Islamic University in Madinah Department of Chemistry CHAPTER


- Slides: 41

Islamic University in Madinah Department of Chemistry CHAPTER – 1 PART-3 PETROCHEMICALS PREPARED BY DR. KHALID AHMAD SHADID CHEMISTRY DEPARTMENT – ISLAMIC UNIVERSITY IN MADINAH 1

PETROCHEMICALS Petrochemicals: Chemical compound or Elements derived partially or entirely from petroleum or natural gas and are intended for Chemical markets. Primary petrochemicals are divided into three groups depending on their chemical structure. v Olefins include ethylene, propylene and butadiene. v Aromatics include benzene, toluene and xylenes. v Synthesis gas is a mixture of carbon monoxide and hydrogen used to make ammonia and methanol. Five main molecules are: (Five big of small molecules) Ethylene, Benzene, Propene, Methane, and then butane 2 Another main molecules: isobutene, butadiene, isoprene, toluene and xylene

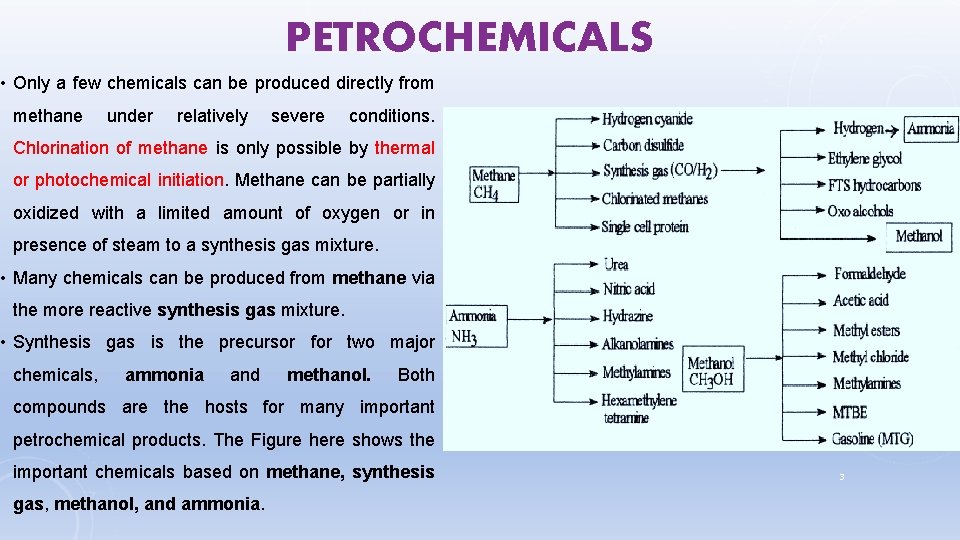
PETROCHEMICALS • Only a few chemicals can be produced directly from methane under relatively severe conditions. Chlorination of methane is only possible by thermal or photochemical initiation. Methane can be partially oxidized with a limited amount of oxygen or in presence of steam to a synthesis gas mixture. • Many chemicals can be produced from methane via the more reactive synthesis gas mixture. • Synthesis gas is the precursor for two major chemicals, ammonia and methanol. Both compounds are the hosts for many important petrochemical products. The Figure here shows the important chemicals based on methane, synthesis gas, methanol, and ammonia. 3

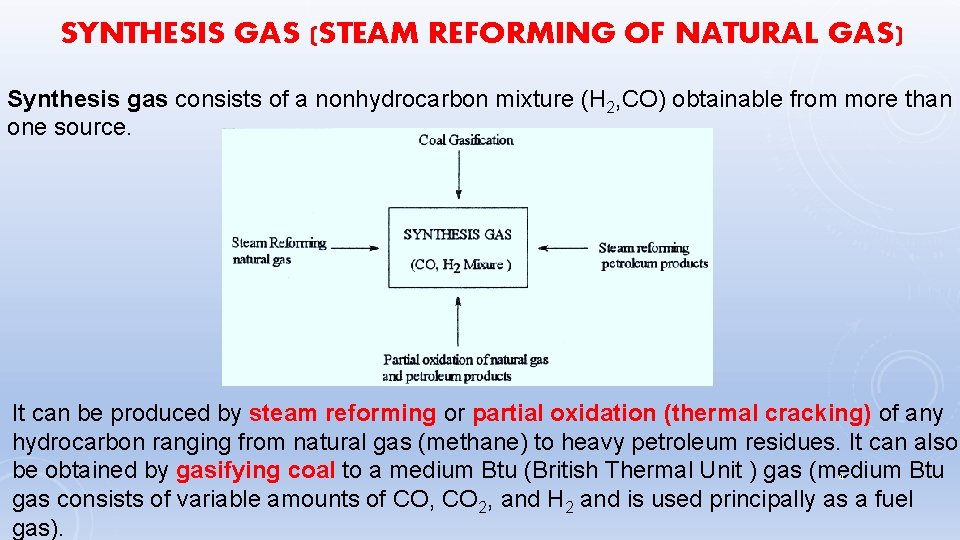
SYNTHESIS GAS (STEAM REFORMING OF NATURAL GAS) Synthesis gas consists of a nonhydrocarbon mixture (H 2, CO) obtainable from more than one source. It can be produced by steam reforming or partial oxidation (thermal cracking) of any hydrocarbon ranging from natural gas (methane) to heavy petroleum residues. It can also be obtained by gasifying coal to a medium Btu (British Thermal Unit ) gas (medium Btu 4 gas consists of variable amounts of CO, CO 2, and H 2 and is used principally as a fuel gas).

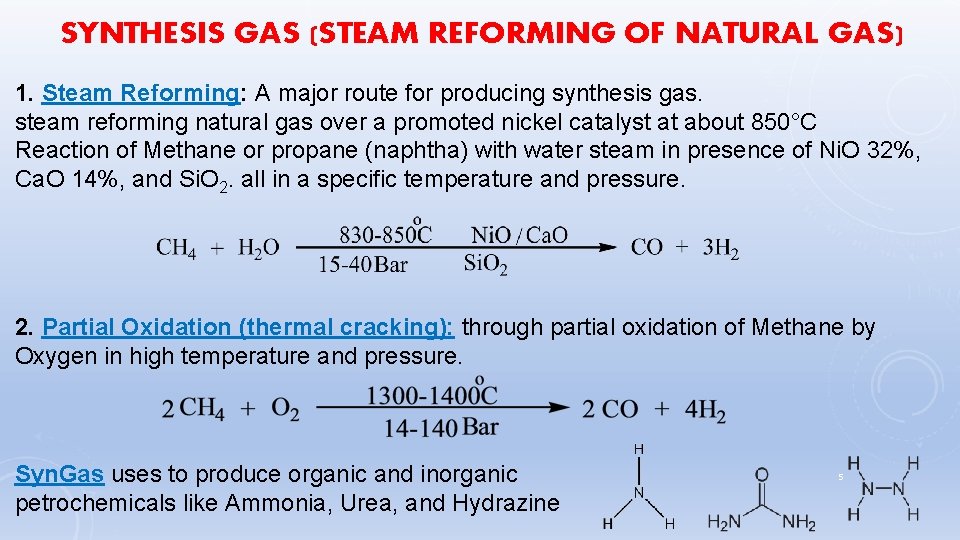
SYNTHESIS GAS (STEAM REFORMING OF NATURAL GAS) 1. Steam Reforming: A major route for producing synthesis gas. steam reforming natural gas over a promoted nickel catalyst at about 850°C Reaction of Methane or propane (naphtha) with water steam in presence of Ni. O 32%, Ca. O 14%, and Si. O 2. all in a specific temperature and pressure. 2. Partial Oxidation (thermal cracking): through partial oxidation of Methane by Oxygen in high temperature and pressure. Syn. Gas uses to produce organic and inorganic petrochemicals like Ammonia, Urea, and Hydrazine 5

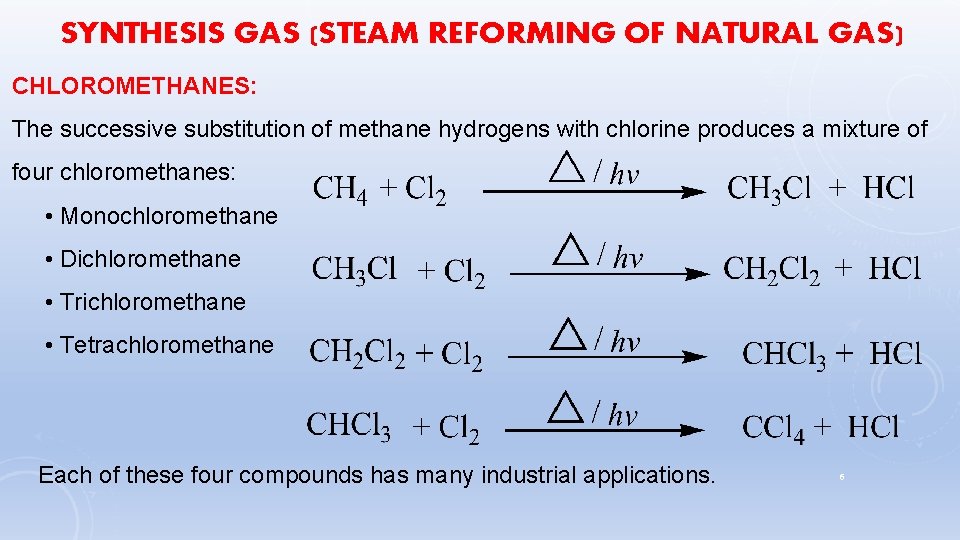
SYNTHESIS GAS (STEAM REFORMING OF NATURAL GAS) CHLOROMETHANES: The successive substitution of methane hydrogens with chlorine produces a mixture of four chloromethanes: • Monochloromethane • Dichloromethane • Trichloromethane • Tetrachloromethane Each of these four compounds has many industrial applications. 6

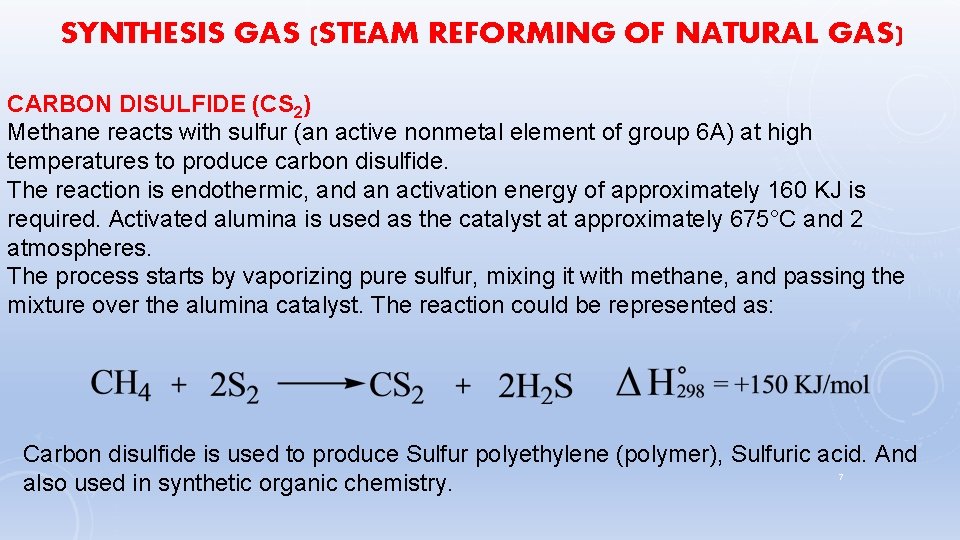
SYNTHESIS GAS (STEAM REFORMING OF NATURAL GAS) CARBON DISULFIDE (CS 2) Methane reacts with sulfur (an active nonmetal element of group 6 A) at high temperatures to produce carbon disulfide. The reaction is endothermic, and an activation energy of approximately 160 KJ is required. Activated alumina is used as the catalyst at approximately 675°C and 2 atmospheres. The process starts by vaporizing pure sulfur, mixing it with methane, and passing the mixture over the alumina catalyst. The reaction could be represented as: Carbon disulfide is used to produce Sulfur polyethylene (polymer), Sulfuric acid. And 7 also used in synthetic organic chemistry.

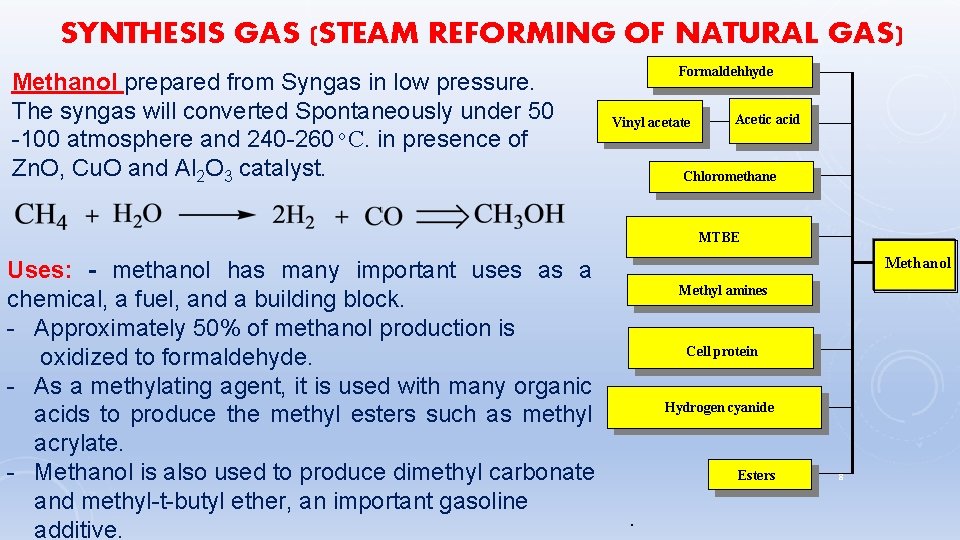
SYNTHESIS GAS (STEAM REFORMING OF NATURAL GAS) Methanol prepared from Syngas in low pressure. The syngas will converted Spontaneously under 50 -100 atmosphere and 240 -260 C. in presence of Zn. O, Cu. O and Al 2 O 3 catalyst. Formaldehhyde Vinyl acetate Chloromethane Uses: - methanol has many important uses as a chemical, a fuel, and a building block. - Approximately 50% of methanol production is oxidized to formaldehyde. - As a methylating agent, it is used with many organic acids to produce the methyl esters such as methyl acrylate. - Methanol is also used to produce dimethyl carbonate and methyl-t-butyl ether, an important gasoline additive. Acetic acid MTBE Methanol Methyl amines Cell protein Hydrogen cyanide Esters . 8

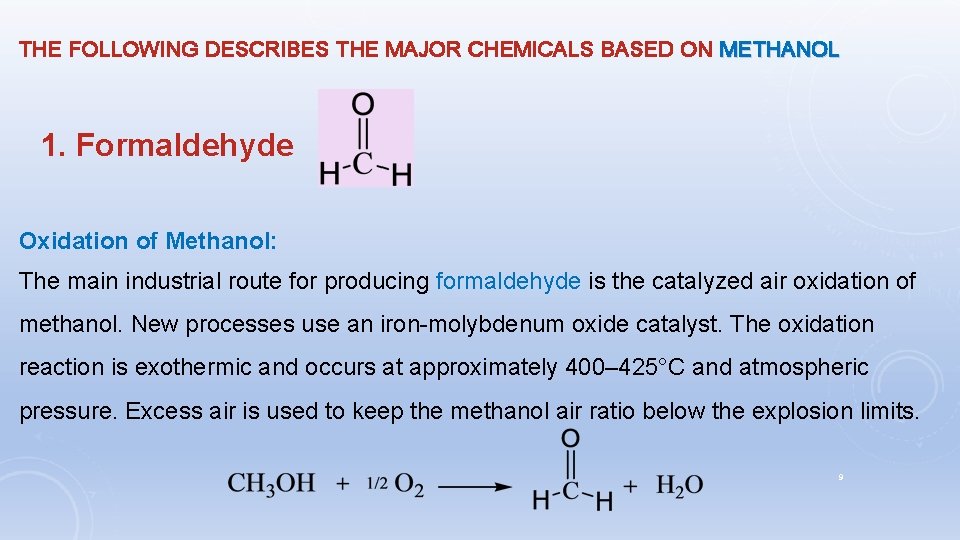
THE FOLLOWING DESCRIBES THE MAJOR CHEMICALS BASED ON METHANOL 1. Formaldehyde Oxidation of Methanol: The main industrial route for producing formaldehyde is the catalyzed air oxidation of methanol. New processes use an iron-molybdenum oxide catalyst. The oxidation reaction is exothermic and occurs at approximately 400– 425°C and atmospheric pressure. Excess air is used to keep the methanol air ratio below the explosion limits. 9

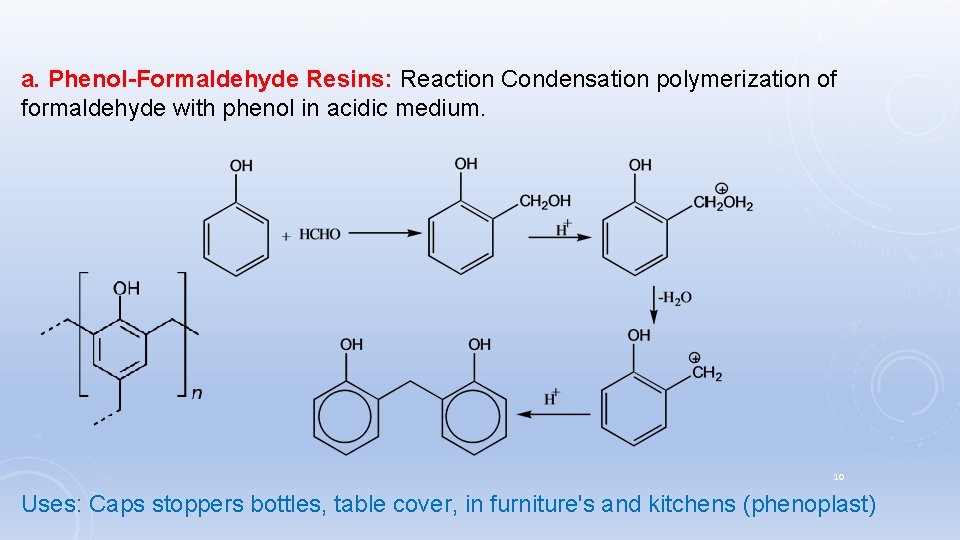
a. Phenol-Formaldehyde Resins: Reaction Condensation polymerization of formaldehyde with phenol in acidic medium. 10 Uses: Caps stoppers bottles, table cover, in furniture's and kitchens (phenoplast)

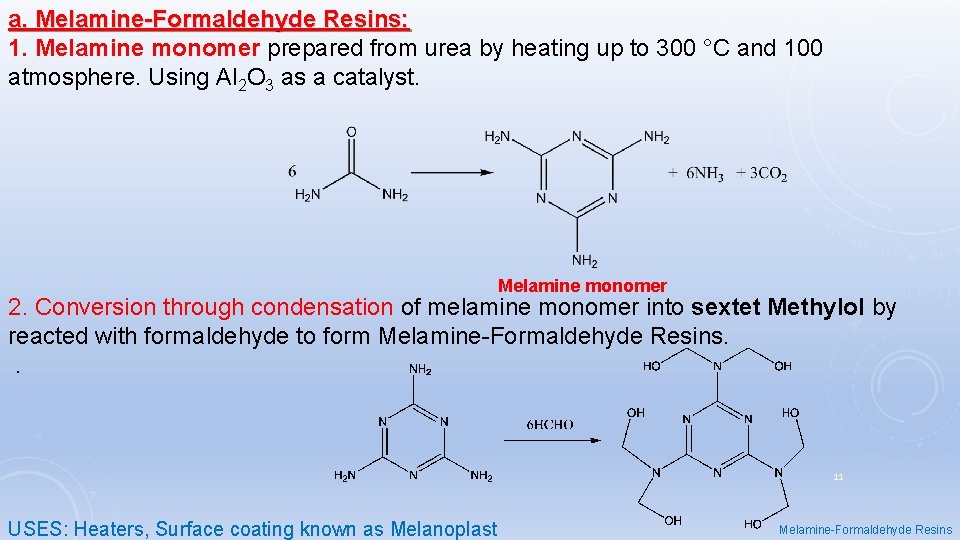
a. Melamine-Formaldehyde Resins: 1. Melamine monomer prepared from urea by heating up to 300 °C and 100 atmosphere. Using Al 2 O 3 as a catalyst. Melamine monomer 2. Conversion through condensation of melamine monomer into sextet Methylol by reacted with formaldehyde to form Melamine-Formaldehyde Resins. . 11 USES: Heaters, Surface coating known as Melanoplast Melamine-Formaldehyde Resins

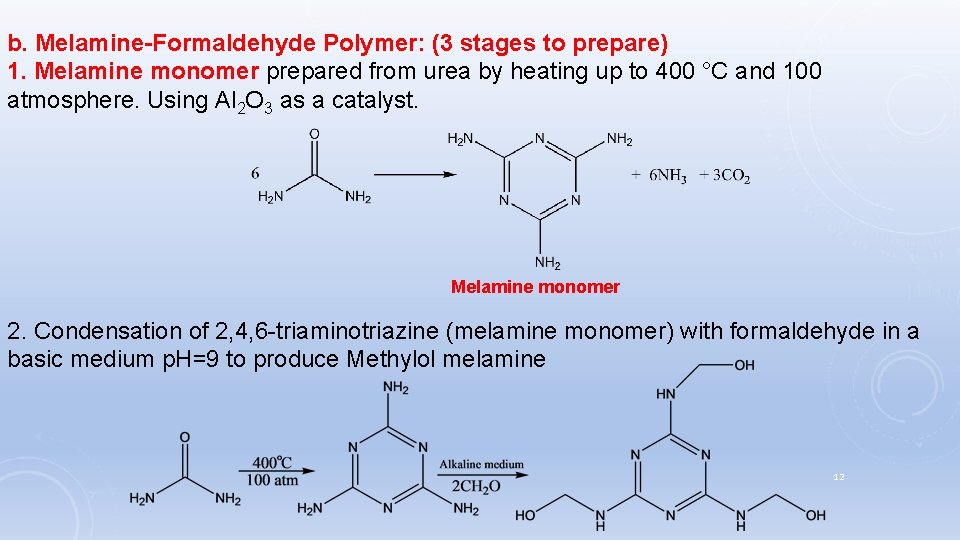
b. Melamine-Formaldehyde Polymer: (3 stages to prepare) 1. Melamine monomer prepared from urea by heating up to 400 °C and 100 atmosphere. Using Al 2 O 3 as a catalyst. Melamine monomer 2. Condensation of 2, 4, 6 -triaminotriazine (melamine monomer) with formaldehyde in a basic medium p. H=9 to produce Methylol melamine 12

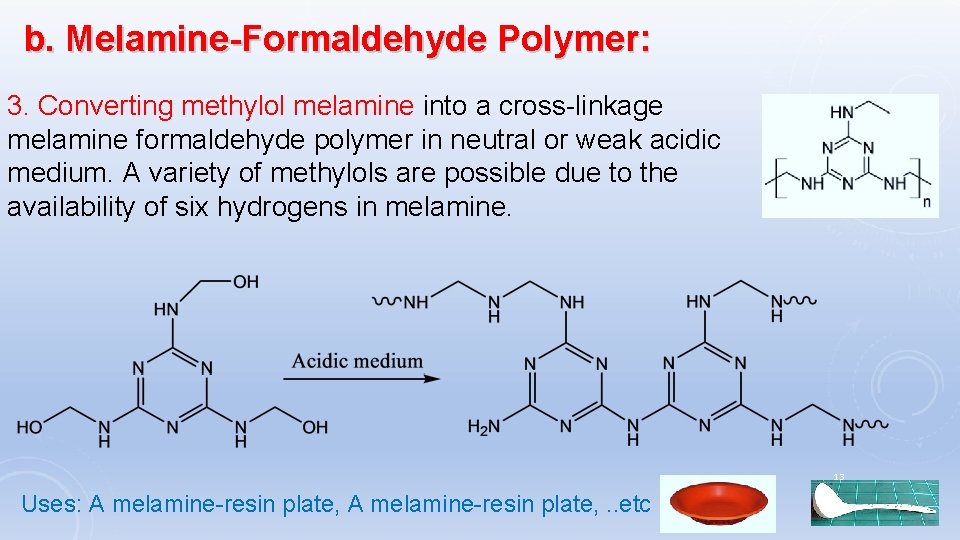
b. Melamine-Formaldehyde Polymer: 3. Converting methylol melamine into a cross-linkage melamine formaldehyde polymer in neutral or weak acidic medium. A variety of methylols are possible due to the availability of six hydrogens in melamine. 13 Uses: A melamine-resin plate, . . etc

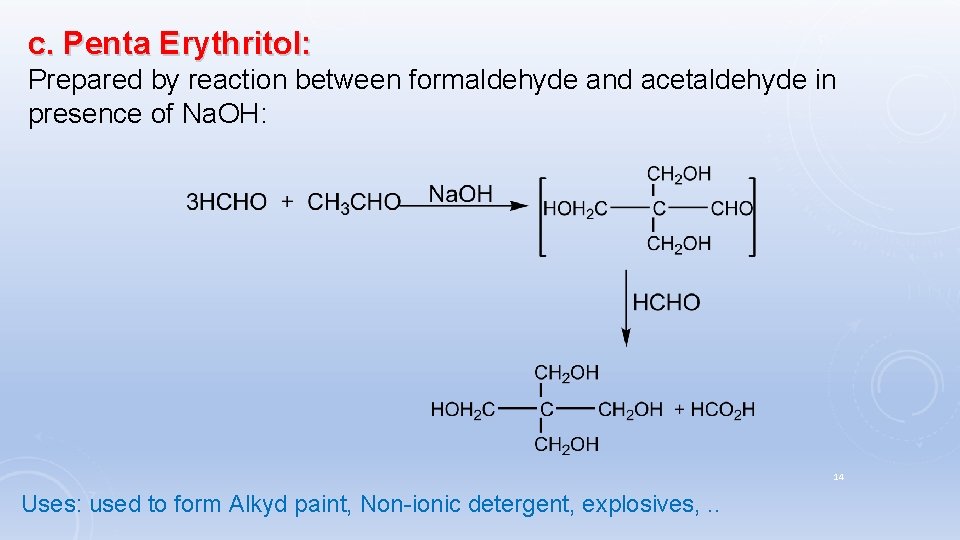
c. Penta Erythritol: Prepared by reaction between formaldehyde and acetaldehyde in presence of Na. OH: 14 Uses: used to form Alkyd paint, Non-ionic detergent, explosives, . .

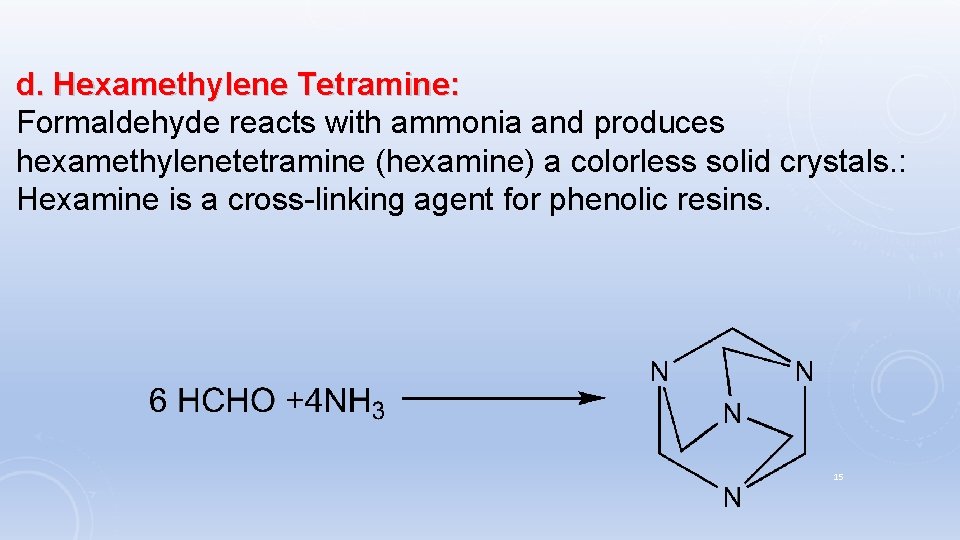
d. Hexamethylene Tetramine: Formaldehyde reacts with ammonia and produces hexamethylenetetramine (hexamine) a colorless solid crystals. : Hexamine is a cross-linking agent for phenolic resins. 15

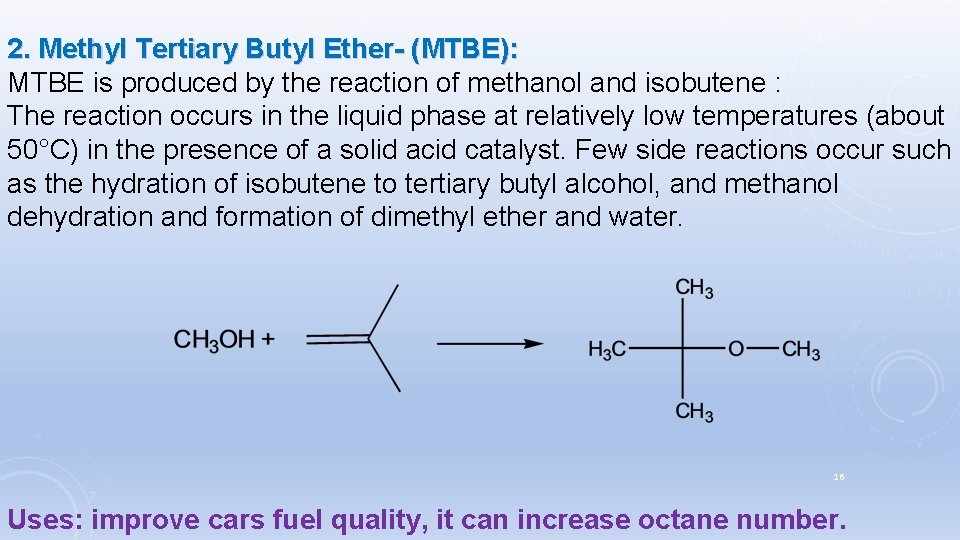
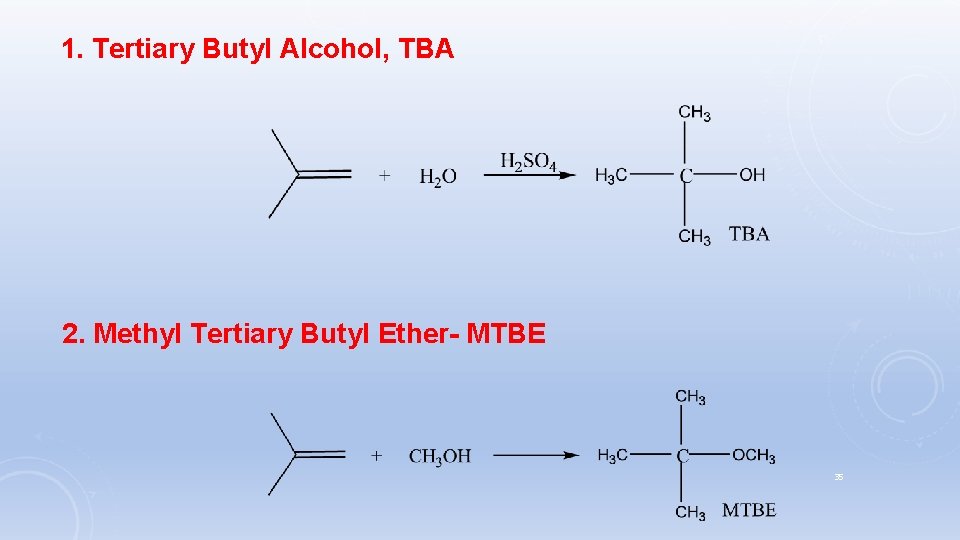
2. Methyl Tertiary Butyl Ether- (MTBE): MTBE is produced by the reaction of methanol and isobutene : The reaction occurs in the liquid phase at relatively low temperatures (about 50°C) in the presence of a solid acid catalyst. Few side reactions occur such as the hydration of isobutene to tertiary butyl alcohol, and methanol dehydration and formation of dimethyl ether and water. 16 Uses: improve cars fuel quality, it can increase octane number.

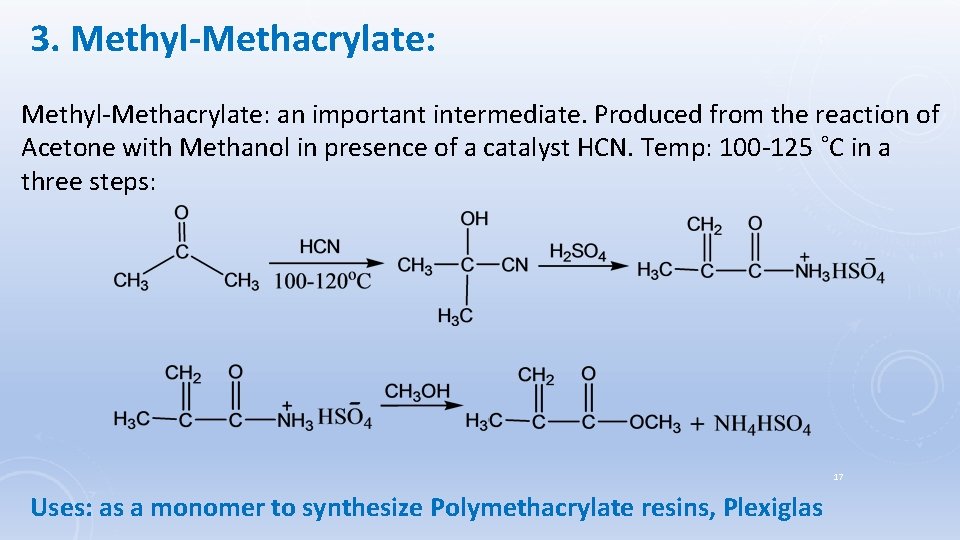
3. Methyl-Methacrylate: an important intermediate. Produced from the reaction of Acetone with Methanol in presence of a catalyst HCN. Temp: 100 -125 ˚C in a three steps: 17 Uses: as a monomer to synthesize Polymethacrylate resins, Plexiglas

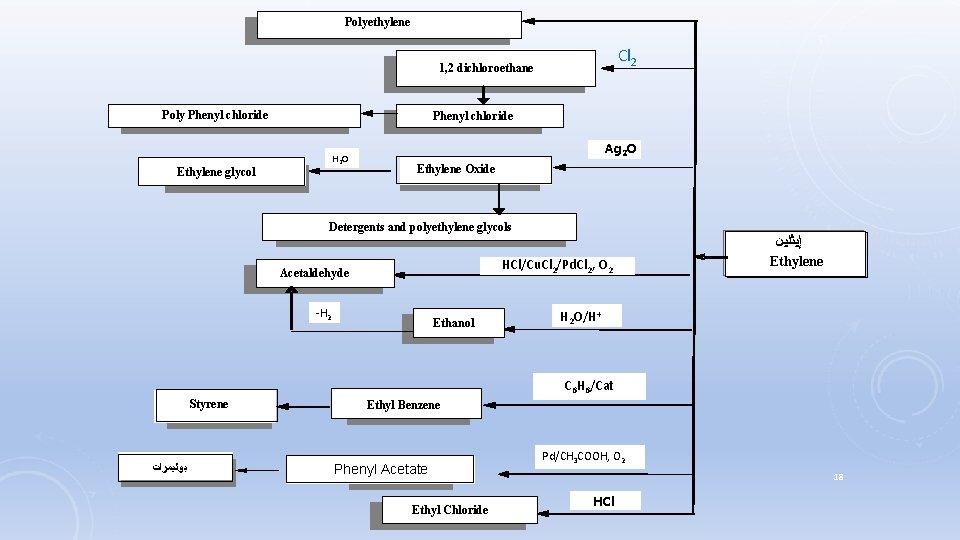
Polyethylene Cl 2 1, 2 dichloroethane Poly Phenyl chloride H 2 O Ethylene glycol Ag 2 O Ethylene Oxide Detergents and polyethylene glycols HCl/Cu. Cl 2/Pd. Cl 2, O 2 Acetaldehyde -H 2 Ethanol ﺇﻴﺜﻠﻴﻦ Ethylene H 2 O/H+ C 6 H 6/Cat Styrene ﺑﻮﻟﻴﻤﺮﺍﺕ Ethyl Benzene Phenyl Acetate Ethyl Chloride Pd/CH 3 COOH, O 2 18 HCl

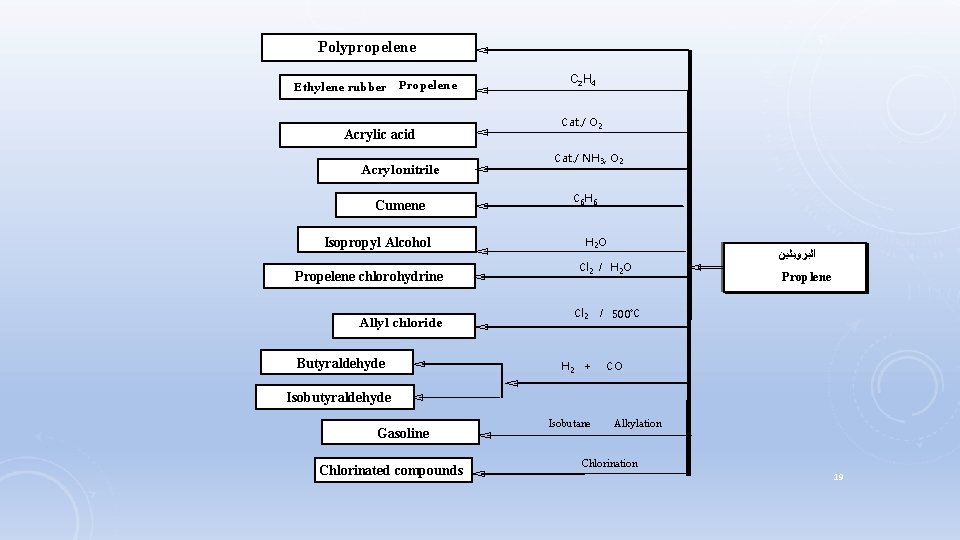
Polypropelene Ethylene rubber Propelene Acrylic acid Acrylonitrile Cumene Isopropyl Alcohol Propelene chlorohydrine Allyl chloride Butyraldehyde C 2 H 4 Cat. / O 2 Cat. / NH 3, O 2 C 6 H 6 H 2 O Cl 2 / H 2 O Cl 2 H 2 + ﺍﻟﺒﺮﻭﺑﻠﻴﻦ Proplene / 500˚C CO Isobutyraldehyde Gasoline Chlorinated compounds Isobutane Alkylation - Chlorination 19

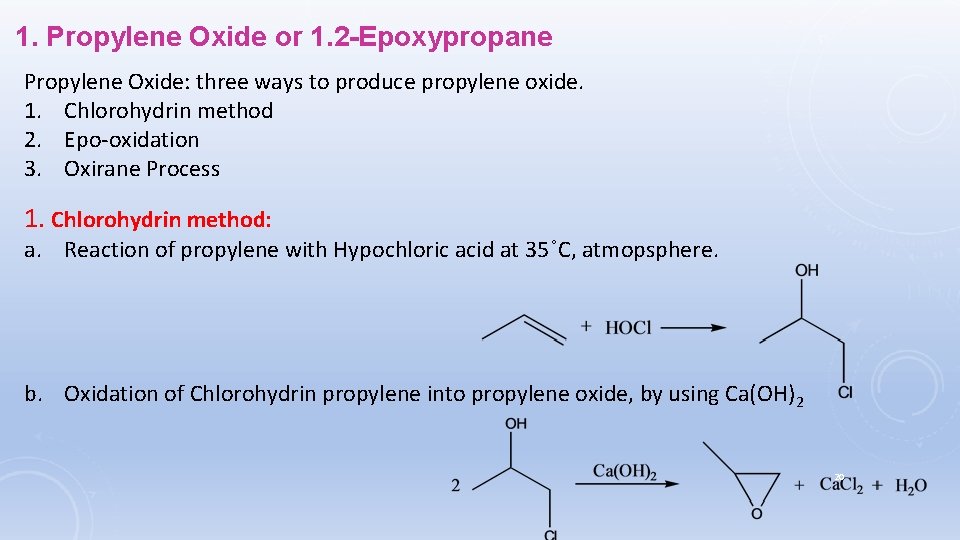
1. Propylene Oxide or 1. 2 -Epoxypropane Propylene Oxide: three ways to produce propylene oxide. 1. Chlorohydrin method 2. Epo-oxidation 3. Oxirane Process 1. Chlorohydrin method: a. Reaction of propylene with Hypochloric acid at 35˚C, atmopsphere. b. Oxidation of Chlorohydrin propylene into propylene oxide, by using Ca(OH)2 20

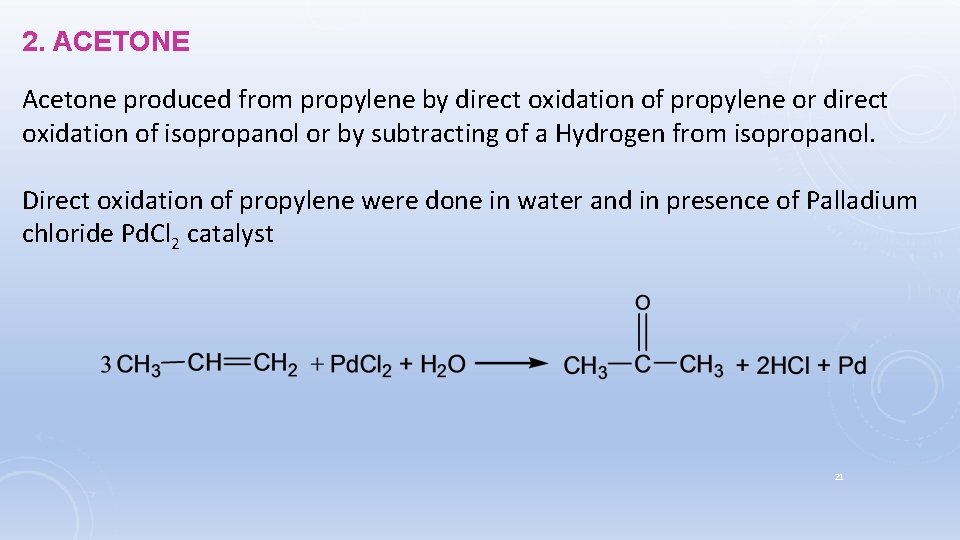
2. ACETONE Acetone produced from propylene by direct oxidation of propylene or direct oxidation of isopropanol or by subtracting of a Hydrogen from isopropanol. Direct oxidation of propylene were done in water and in presence of Palladium chloride Pd. Cl 2 catalyst 21

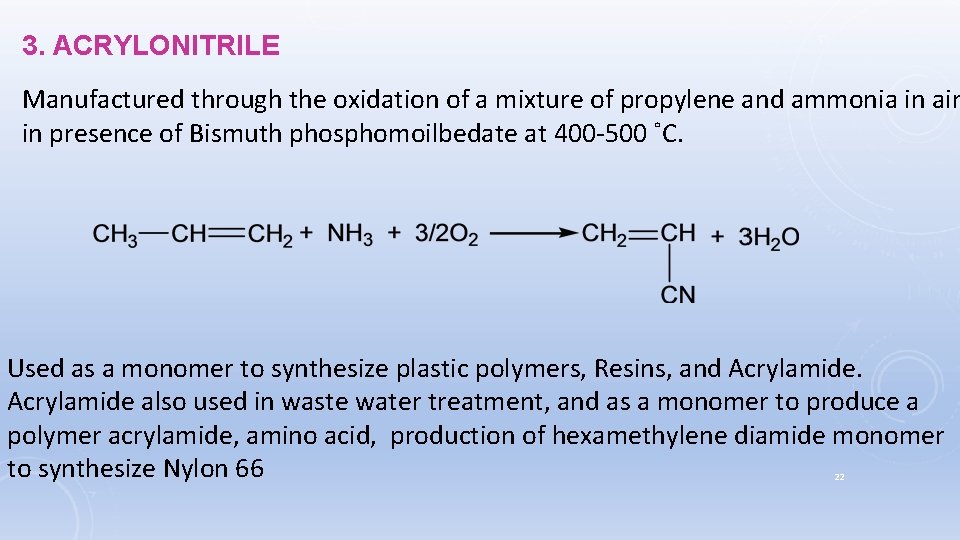
3. ACRYLONITRILE Manufactured through the oxidation of a mixture of propylene and ammonia in air in presence of Bismuth phosphomoilbedate at 400 -500 ˚C. Used as a monomer to synthesize plastic polymers, Resins, and Acrylamide also used in waste water treatment, and as a monomer to produce a polymer acrylamide, amino acid, production of hexamethylene diamide monomer to synthesize Nylon 66 22

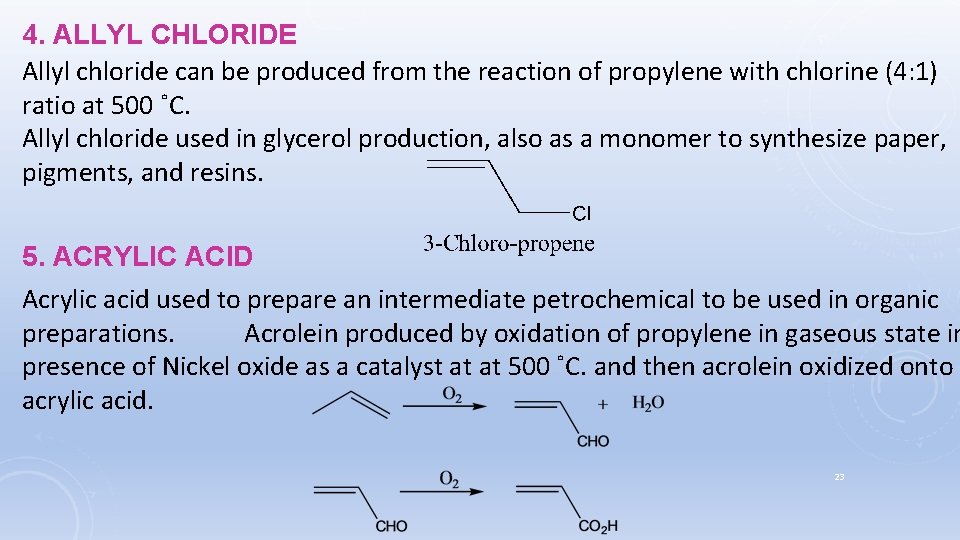
4. ALLYL CHLORIDE Allyl chloride can be produced from the reaction of propylene with chlorine (4: 1) ratio at 500 ˚C. Allyl chloride used in glycerol production, also as a monomer to synthesize paper, pigments, and resins. 5. ACRYLIC ACID Acrylic acid used to prepare an intermediate petrochemical to be used in organic preparations. Acrolein produced by oxidation of propylene in gaseous state in presence of Nickel oxide as a catalyst at at 500 ˚C. and then acrolein oxidized onto acrylic acid. 23

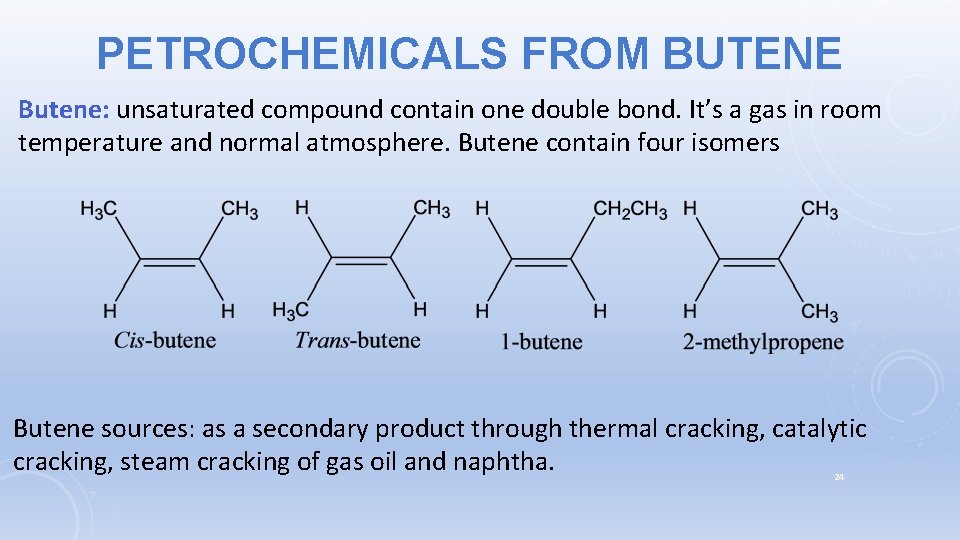
PETROCHEMICALS FROM BUTENE Butene: unsaturated compound contain one double bond. It’s a gas in room temperature and normal atmosphere. Butene contain four isomers Butene sources: as a secondary product through thermal cracking, catalytic cracking, steam cracking of gas oil and naphtha. 24

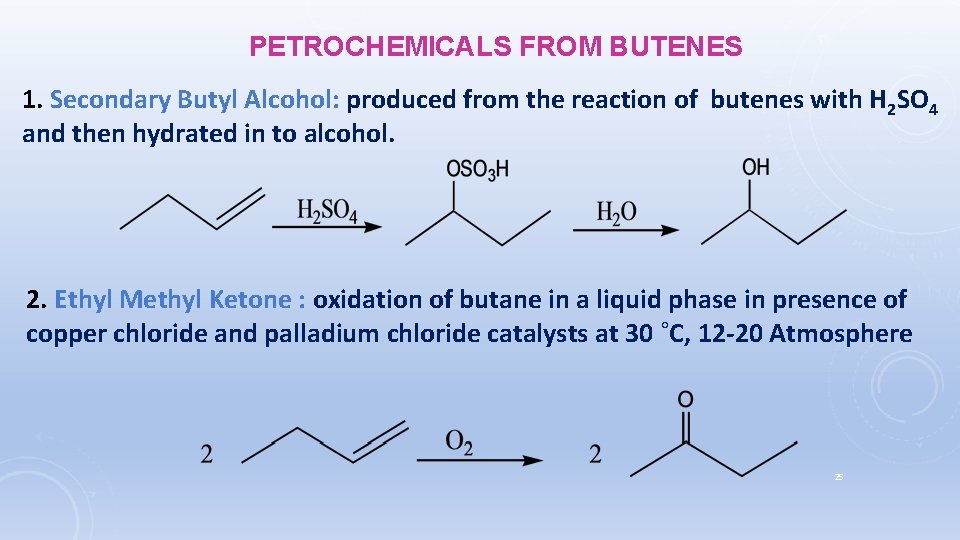
PETROCHEMICALS FROM BUTENES 1. Secondary Butyl Alcohol: produced from the reaction of butenes with H 2 SO 4 and then hydrated in to alcohol. 2. Ethyl Methyl Ketone : oxidation of butane in a liquid phase in presence of copper chloride and palladium chloride catalysts at 30 ˚C, 12 -20 Atmosphere 25

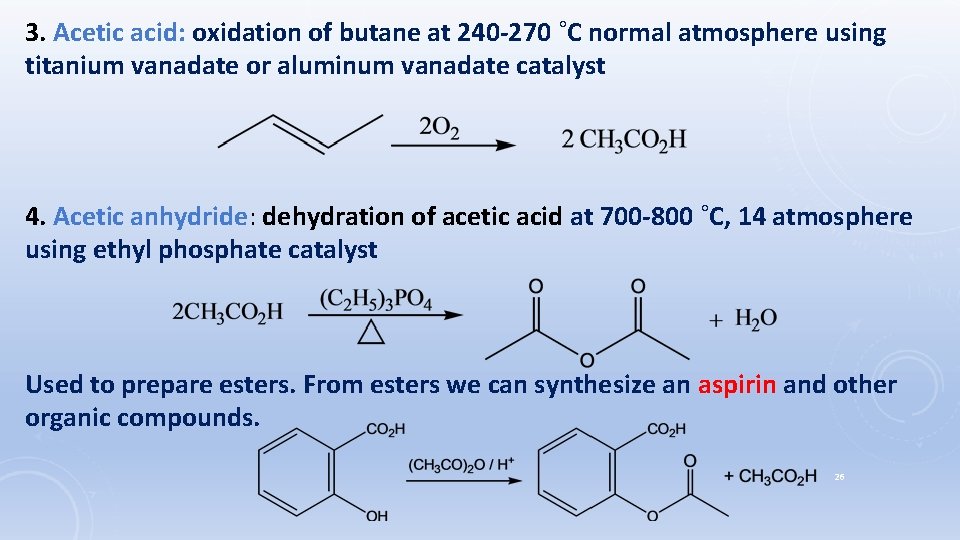
3. Acetic acid: oxidation of butane at 240 -270 ˚C normal atmosphere using titanium vanadate or aluminum vanadate catalyst 4. Acetic anhydride: dehydration of acetic acid at 700 -800 ˚C, 14 atmosphere using ethyl phosphate catalyst Used to prepare esters. From esters we can synthesize an aspirin and other organic compounds. 26

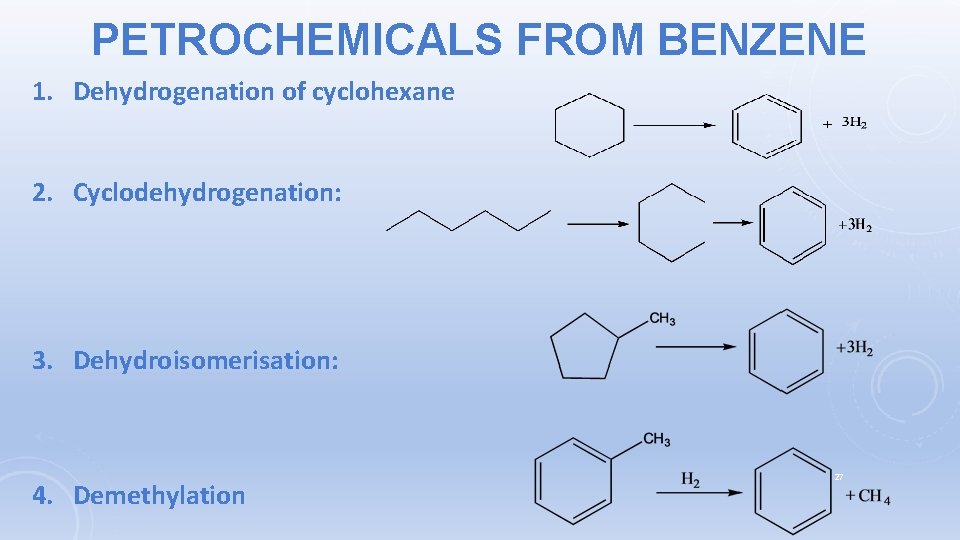
PETROCHEMICALS FROM BENZENE 1. Dehydrogenation of cyclohexane 2. Cyclodehydrogenation: 3. Dehydroisomerisation: 4. Demethylation 27

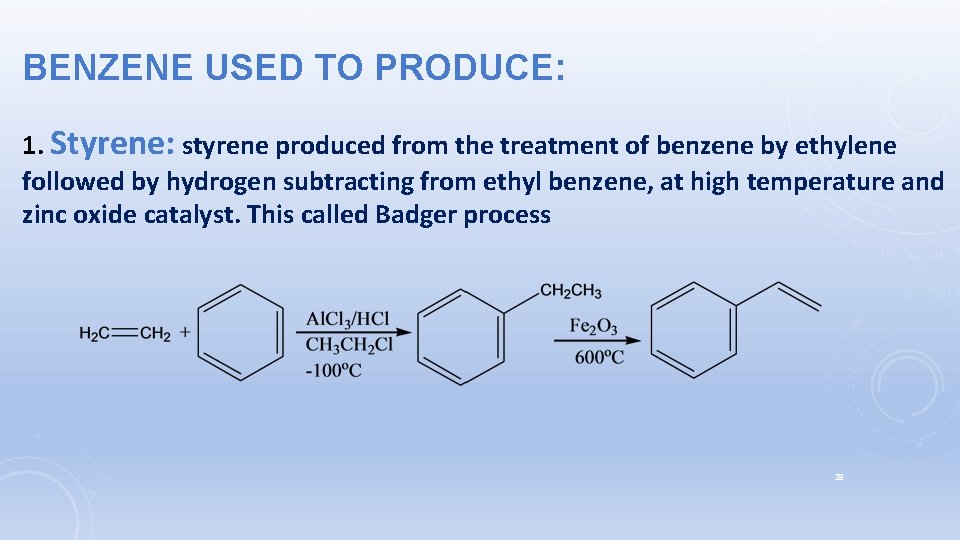
BENZENE USED TO PRODUCE: 1. Styrene: styrene produced from the treatment of benzene by ethylene followed by hydrogen subtracting from ethyl benzene, at high temperature and zinc oxide catalyst. This called Badger process 28

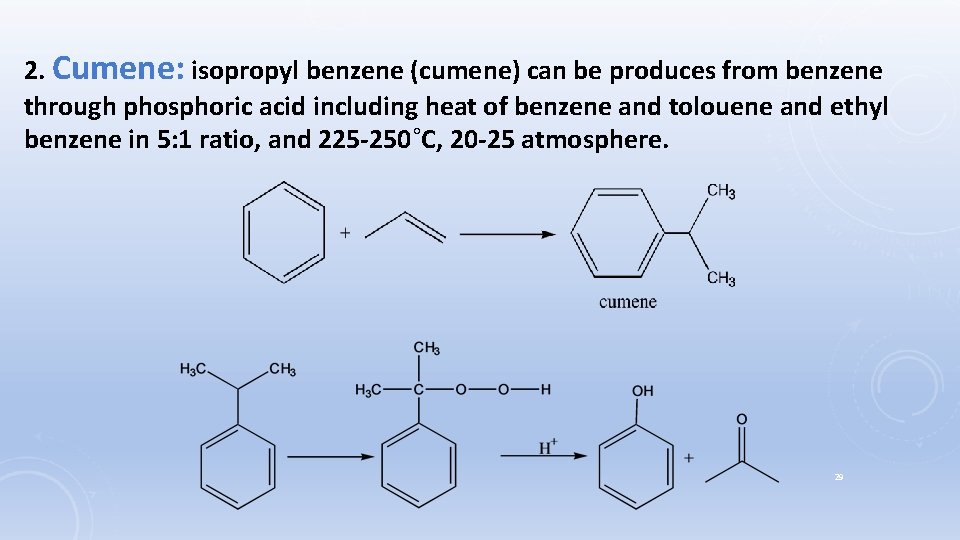
2. Cumene: isopropyl benzene (cumene) can be produces from benzene through phosphoric acid including heat of benzene and tolouene and ethyl benzene in 5: 1 ratio, and 225 -250˚C, 20 -25 atmosphere. 29

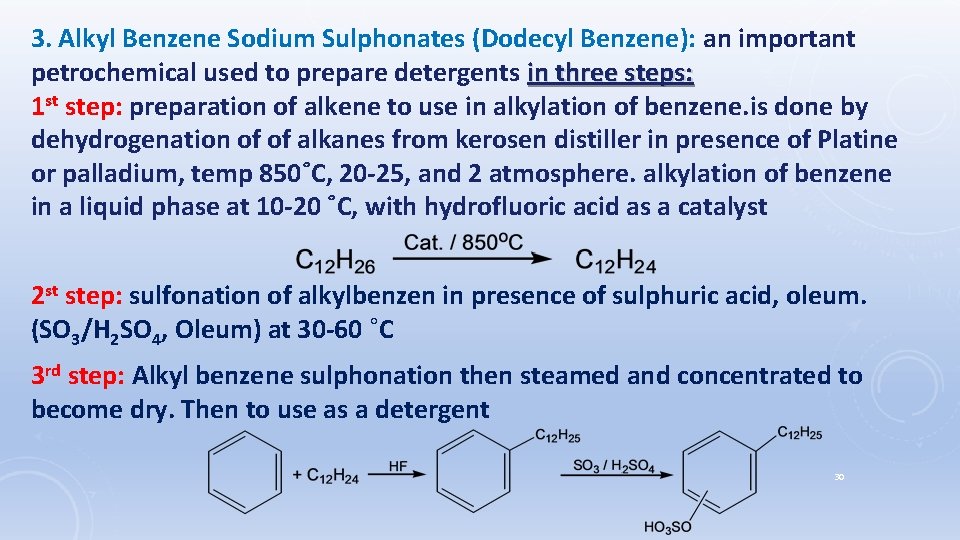
3. Alkyl Benzene Sodium Sulphonates (Dodecyl Benzene): an important petrochemical used to prepare detergents in three steps: 1 st step: preparation of alkene to use in alkylation of benzene. is done by dehydrogenation of of alkanes from kerosen distiller in presence of Platine or palladium, temp 850˚C, 20 -25, and 2 atmosphere. alkylation of benzene in a liquid phase at 10 -20 ˚C, with hydrofluoric acid as a catalyst 2 st step: sulfonation of alkylbenzen in presence of sulphuric acid, oleum. (SO 3/H 2 SO 4, Oleum) at 30 -60 ˚C 3 rd step: Alkyl benzene sulphonation then steamed and concentrated to become dry. Then to use as a detergent 30

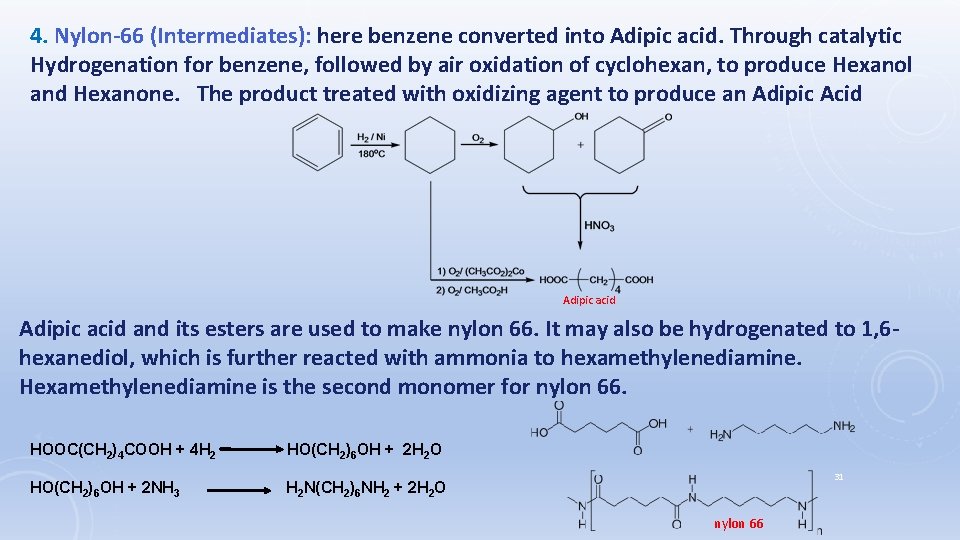
4. Nylon-66 (Intermediates): here benzene converted into Adipic acid. Through catalytic Hydrogenation for benzene, followed by air oxidation of cyclohexan, to produce Hexanol and Hexanone. The product treated with oxidizing agent to produce an Adipic Acid Adipic acid and its esters are used to make nylon 66. It may also be hydrogenated to 1, 6 hexanediol, which is further reacted with ammonia to hexamethylenediamine. Hexamethylenediamine is the second monomer for nylon 66. HOOC(CH 2)4 COOH + 4 H 2 HO(CH 2)6 OH + 2 H 2 O HO(CH 2)6 OH + 2 NH 3 H 2 N(CH 2)6 NH 2 + 2 H 2 O 31 nylon 66

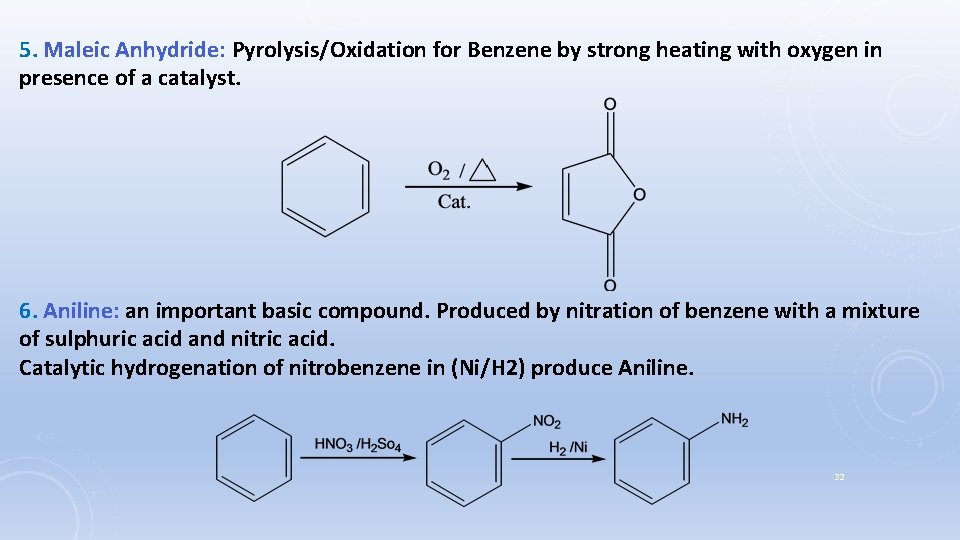
5. Maleic Anhydride: Pyrolysis/Oxidation for Benzene by strong heating with oxygen in presence of a catalyst. 6. Aniline: an important basic compound. Produced by nitration of benzene with a mixture of sulphuric acid and nitric acid. Catalytic hydrogenation of nitrobenzene in (Ni/H 2) produce Aniline. 32

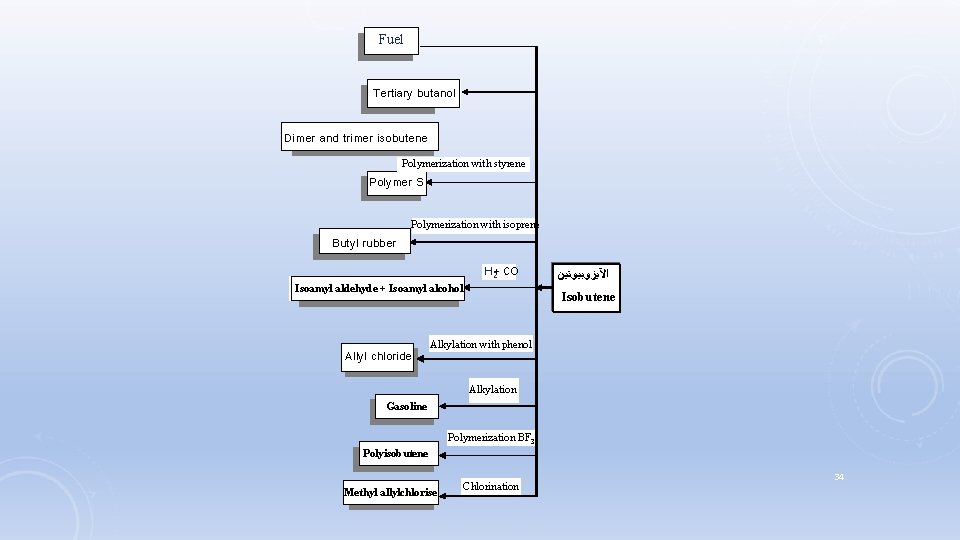
MISCELLANEOUS PETROCHEMICALS Five main molecules are: (Five big of small molecules) Ethylene, Benzene, Propene, Methane, and then butane Another main molecules: Isobutene, Butadiene, Isoprene, Toluene and xylenes 1. Isobutene: Isobutene Produced as a secondary product. Through a thermal cracking, catalytic cracking, and steam cracking of a liquid of Gas oil and naphta. 33

Fuel Tertiary butanol Dimer and trimer isobutene Polymerization with styrene Polymer S Polymerization with isoprene Butyl rubber H 2+ CO Isoamyl aldehyde + Isoamyl alcohol Allyl chloride ﺍﻵﻴﺰﻭﺑﻴﻮﺗﻴﻦ Isobutene Alkylation with phenol Alkylation Gasoline Polymerization BF 3 Polyisobutene Methyl allylchlorise Chlorination 34

1. Tertiary Butyl Alcohol, TBA 2. Methyl Tertiary Butyl Ether- MTBE 35

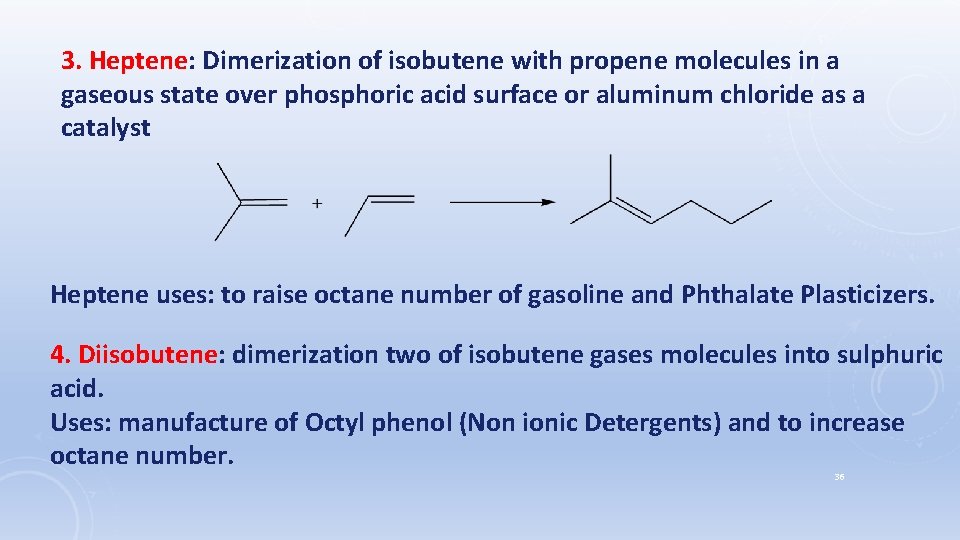
3. Heptene: Dimerization of isobutene with propene molecules in a gaseous state over phosphoric acid surface or aluminum chloride as a catalyst Heptene uses: to raise octane number of gasoline and Phthalate Plasticizers. 4. Diisobutene: dimerization two of isobutene gases molecules into sulphuric acid. Uses: manufacture of Octyl phenol (Non ionic Detergents) and to increase octane number. 36

5. Butyl Rubber: Butyl rubber vulcanizates have tensile strengths up to 2, 000 psi, and are characterized by low permeability to air and a high resistance to many chemicals and to oxidation. These properties make it a suitable rubber for the production of tire inner tubes and inner liners of tubeless tires. The major use of butyl rubber is for inner tubes. Other uses include wire and cable insulation, steam hoses, mechanical goods, and adhesives. Chlorinated butyl is a low molecular weight polymer used as an adhesive and a sealant. 37

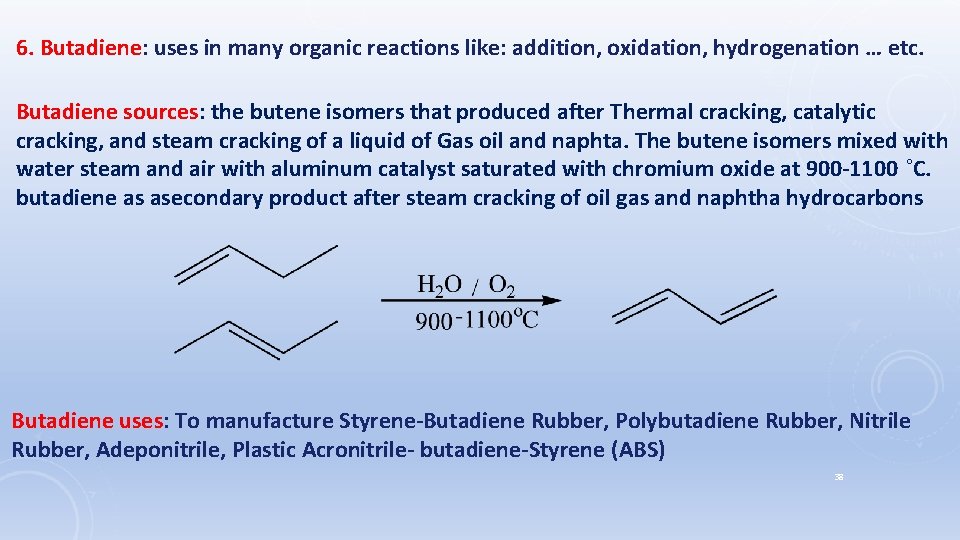
6. Butadiene: uses in many organic reactions like: addition, oxidation, hydrogenation … etc. Butadiene sources: the butene isomers that produced after Thermal cracking, catalytic cracking, and steam cracking of a liquid of Gas oil and naphta. The butene isomers mixed with water steam and air with aluminum catalyst saturated with chromium oxide at 900 -1100 ˚C. butadiene as asecondary product after steam cracking of oil gas and naphtha hydrocarbons Butadiene uses: To manufacture Styrene-Butadiene Rubber, Polybutadiene Rubber, Nitrile Rubber, Adeponitrile, Plastic Acronitrile- butadiene-Styrene (ABS) 38

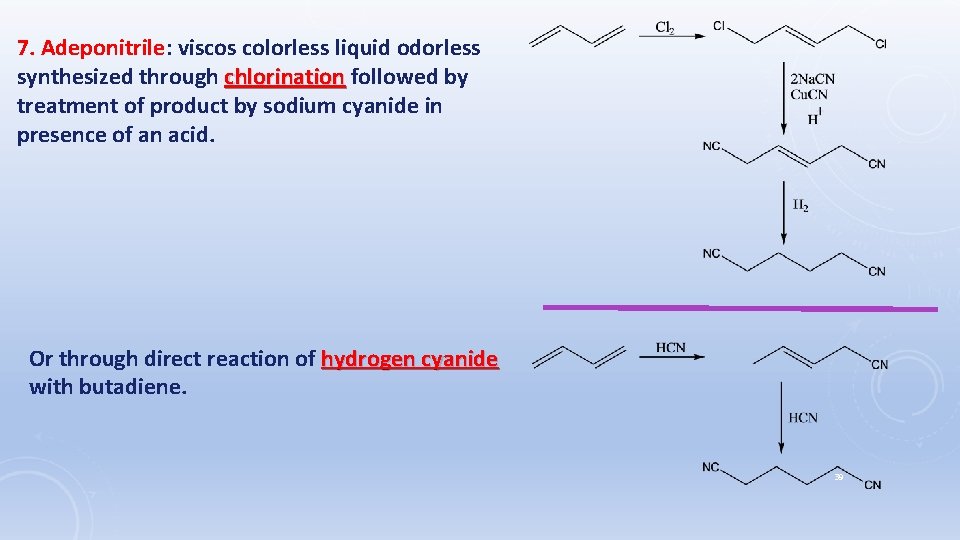
7. Adeponitrile: viscos colorless liquid odorless synthesized through chlorination followed by treatment of product by sodium cyanide in presence of an acid. Or through direct reaction of hydrogen cyanide with butadiene. 39

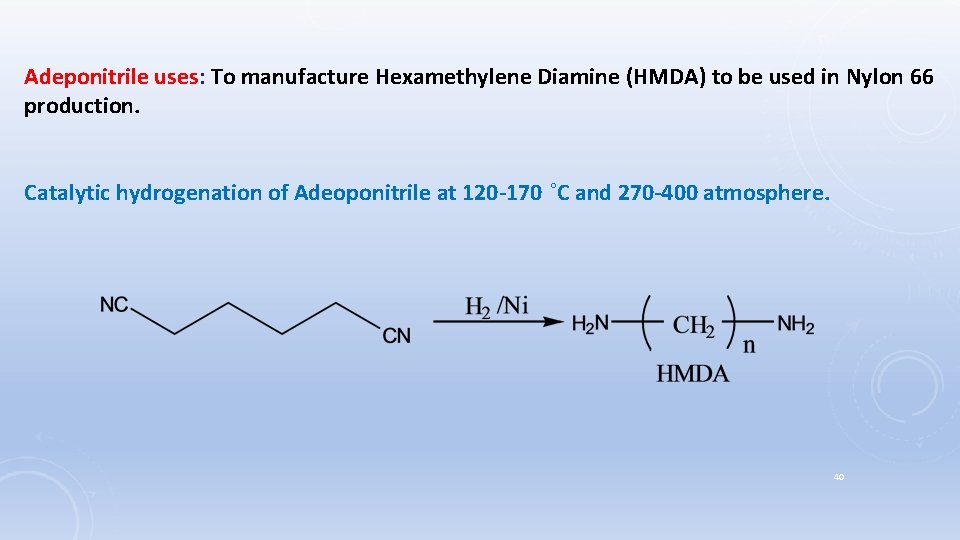
Adeponitrile uses: To manufacture Hexamethylene Diamine (HMDA) to be used in Nylon 66 production. Catalytic hydrogenation of Adeoponitrile at 120 -170 ˚C and 270 -400 atmosphere. 40

Thank You 41
 Peta konsep dakwah nabi muhammad
Peta konsep dakwah nabi muhammad Manipal university chemistry department
Manipal university chemistry department Islamic online university malaysia
Islamic online university malaysia Islamic university college
Islamic university college Tempoh dakwah secara sembunyi
Tempoh dakwah secara sembunyi Isi piagam madinah
Isi piagam madinah Dakwah rasulullah periode madinah berlangsung selama
Dakwah rasulullah periode madinah berlangsung selama Nasheed madina
Nasheed madina Metode dakwah nabi di madinah
Metode dakwah nabi di madinah Membangun masyarakat madinah
Membangun masyarakat madinah Siwy madinah
Siwy madinah Dawateislami istikhara bradford
Dawateislami istikhara bradford Madinah
Madinah Peta hijrah nabi
Peta hijrah nabi Ringkasan piagam madinah
Ringkasan piagam madinah Kerajaan islam di madinah
Kerajaan islam di madinah Chemistry department usf
Chemistry department usf Keralastec
Keralastec Texas tech lab explosion
Texas tech lab explosion Chapter 19 islamic gunpowder empires
Chapter 19 islamic gunpowder empires Chapter 9 lesson 3 islamic civilization
Chapter 9 lesson 3 islamic civilization Chapter 27 the islamic empires
Chapter 27 the islamic empires Chapter 27 the islamic empires
Chapter 27 the islamic empires Ib chemistry organic chemistry
Ib chemistry organic chemistry Organic vs inorganic chemistry
Organic vs inorganic chemistry Department of law university of jammu
Department of law university of jammu Department of geology university of dhaka
Department of geology university of dhaka Narrativistic
Narrativistic University of bridgeport it department
University of bridgeport it department Iowa state math department
Iowa state math department Department of physics university of tokyo
Department of physics university of tokyo Texas state university psychology department
Texas state university psychology department Department of information engineering university of padova
Department of information engineering university of padova Department of information engineering university of padova
Department of information engineering university of padova Syracuse university psychology department
Syracuse university psychology department Jackson state university finance department
Jackson state university finance department Columbia university department of computer science
Columbia university department of computer science Michigan state university physics department
Michigan state university physics department Columbia university cs department
Columbia university cs department University of sargodha engineering department
University of sargodha engineering department Stanford university philosophy department
Stanford university philosophy department University of chemistry and technology prague
University of chemistry and technology prague