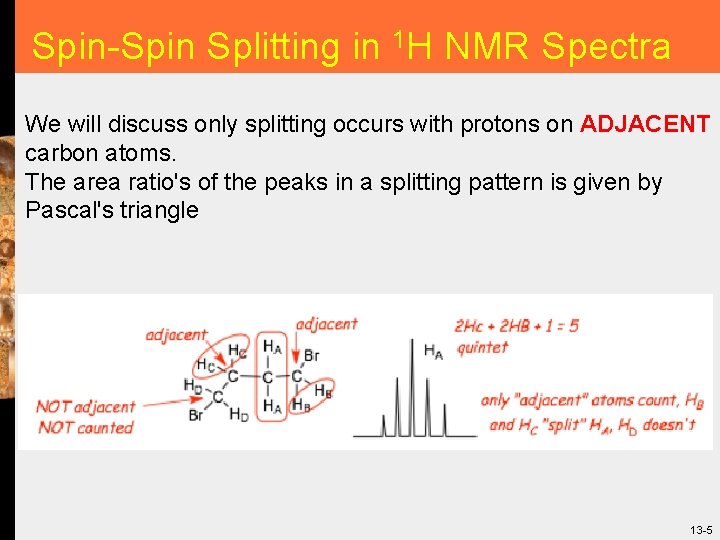
Spin-Spin Splitting in 1 H NMR Spectra We will discuss only splitting occurs with protons on ADJACENT carbon atoms. The area ratio's of the peaks in a splitting pattern is given by Pascal's triangle 13 -5

Signal Splitting; the (n + 1) Rule § § § Peak: The units into which an NMR signal is split; singlet (s), doublet (d), triplet (t), quartet (q), multiplet (m) and so forth. Signal splitting: Splitting of an NMR signal into a set of peaks by the influence of neighboring nonequivalent hydrogens. (n + 1) rule: If a hydrogen has n hydrogens nonequivalent to it but equivalent among themselves on the same or adjacent atom(s), its 1 H-NMR signal is split into (n + 1) peaks. 13 -6

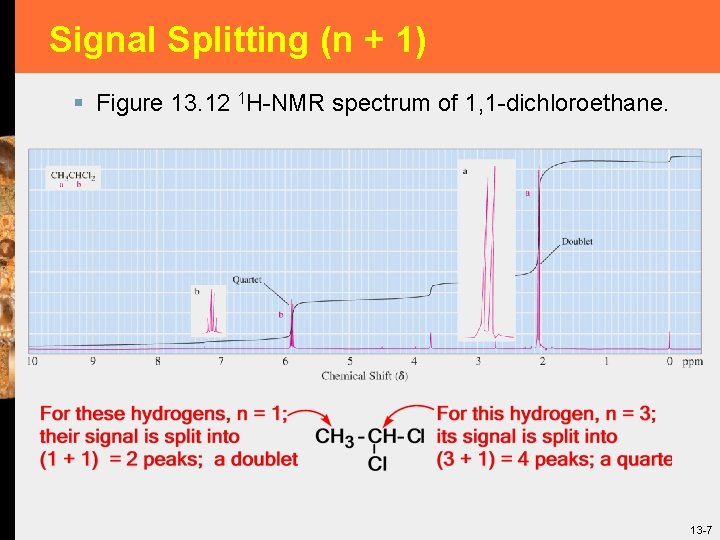
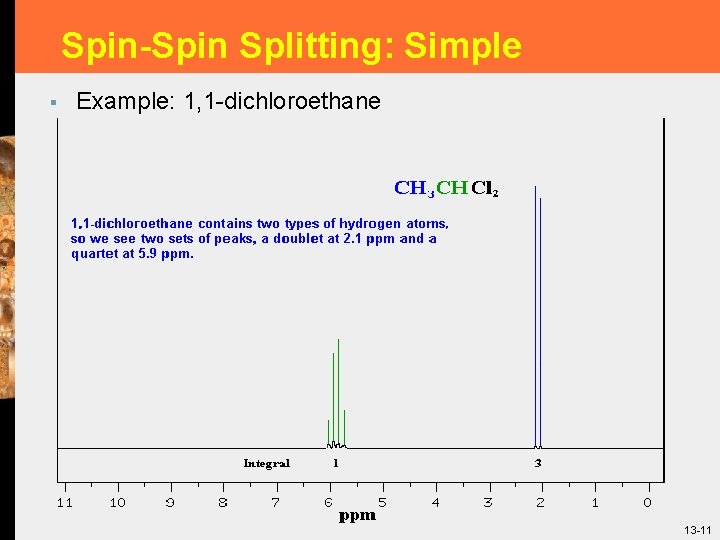
Signal Splitting (n + 1) § Figure 13. 12 1 H-NMR spectrum of 1, 1 -dichloroethane. 13 -7

Origins of Signal Splitting § § Signal coupling: An interaction in which the nuclear spins of adjacent atoms influence each other and lead to the splitting of NMR signals. Coupling constant (J) : The separation on an NMR spectrum (in hertz) between adjacent peaks in a multiplet. § A quantitative measure of the spin-spin coupling with adjacent nuclei. 13 -8

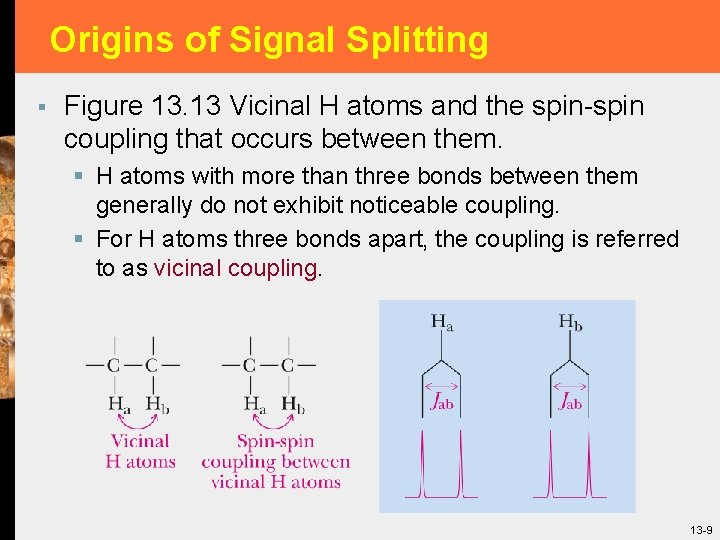
Origins of Signal Splitting § Figure 13. 13 Vicinal H atoms and the spin-spin coupling that occurs between them. § H atoms with more than three bonds between them generally do not exhibit noticeable coupling. § For H atoms three bonds apart, the coupling is referred to as vicinal coupling. 13 -9

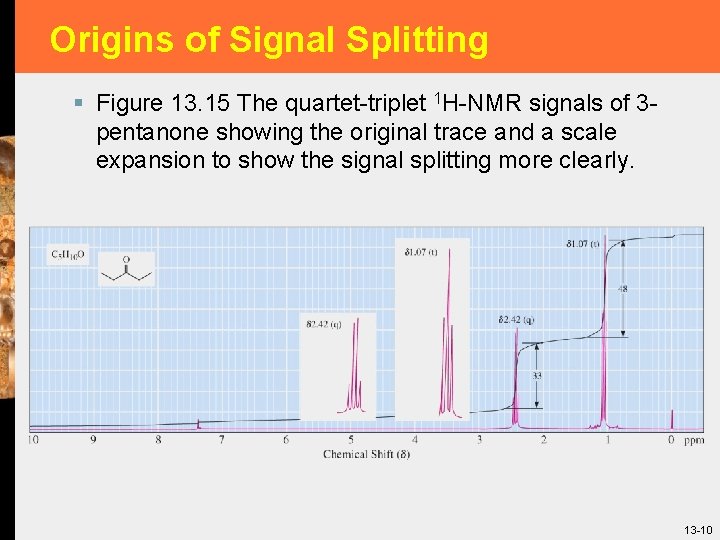
Origins of Signal Splitting § Figure 13. 15 The quartet-triplet 1 H-NMR signals of 3 pentanone showing the original trace and a scale expansion to show the signal splitting more clearly. 13 -10

Spin-Spin Splitting: Simple § Example: 1, 1 -dichloroethane 13 -11

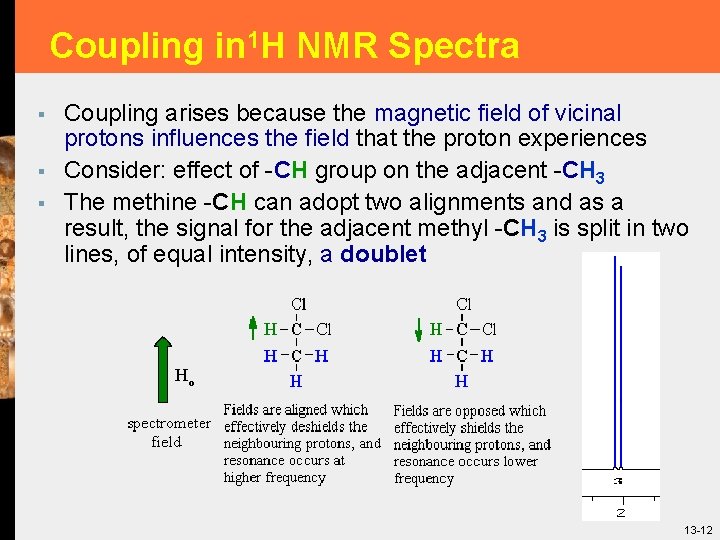
Coupling in 1 H NMR Spectra § § § Coupling arises because the magnetic field of vicinal protons influences the field that the proton experiences Consider: effect of -CH group on the adjacent -CH 3 The methine -CH can adopt two alignments and as a result, the signal for the adjacent methyl -CH 3 is split in two lines, of equal intensity, a doublet 13 -12

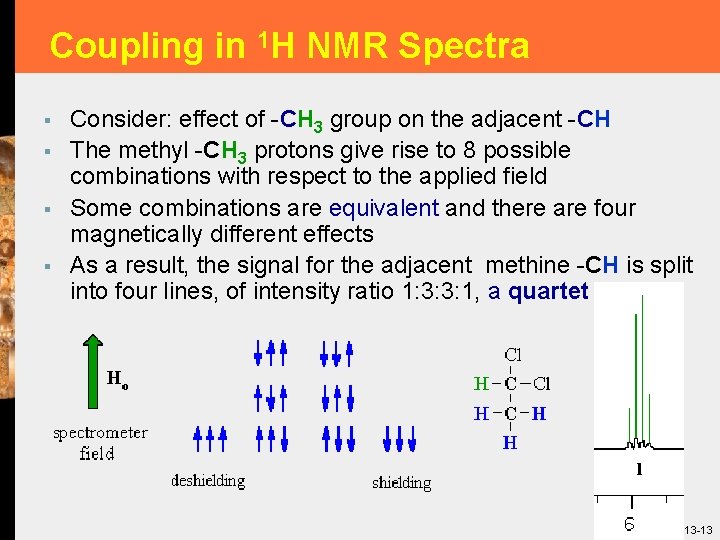
Coupling in 1 H NMR Spectra § § Consider: effect of -CH 3 group on the adjacent -CH The methyl -CH 3 protons give rise to 8 possible combinations with respect to the applied field Some combinations are equivalent and there are four magnetically different effects As a result, the signal for the adjacent methine -CH is split into four lines, of intensity ratio 1: 3: 3: 1, a quartet 13 -13

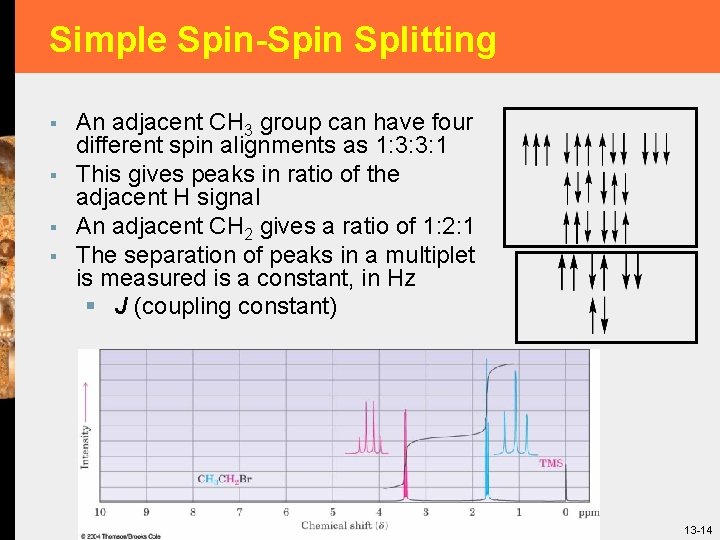
Simple Spin-Spin Splitting § § An adjacent CH 3 group can have four different spin alignments as 1: 3: 3: 1 This gives peaks in ratio of the adjacent H signal An adjacent CH 2 gives a ratio of 1: 2: 1 The separation of peaks in a multiplet is measured is a constant, in Hz § J (coupling constant) 13 -14

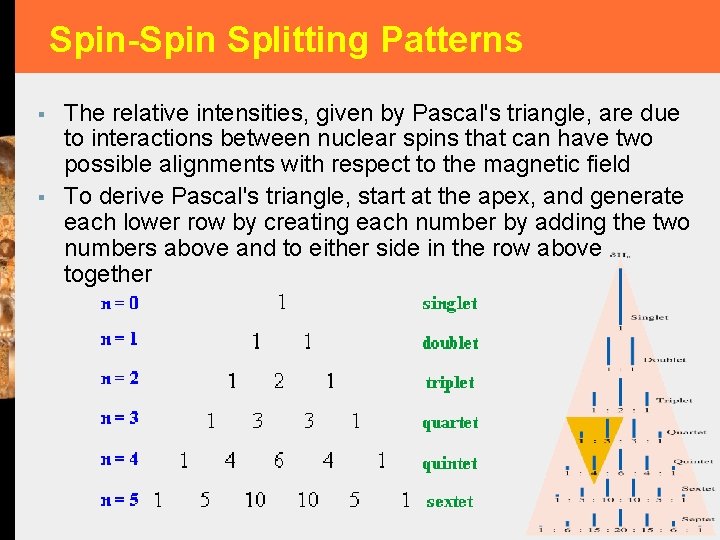
Spin-Spin Splitting Patterns § § The relative intensities, given by Pascal's triangle, are due to interactions between nuclear spins that can have two possible alignments with respect to the magnetic field To derive Pascal's triangle, start at the apex, and generate each lower row by creating each number by adding the two numbers above and to either side in the row above together 13 -15

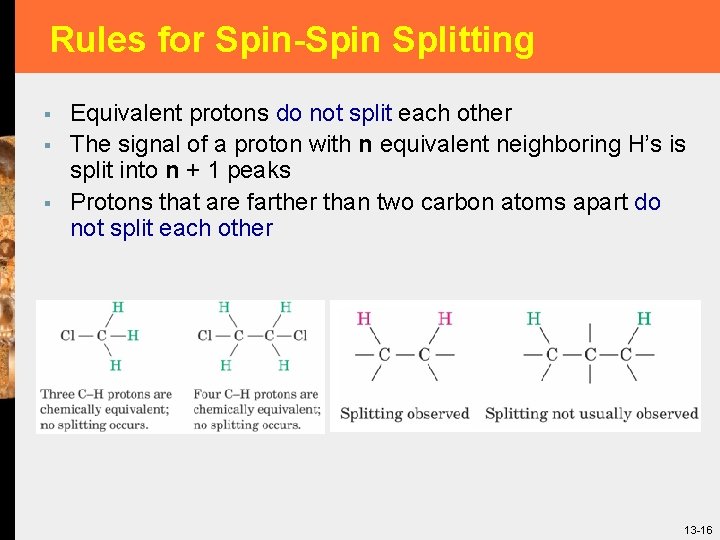
Rules for Spin-Spin Splitting § § § Equivalent protons do not split each other The signal of a proton with n equivalent neighboring H’s is split into n + 1 peaks Protons that are farther than two carbon atoms apart do not split each other 13 -16

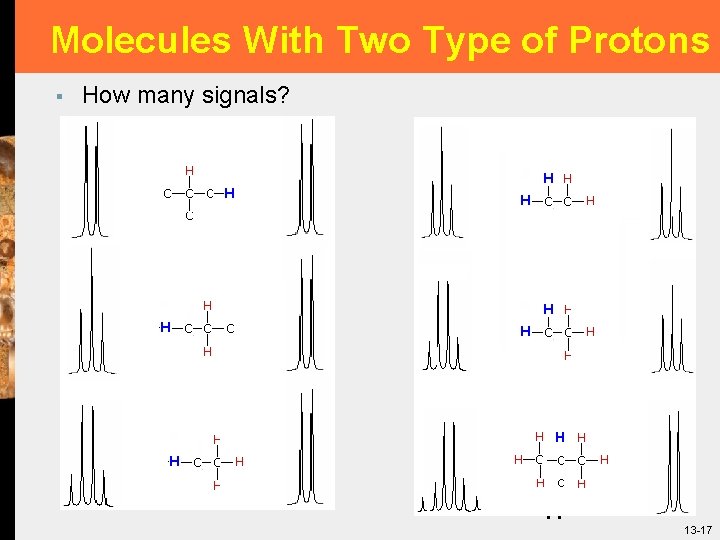
Molecules With Two Type of Protons § How many signals? 17 13 -17

Rules for Spin-Spin Splitting § Two groups of protons coupled to each other have the same coupling constant, J § J coupling constant is the distance between peaks in a multiplet (in Hz) § Peaks for protons that split each other will always have the same coupling constant § J useful in determining which peaks are adjacent to each other § Multiplets will often be skewed in the direction of the peak to which they are coupled 13 -18

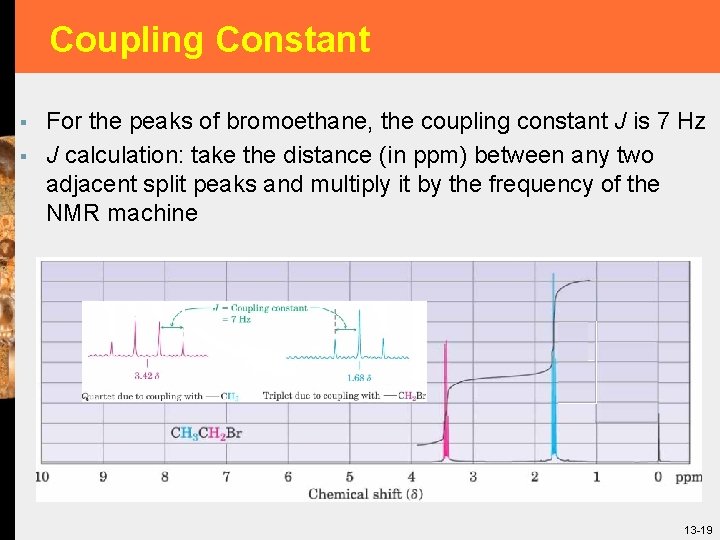
Coupling Constant § § For the peaks of bromoethane, the coupling constant J is 7 Hz J calculation: take the distance (in ppm) between any two adjacent split peaks and multiply it by the frequency of the NMR machine 13 -19

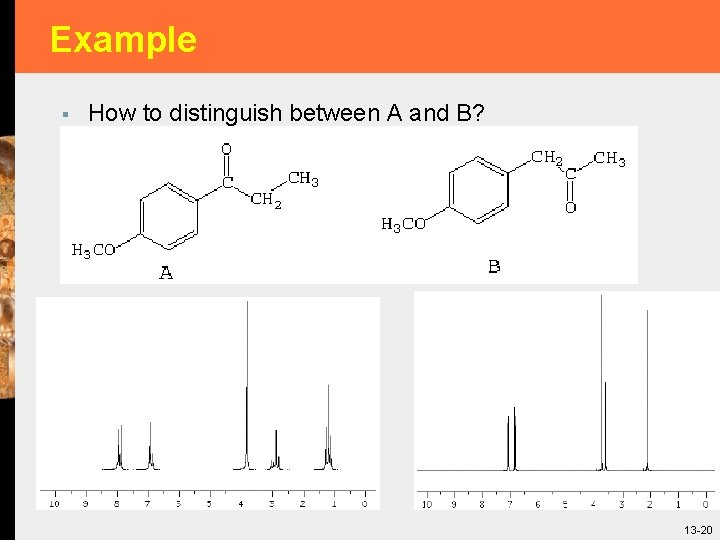
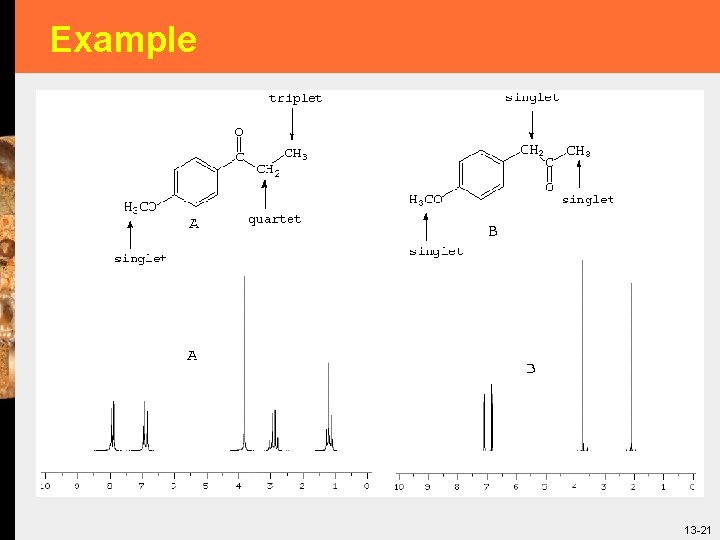
Example § How to distinguish between A and B? 13 -20

Example 13 -21

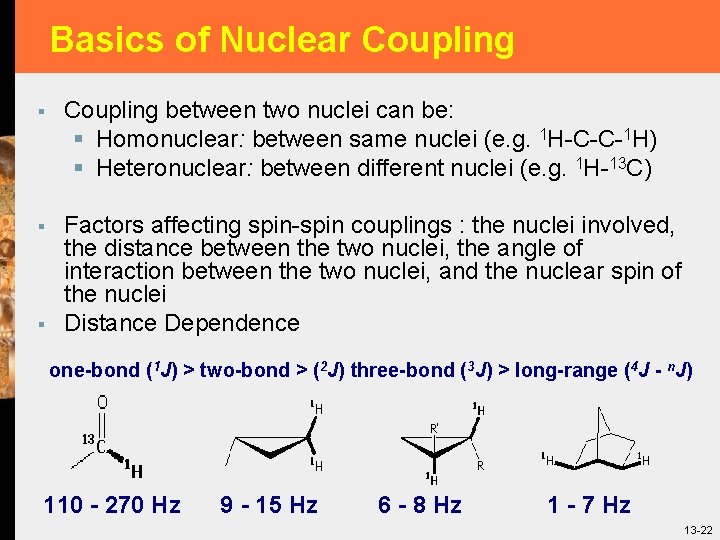
Basics of Nuclear Coupling § Coupling between two nuclei can be: § Homonuclear: between same nuclei (e. g. 1 H-C-C-1 H) § Heteronuclear: between different nuclei (e. g. 1 H-13 C) § Factors affecting spin-spin couplings : the nuclei involved, the distance between the two nuclei, the angle of interaction between the two nuclei, and the nuclear spin of the nuclei Distance Dependence § one-bond (1 J) > two-bond > (2 J) three-bond (3 J) > long-range (4 J - n. J) 110 - 270 Hz 9 - 15 Hz 6 - 8 Hz 1 - 7 Hz 13 -22

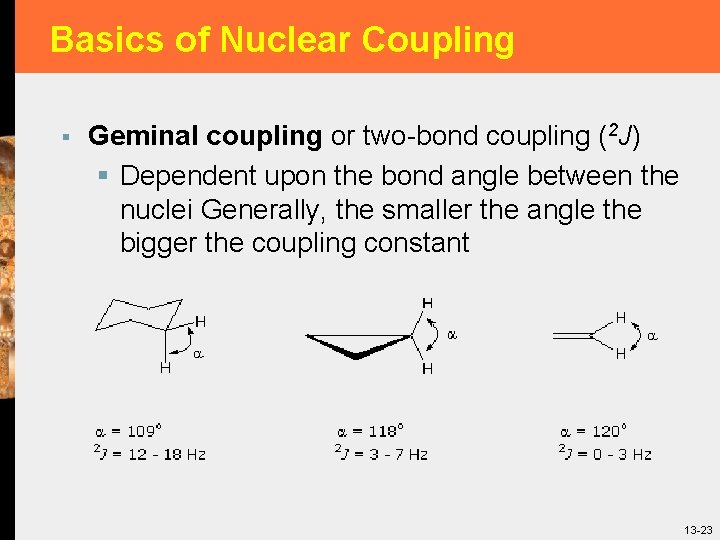
Basics of Nuclear Coupling § Geminal coupling or two-bond coupling (2 J) § Dependent upon the bond angle between the nuclei Generally, the smaller the angle the bigger the coupling constant 13 -23

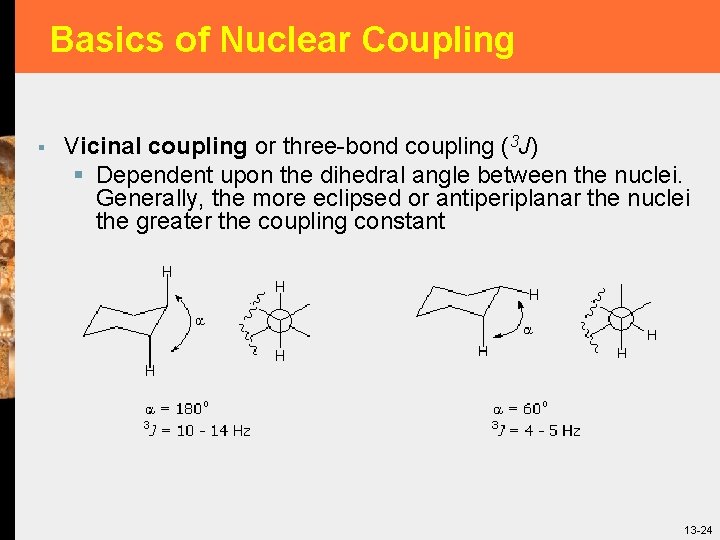
Basics of Nuclear Coupling § Vicinal coupling or three-bond coupling (3 J) § Dependent upon the dihedral angle between the nuclei. Generally, the more eclipsed or antiperiplanar the nuclei the greater the coupling constant 13 -24

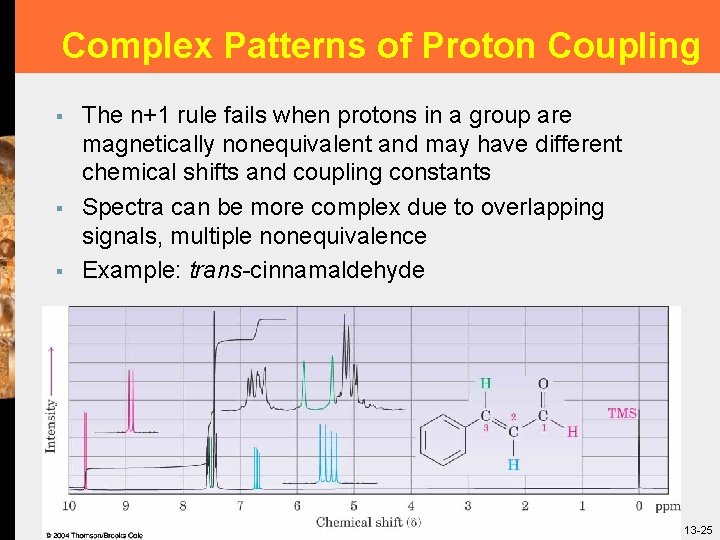
Complex Patterns of Proton Coupling § § § The n+1 rule fails when protons in a group are magnetically nonequivalent and may have different chemical shifts and coupling constants Spectra can be more complex due to overlapping signals, multiple nonequivalence Example: trans-cinnamaldehyde 25 13 -25

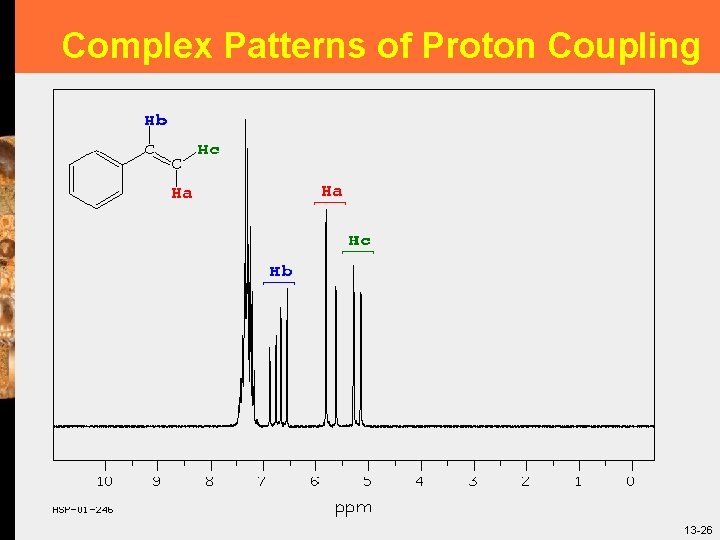
Complex Patterns of Proton Coupling 13 -26

Complex Patterns of Proton Coupling § § Doublet-of-doublets! Hb is split by Ha with a J of around 15 Hz. It is further split by proton Hc with a J of around 10 Hz. One way that you know it is not a quartet is that the peaks are all (roughly) the same height Because of the small coupling between Ha and Hc, one of the splittings is very small, and consequently is difficult to see in the real spectrum 13 -27

Coupling of Protons on Oxygen § § § Normally, no coupling is observed between the O-H and H’s on the carbon atom to which the hydroxyl group is attached The average time of residence of a proton on oxygen is 10 -5 seconds. About 10 -2 to 10 -3 second is required for an NMR transition event to be recorded. Thus, the hydroxyl proton is unattached more frequently than it is attached to oxygen, and the spin interaction between the hydroxyl proton and any other protons is effectively decoupled Likewise, protons on nitrogen also exchange quickly enough to be uncoupled from other protons in the molecule 13 -28

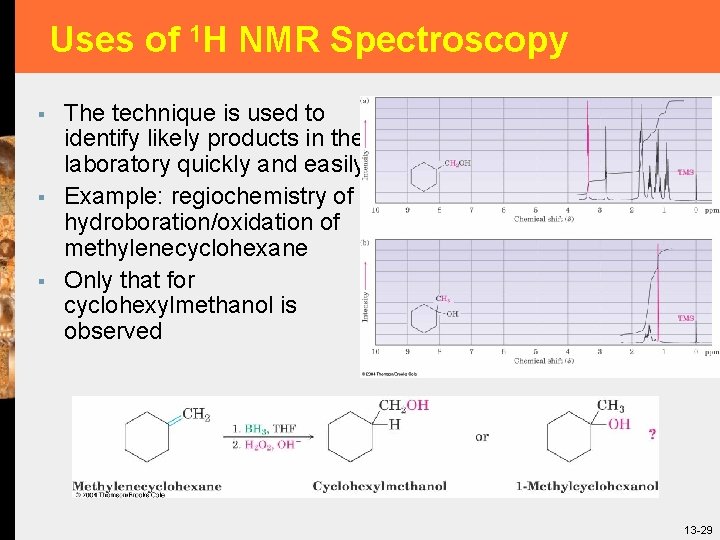
Uses of 1 H NMR Spectroscopy § § § The technique is used to identify likely products in the laboratory quickly and easily Example: regiochemistry of hydroboration/oxidation of methylenecyclohexane Only that for cyclohexylmethanol is observed 13 -29

13 C NMR Spectroscopy 13 -30

13 C § § § NMR Spectroscopy: Background Like 1 H, 13 C has I = ½. For 12 C, I = 0, no NMR signal observed 13 C has only about 1. 1% natural abundance. As a result, C is about 400 times less sensitive than H nucleus to the NMR phenomena 13 C resonances are 0 to 220 ppm downfield from TMS Similar factors affect the chemical shifts in 13 C as seen for 1 H NMR Long relaxation times (excited state to ground state) mean no integrations 13 -31

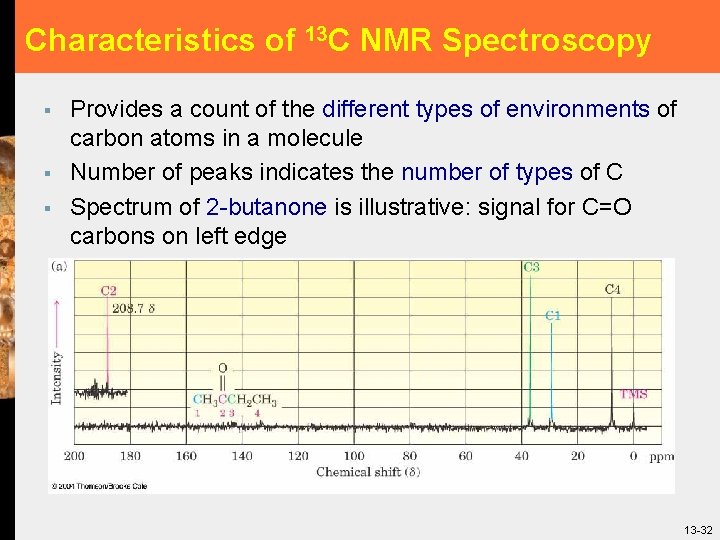
Characteristics of 13 C NMR Spectroscopy § § § Provides a count of the different types of environments of carbon atoms in a molecule Number of peaks indicates the number of types of C Spectrum of 2 -butanone is illustrative: signal for C=O carbons on left edge 13 -32

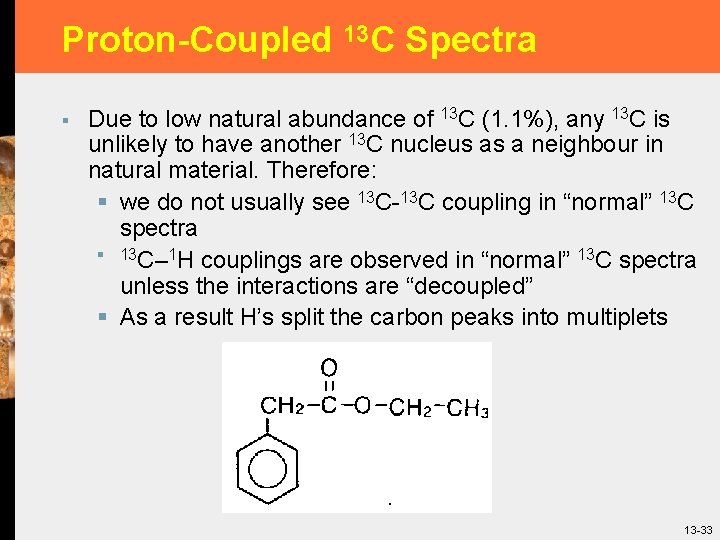
Proton-Coupled 13 C Spectra § Due to low natural abundance of 13 C (1. 1%), any 13 C is unlikely to have another 13 C nucleus as a neighbour in natural material. Therefore: § we do not usually see 13 C-13 C coupling in “normal” 13 C spectra § 13 C– 1 H couplings are observed in “normal” 13 C spectra unless the interactions are “decoupled” § As a result H’s split the carbon peaks into multiplets 13 -33

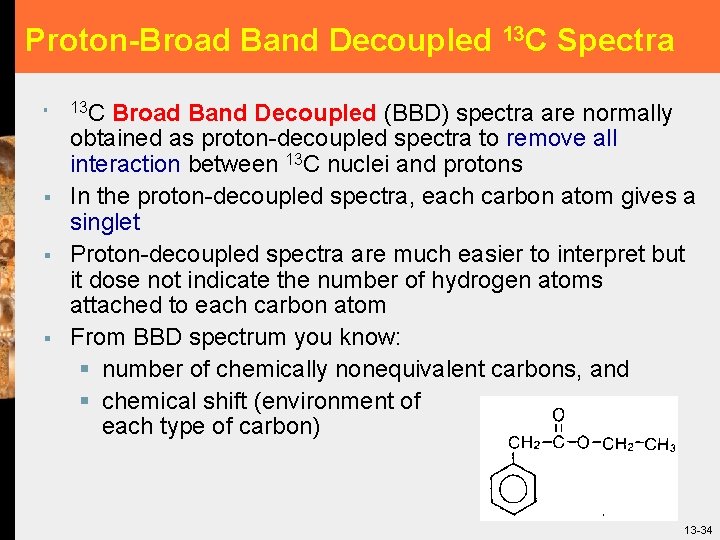
Proton-Broad Band Decoupled 13 C Spectra § § 13 C Broad Band Decoupled (BBD) spectra are normally obtained as proton-decoupled spectra to remove all interaction between 13 C nuclei and protons In the proton-decoupled spectra, each carbon atom gives a singlet Proton-decoupled spectra are much easier to interpret but it dose not indicate the number of hydrogen atoms attached to each carbon atom From BBD spectrum you know: § number of chemically nonequivalent carbons, and § chemical shift (environment of each type of carbon) 34 13 -34

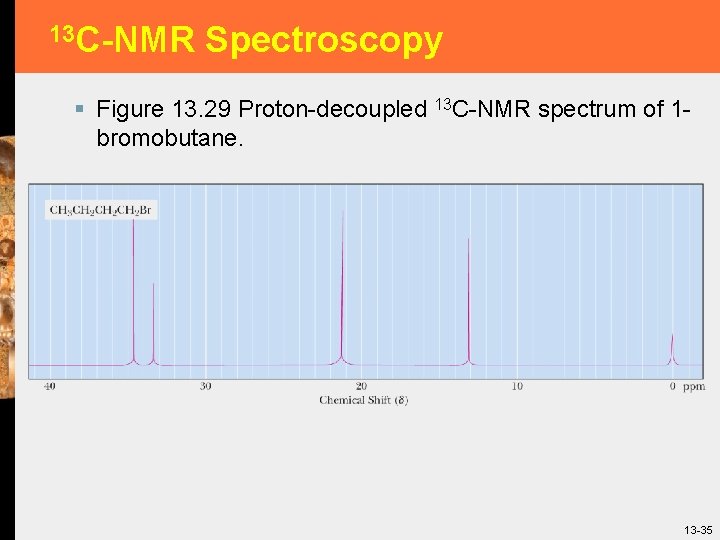
13 C-NMR Spectroscopy § Figure 13. 29 Proton-decoupled 13 C-NMR spectrum of 1 bromobutane. 13 -35

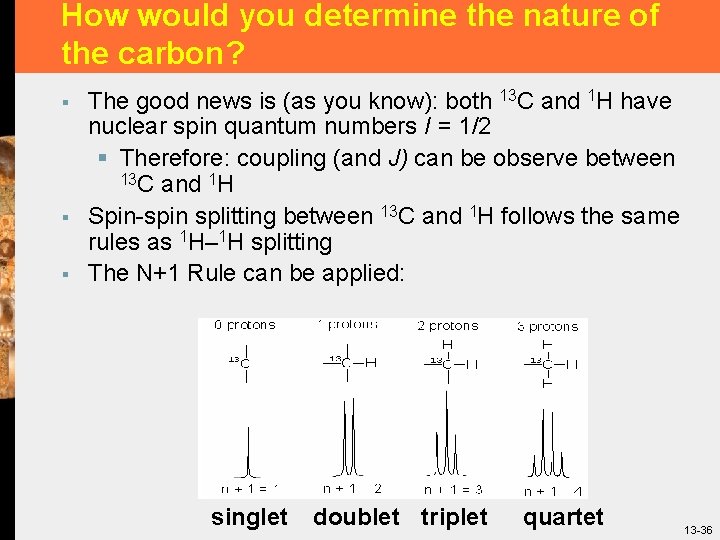
How would you determine the nature of the carbon? § § § The good news is (as you know): both 13 C and 1 H have nuclear spin quantum numbers I = 1/2 § Therefore: coupling (and J) can be observe between 13 C and 1 H Spin-spin splitting between 13 C and 1 H follows the same rules as 1 H– 1 H splitting The N+1 Rule can be applied: singlet doublet triplet quartet 13 -36

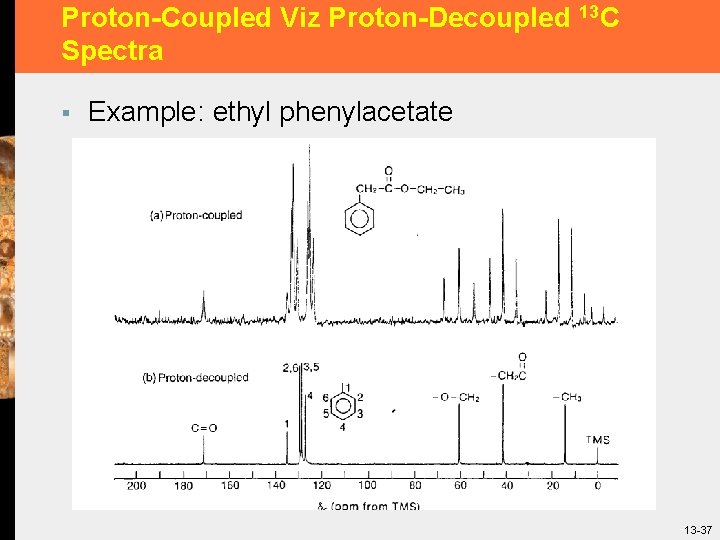
Proton-Coupled Viz Proton-Decoupled 13 C Spectra § Example: ethyl phenylacetate 13 -37

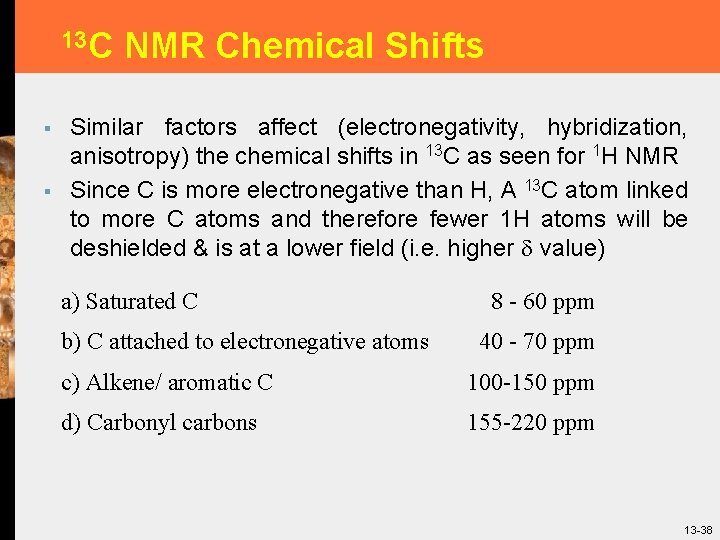
13 C § § NMR Chemical Shifts Similar factors affect (electronegativity, hybridization, anisotropy) the chemical shifts in 13 C as seen for 1 H NMR Since C is more electronegative than H, A 13 C atom linked to more C atoms and therefore fewer 1 H atoms will be deshielded & is at a lower field (i. e. higher value) a) Saturated C b) C attached to electronegative atoms 8 - 60 ppm 40 - 70 ppm c) Alkene/ aromatic C 100 -150 ppm d) Carbonyl carbons 155 -220 ppm 13 -38

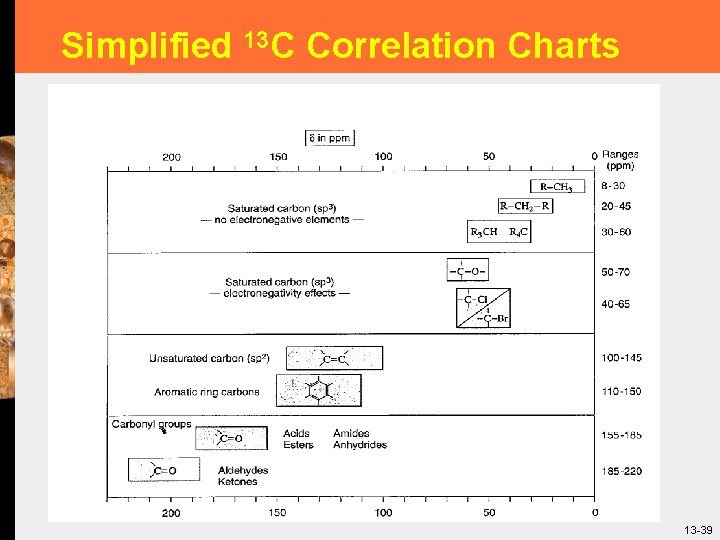
Simplified 13 C Correlation Charts 39 13 -39

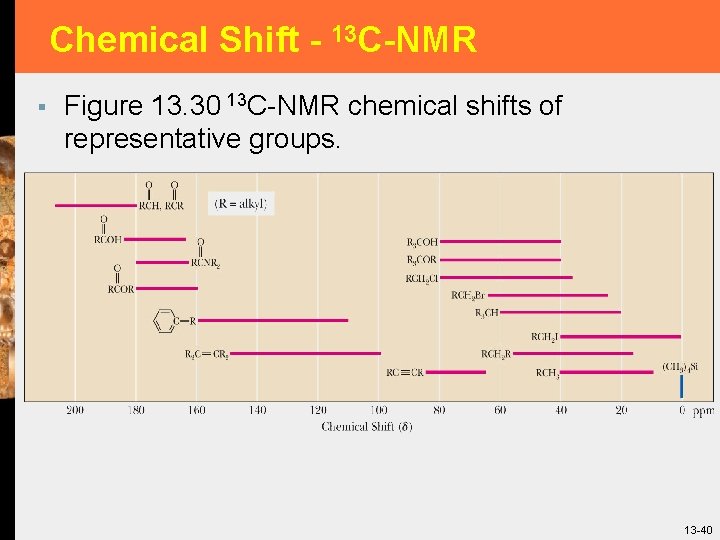
Chemical Shift - 13 C-NMR § Figure 13. 30 13 C-NMR chemical shifts of representative groups. 13 -40

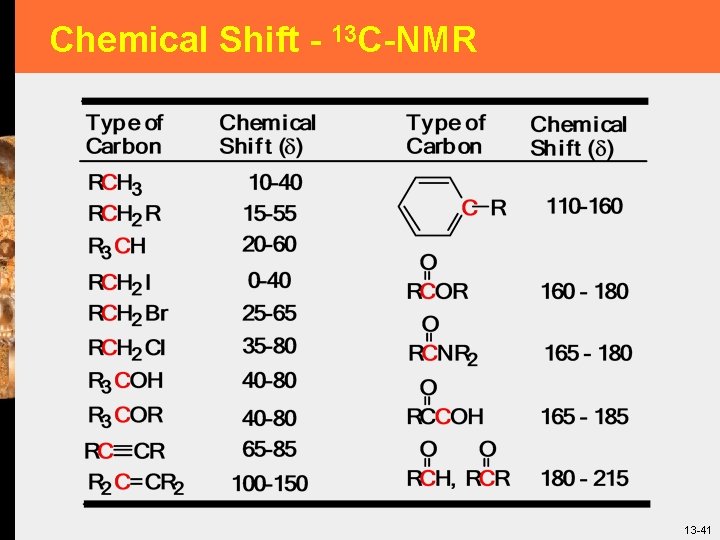
Chemical Shift - 13 C-NMR 13 -41

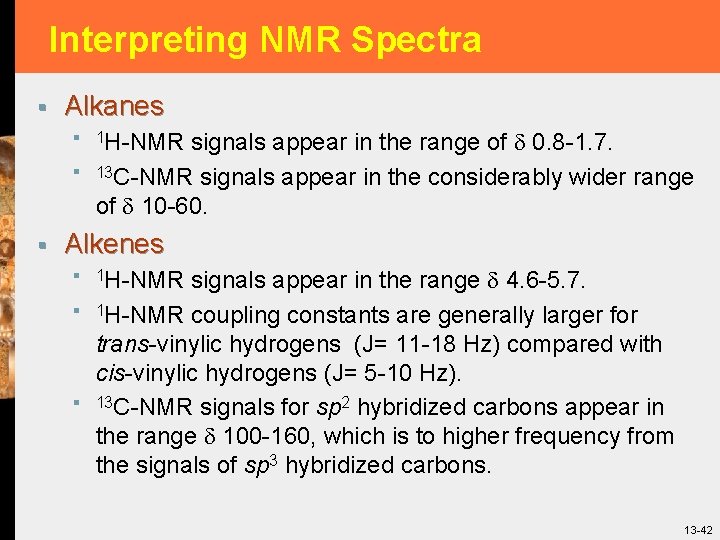
Interpreting NMR Spectra § Alkanes § 1 H-NMR signals appear in the range of 0. 8 -1. 7. § 13 C-NMR signals appear in the considerably wider range of 10 -60. § Alkenes § 1 H-NMR signals appear in the range 4. 6 -5. 7. § 1 H-NMR coupling constants are generally larger for § trans-vinylic hydrogens (J= 11 -18 Hz) compared with cis-vinylic hydrogens (J= 5 -10 Hz). 13 C-NMR signals for sp 2 hybridized carbons appear in the range 100 -160, which is to higher frequency from the signals of sp 3 hybridized carbons. 13 -42

Interpreting NMR Spectra § Figure 13. 31 1 H-NMR spectrum of vinyl acetate. 13 -43

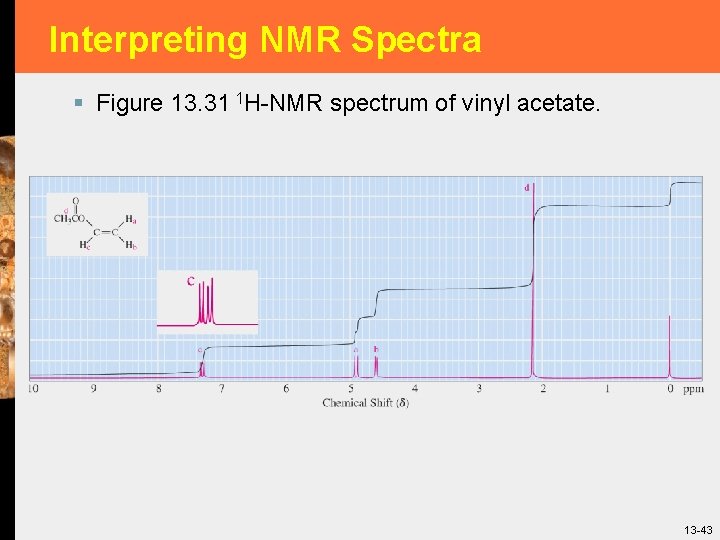
Interpreting NMR Spectra § § Alcohols 1 H-NMR O-H chemical shift often appears in the range 3. 0 -4. 0, but may be as low as 0. 5. § 1 H-NMR chemical shifts of hydrogens on the carbon bearing the -OH group are deshielded by the electronwithdrawing inductive effect of the oxygen and appear in the range 3. 0 -4. 0. § Ethers § A distinctive feature in the 1 H-NMR spectra of ethers is the chemical shift, 3. 3 -4. 0, of hydrogens on the carbons bonded to the ether oxygen. 13 -44

Interpreting NMR Spectra § Figure 13. 32 1 H-NMR spectrum of 1 -propanol. 13 -45

Interpreting NMR Spectra § Aldehydes and ketones § 1 H-NMR: aldehyde hydrogens appear at 9. 5 -10. 1. § 1 H-NMR: a-hydrogens of aldehydes and ketones appear § § at 2. 2 -2. 6. 13 C-NMR: carbonyl carbons appear at 180 -215. Amines § 1 H-NMR: amine hydrogens appear at 0. 5 -5. 0 depending on conditions. 13 -46

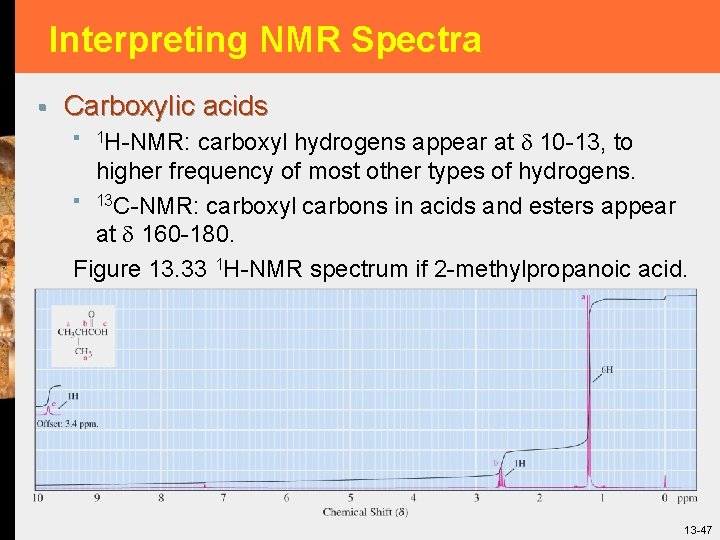
Interpreting NMR Spectra § Carboxylic acids § 1 H-NMR: carboxyl hydrogens appear at 10 -13, to higher frequency of most other types of hydrogens. § 13 C-NMR: carboxyl carbons in acids and esters appear at 160 -180. Figure 13. 33 1 H-NMR spectrum if 2 -methylpropanoic acid. 13 -47

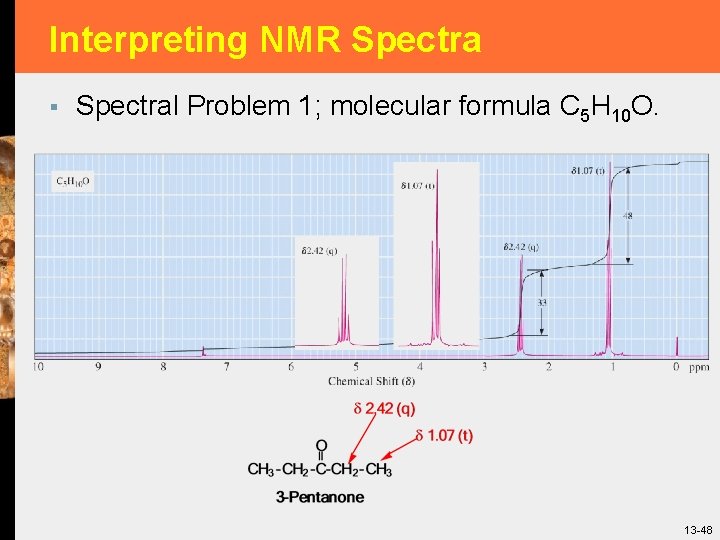
Interpreting NMR Spectra § Spectral Problem 1; molecular formula C 5 H 10 O. 13 -48

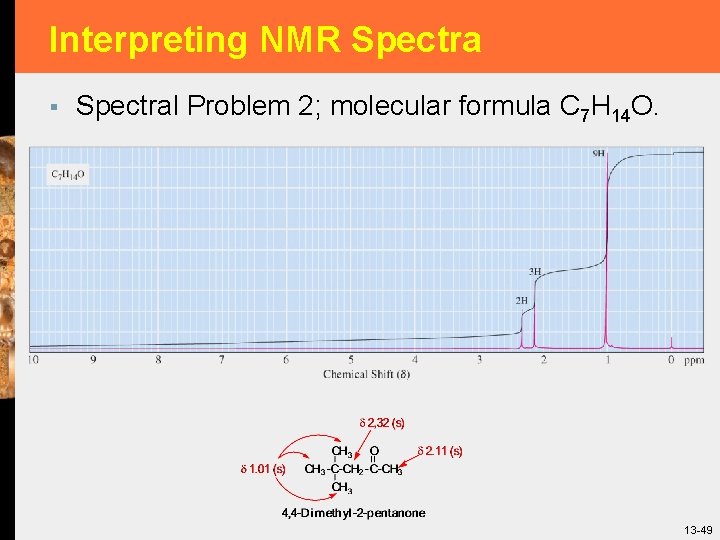
Interpreting NMR Spectra § Spectral Problem 2; molecular formula C 7 H 14 O. 13 -49

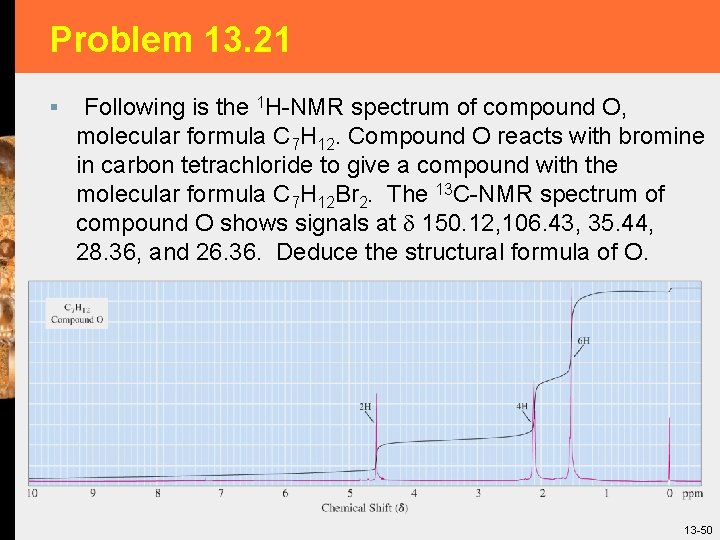
Problem 13. 21 § Following is the 1 H-NMR spectrum of compound O, molecular formula C 7 H 12. Compound O reacts with bromine in carbon tetrachloride to give a compound with the molecular formula C 7 H 12 Br 2. The 13 C-NMR spectrum of compound O shows signals at 150. 12, 106. 43, 35. 44, 28. 36, and 26. 36. Deduce the structural formula of O. 13 -50

Problem 13. 21 § The molecular formula, C 7 H 12, indicates a hydrogen deficiency of 2, so there are two rings and/or pi bonds. Compound O reacts with only one mole of bromine, so there is only one double bond and there then must be one ring. Furthermore, the 13 C-NMR shows only two sp 2 resonances, another evidence that there is only one double bond. 13 -51

Good Luck With thanks to Dr. Ziad Ali, Taibah University for providing NMR resources 13 -52
 Peta konsep dakwah nabi muhammad saw di mekah
Peta konsep dakwah nabi muhammad saw di mekah Manipal university chemistry department
Manipal university chemistry department Lesson 15 nuclear quest nuclear reactions
Lesson 15 nuclear quest nuclear reactions Fisión nuclear vs fision nuclear
Fisión nuclear vs fision nuclear Chernobyl nuclear disaster webquest
Chernobyl nuclear disaster webquest Chemistry
Chemistry Application of nuclear chemistry
Application of nuclear chemistry Application of nuclear chemistry
Application of nuclear chemistry What is nuclear charge in chemistry
What is nuclear charge in chemistry 12x12x12x12x12x12x12
12x12x12x12x12x12x12 Chapter 25 nuclear chemistry answer key
Chapter 25 nuclear chemistry answer key Anatomy of a wave
Anatomy of a wave Applications of nuclear chemistry
Applications of nuclear chemistry T half life formula
T half life formula Rectangle real life examples
Rectangle real life examples Chapter 21 review nuclear chemistry
Chapter 21 review nuclear chemistry Copyright
Copyright Nuclear chemistry
Nuclear chemistry Irradiated food
Irradiated food Chapter 25 nuclear chemistry
Chapter 25 nuclear chemistry Chapter 25 nuclear chemistry
Chapter 25 nuclear chemistry Chapter 10 nuclear chemistry
Chapter 10 nuclear chemistry Chapter 10 nuclear chemistry
Chapter 10 nuclear chemistry Islamic online university malaysia
Islamic online university malaysia Islamic university college
Islamic university college Tempoh nabi berdakwah di mekah
Tempoh nabi berdakwah di mekah Isi piagam madinah
Isi piagam madinah Dakwah rasulullah periode madinah berlangsung selama
Dakwah rasulullah periode madinah berlangsung selama Nasheed madina
Nasheed madina Metode dakwah nabi di madinah
Metode dakwah nabi di madinah Membangun masyarakat madinah
Membangun masyarakat madinah Siwy madinah
Siwy madinah Darul madinah pre nursery syllabus
Darul madinah pre nursery syllabus Madinah
Madinah Perjanjian aqabah pertama
Perjanjian aqabah pertama Piagam madinah
Piagam madinah Kerajaan islam di madinah
Kerajaan islam di madinah Usf chemistry
Usf chemistry Nit calicut chemistry
Nit calicut chemistry Texas tech chemistry department
Texas tech chemistry department Ib chemistry functional groups
Ib chemistry functional groups Organic vs inorganic chemistry
Organic vs inorganic chemistry Department of law university of jammu
Department of law university of jammu Department of geology university of dhaka
Department of geology university of dhaka Narrativistic
Narrativistic University of bridgeport it department
University of bridgeport it department Isabel darcy
Isabel darcy Department of physics university of tokyo
Department of physics university of tokyo Texas state majors
Texas state majors Department of information engineering university of padova
Department of information engineering university of padova Department of information engineering university of padova
Department of information engineering university of padova Syracuse university psychology department
Syracuse university psychology department Jackson state university finance department
Jackson state university finance department