Islamic University in Madinah Department of Chemistry Nuclear




- Slides: 18

Islamic University in Madinah Department of Chemistry Nuclear Magnetic Resonance Spectroscopy Part-2 Prepared By Dr. Khalid Ahmad Shadid 13 -1

13 -2

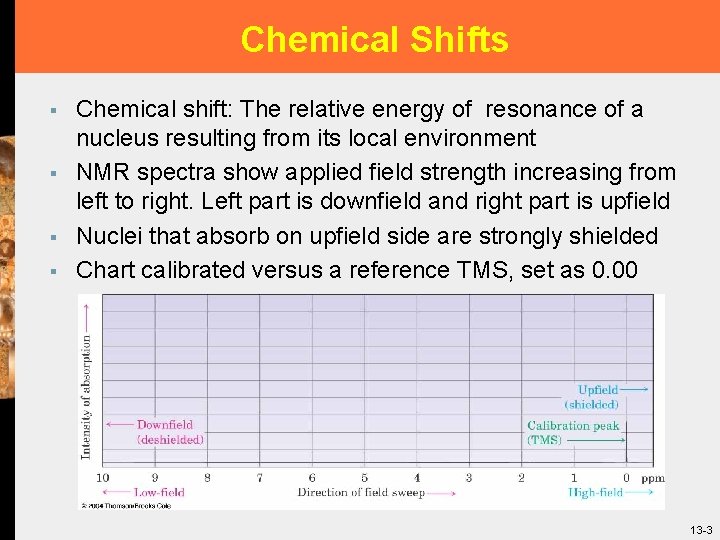
Chemical Shifts § § Chemical shift: The relative energy of resonance of a nucleus resulting from its local environment NMR spectra show applied field strength increasing from left to right. Left part is downfield and right part is upfield Nuclei that absorb on upfield side are strongly shielded Chart calibrated versus a reference TMS, set as 0. 00 13 -3


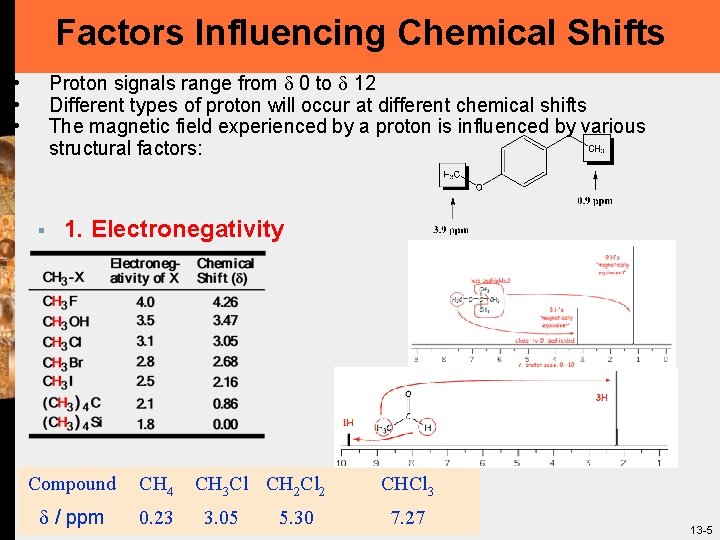
Factors Influencing Chemical Shifts Proton signals range from 0 to 12 Different types of proton will occur at different chemical shifts The magnetic field experienced by a proton is influenced by various structural factors: • • • § 1. Electronegativity Compound CH 4 / ppm 0. 23 CH 3 Cl CH 2 Cl 2 3. 05 5. 30 CHCl 3 7. 27 13 -5

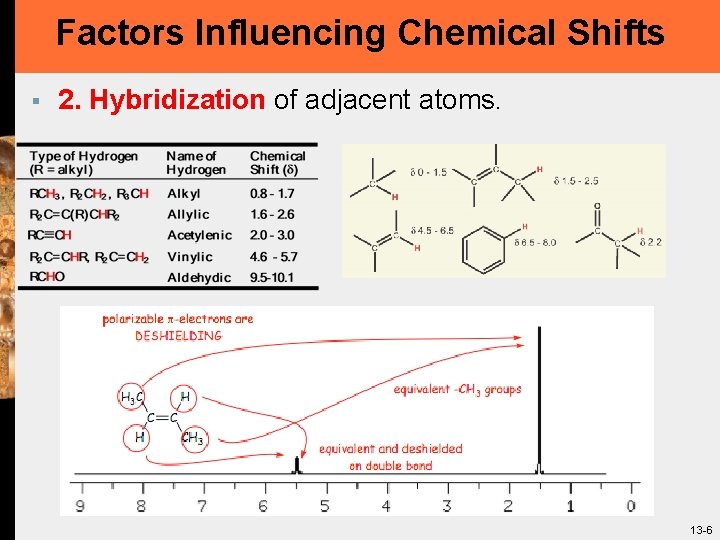
Factors Influencing Chemical Shifts § 2. Hybridization of adjacent atoms. 13 -6

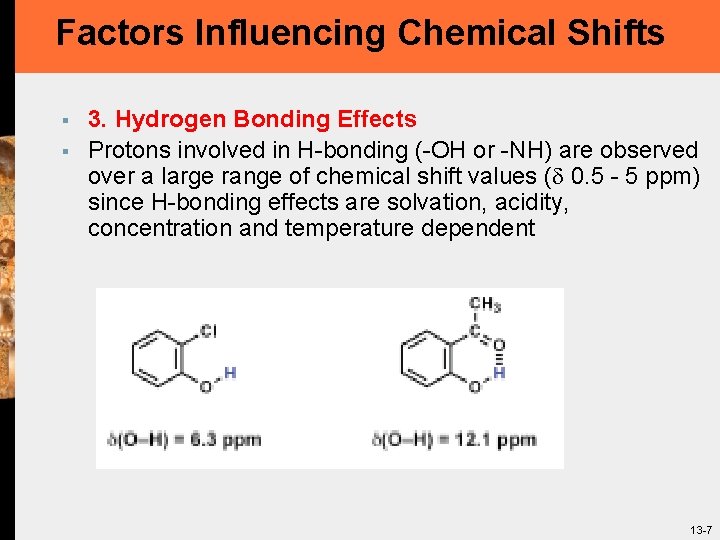
Factors Influencing Chemical Shifts § § 3. Hydrogen Bonding Effects Protons involved in H-bonding (-OH or -NH) are observed over a large range of chemical shift values ( 0. 5 - 5 ppm) since H-bonding effects are solvation, acidity, concentration and temperature dependent 13 -7

Factors Influencing Chemical Shifts 4. Magnetic anisotropy Effect: § § Magnetic anisotropy: "non-uniform magnetic field“ Electrons in p systems (e. g. aromatics, alkenes, alkynes, carbonyls etc. ) interact with the B 0 which induces a magnetic field that causes the anisotropy As a result, the nearby protons will experience 3 fields: the applied field, the shielding field of the valence electrons and the field due to the p system Depending on the position of the proton in this third field, it can be either shielded (smaller ) or deshielded (larger ) 13 -8

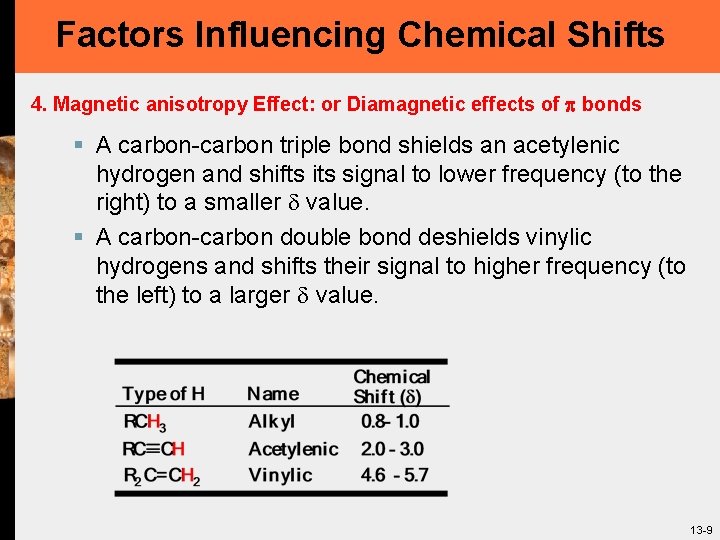
Factors Influencing Chemical Shifts 4. Magnetic anisotropy Effect: or Diamagnetic effects of bonds § A carbon-carbon triple bond shields an acetylenic hydrogen and shifts its signal to lower frequency (to the right) to a smaller value. § A carbon-carbon double bond deshields vinylic hydrogens and shifts their signal to higher frequency (to the left) to a larger value. 13 -9

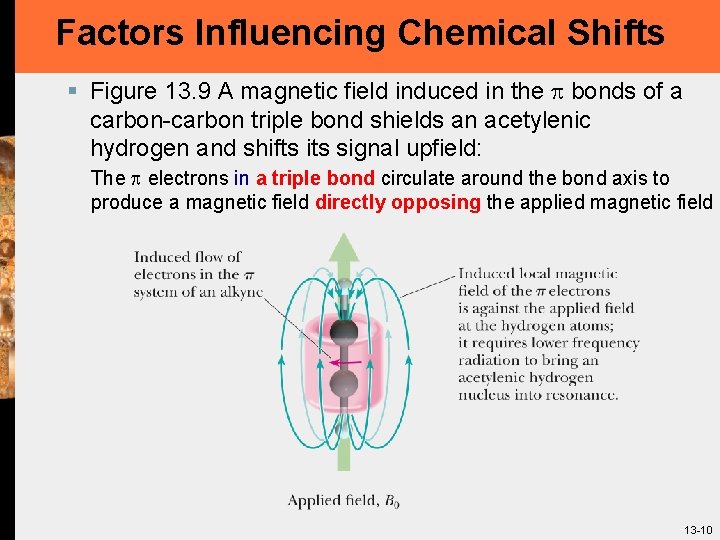
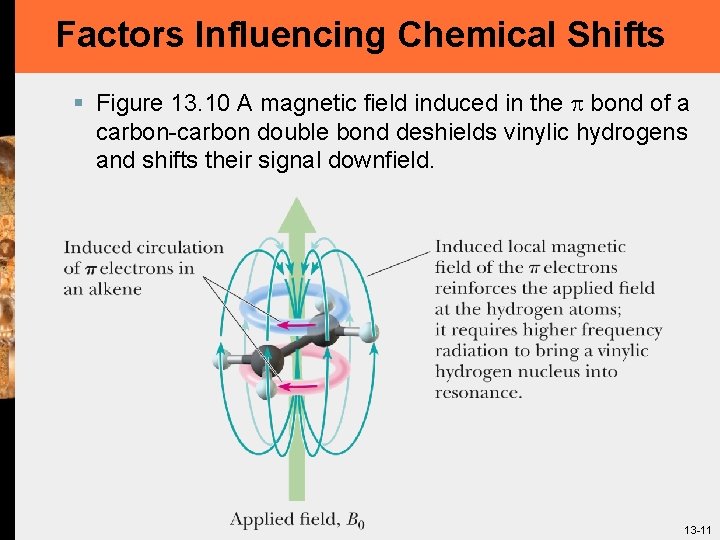
Factors Influencing Chemical Shifts § Figure 13. 9 A magnetic field induced in the p bonds of a carbon-carbon triple bond shields an acetylenic hydrogen and shifts its signal upfield: The p electrons in a triple bond circulate around the bond axis to produce a magnetic field directly opposing the applied magnetic field 13 -10

Factors Influencing Chemical Shifts § Figure 13. 10 A magnetic field induced in the p bond of a carbon-carbon double bond deshields vinylic hydrogens and shifts their signal downfield. 13 -11

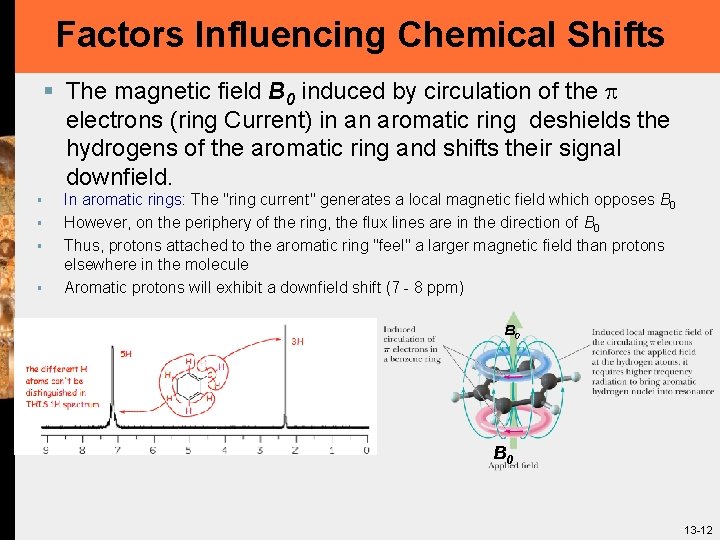
Factors Influencing Chemical Shifts § The magnetic field B 0 induced by circulation of the p electrons (ring Current) in an aromatic ring deshields the hydrogens of the aromatic ring and shifts their signal downfield. § § In aromatic rings: The "ring current" generates a local magnetic field which opposes B 0 However, on the periphery of the ring, the flux lines are in the direction of B 0 Thus, protons attached to the aromatic ring "feel" a larger magnetic field than protons elsewhere in the molecule Aromatic protons will exhibit a downfield shift (7 - 8 ppm) B 0 13 -12

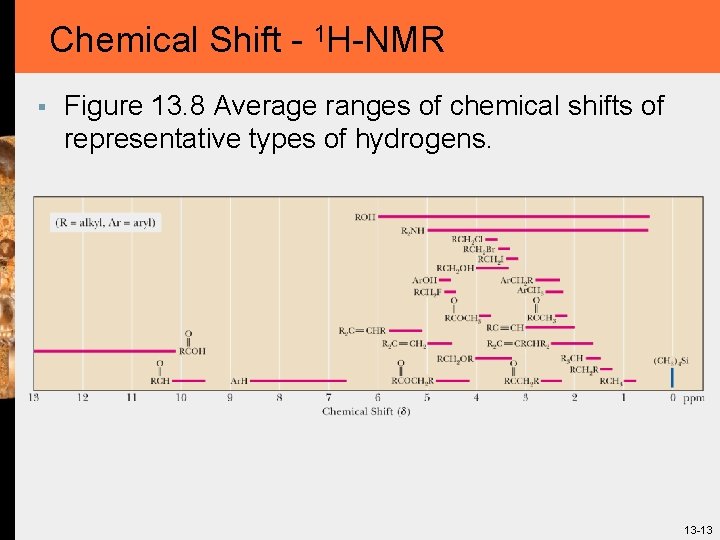
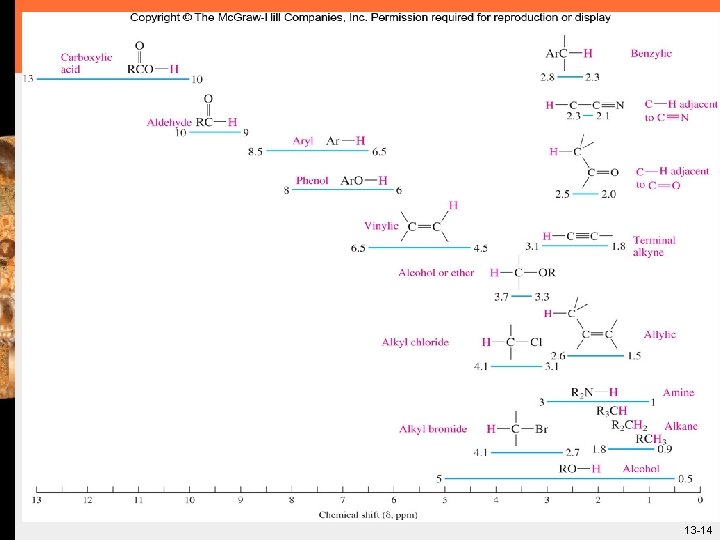
Chemical Shift - 1 H-NMR § Figure 13. 8 Average ranges of chemical shifts of representative types of hydrogens. 13 -13

13 -14

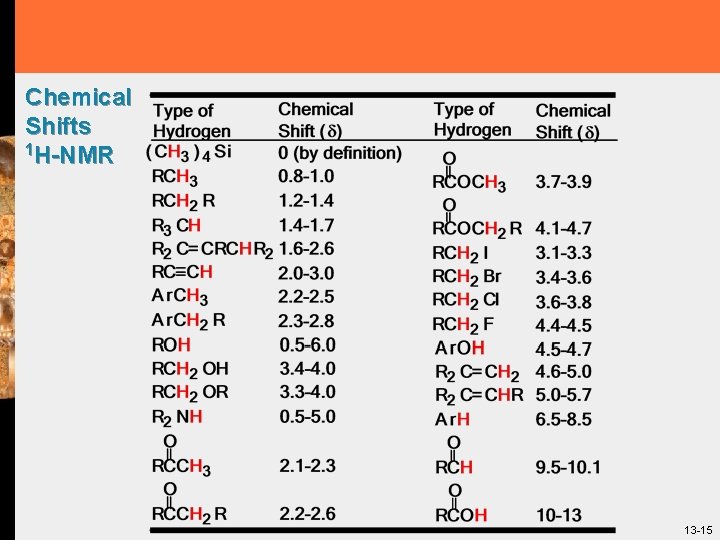
Chemical Shifts 1 H-NMR 13 -15

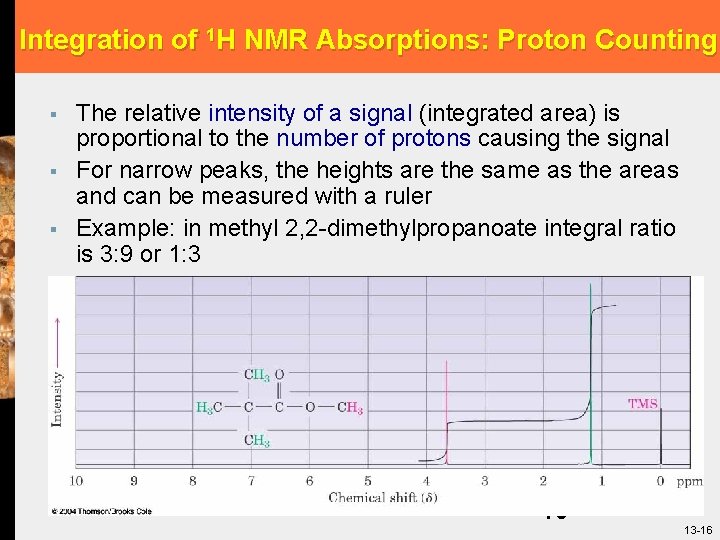
Integration of 1 H NMR Absorptions: Proton Counting § § § The relative intensity of a signal (integrated area) is proportional to the number of protons causing the signal For narrow peaks, the heights are the same as the areas and can be measured with a ruler Example: in methyl 2, 2 -dimethylpropanoate integral ratio is 3: 9 or 1: 3 16 13 -16

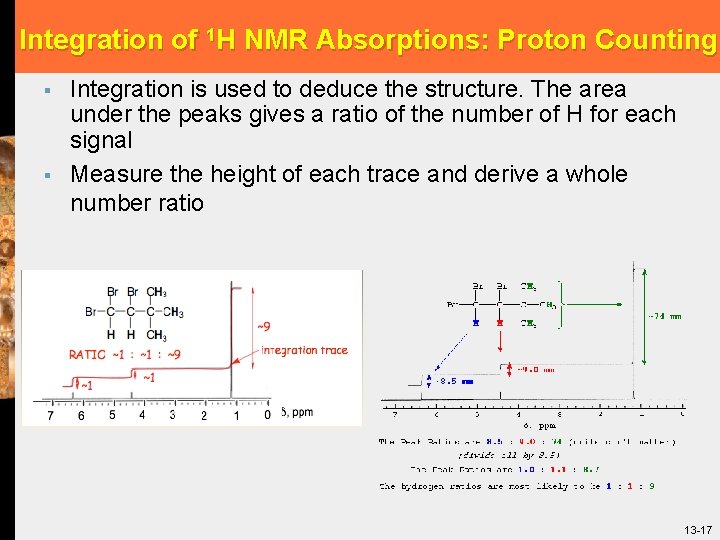
Integration of 1 H NMR Absorptions: Proton Counting § § Integration is used to deduce the structure. The area under the peaks gives a ratio of the number of H for each signal Measure the height of each trace and derive a whole number ratio 13 -17

Good Luck 13 -18
 Peta konsep dakwah periode madinah
Peta konsep dakwah periode madinah Manipal university chemistry department
Manipal university chemistry department Lesson 15 nuclear quest nuclear reactions
Lesson 15 nuclear quest nuclear reactions Fisión nuclear vs fision nuclear
Fisión nuclear vs fision nuclear Chernobyl nuclear disaster webquest
Chernobyl nuclear disaster webquest Nuclear chemistry
Nuclear chemistry Application of nuclear chemistry
Application of nuclear chemistry Application of nuclear chemistry
Application of nuclear chemistry Zeff trend
Zeff trend 12x12x12x12x12x12x12
12x12x12x12x12x12x12 Chapter 25 nuclear chemistry answer key
Chapter 25 nuclear chemistry answer key Nuclear chemistry review worksheet answer key
Nuclear chemistry review worksheet answer key 25 m/s
25 m/s Nuclear chemistry
Nuclear chemistry Rectangle real life examples
Rectangle real life examples Chapter 21 review nuclear chemistry
Chapter 21 review nuclear chemistry Copyright
Copyright Nitrogen-13 decay equation
Nitrogen-13 decay equation Applications of nuclear chemistry
Applications of nuclear chemistry