In the name of God Protein misfolding diseases











- Slides: 27

In the name of God Protein misfolding diseases (Alzheimer’s) Introduction Protein misfolding diseases Alzheimer’s disease Preventing amyloid aggregate formation References Zeinab Monday, July-5 - Mokhtari

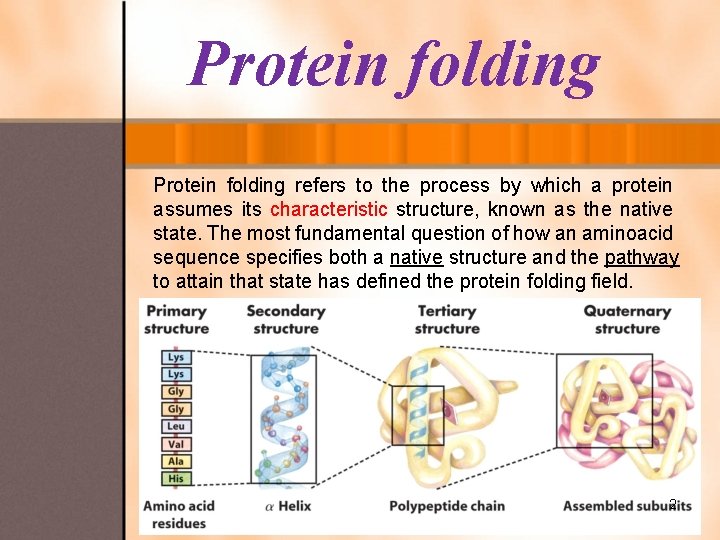
Protein folding refers to the process by which a protein assumes its characteristic structure, known as the native state. The most fundamental question of how an aminoacid sequence specifies both a native structure and the pathway to attain that state has defined the protein folding field. 2

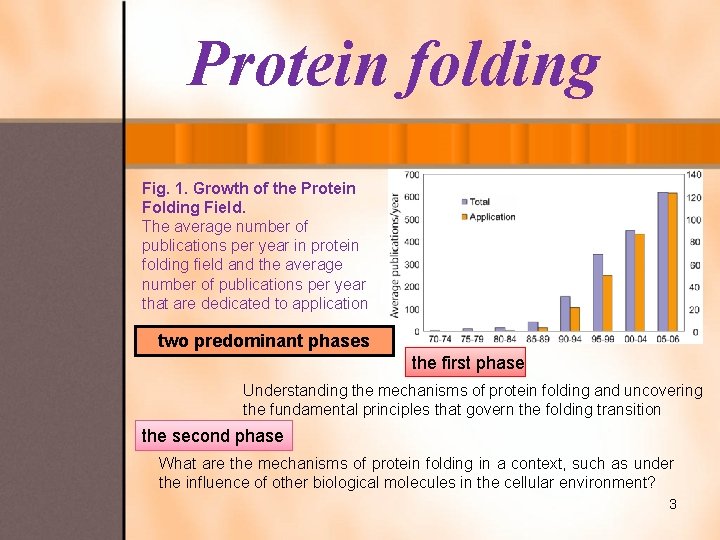
Protein folding Fig. 1. Growth of the Protein Folding Field. The average number of publications per year in protein folding field and the average number of publications per year that are dedicated to application two predominant phases the first phase Understanding the mechanisms of protein folding and uncovering the fundamental principles that govern the folding transition the second phase What are the mechanisms of protein folding in a context, such as under the influence of other biological molecules in the cellular environment? 3

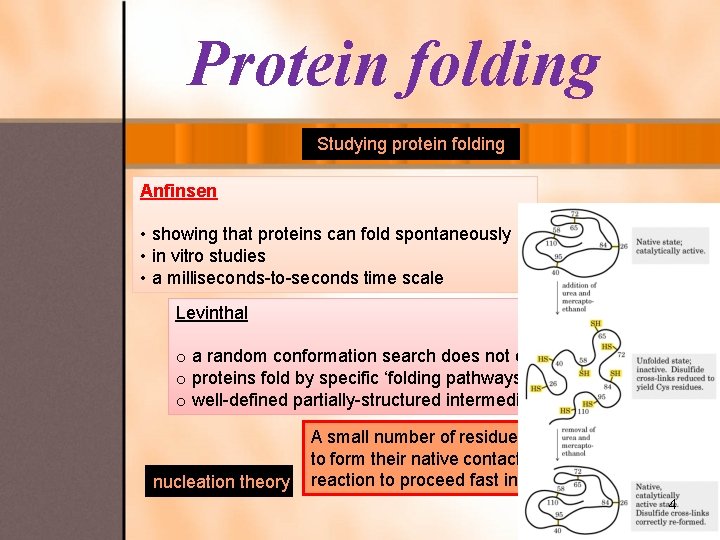
Protein folding Studying protein folding Anfinsen • showing that proteins can fold spontaneously • in vitro studies • a milliseconds-to-seconds time scale Levinthal o a random conformation search does not occur in folding o proteins fold by specific ‘folding pathways’ o well-defined partially-structured intermediate states nucleation theory A small number of residues (folding nucleus) need to form their native contacts in order for the folding reaction to proceed fast into the native state. 4

Protein folding Studying protein folding Experimental methods Protein engineering, nuclear magnetic resonance (NMR), mass spectrometry, hydrogen exchange, fluorescence resonance energy transfer (FRET), and atomic force microscopy (AFM) Computational methods ØThe Fold-Rate server (http: //psfs. cbrc. jp/fold-rate/) ØThe Parasol folding server (http: //parasol. tamu. edu/groups/amatogroup/foldingserver) … The close interplay of computational and experimental efforts has advanced our knowledge of protein folding kinetics, including predicting the protein folding rate, identifying the kinetically-important residues, and characterizing the multiple pathways. 5

Protein folding Two major differences between protein folding in vivo and in vitro : I. protein folding in vivo is usually assisted by molecular machinery, such as chaperones (in an ATP-dependent manner), and often involves small molecule cofactors. II. the concentrations of macromolecular solutes in cells can reach hundreds of grams per liter , but most in vitro studies are performed in buffered solution with <1% of the cellular macromolecule concentration. 6

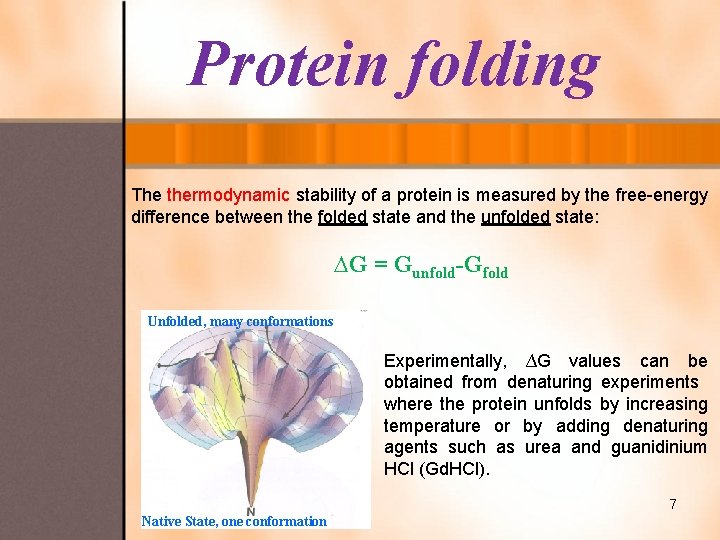
Protein folding The thermodynamic stability of a protein is measured by the free-energy difference between the folded state and the unfolded state: ∆G = Gunfold-Gfold Unfolded, many conformations Experimentally, ∆G values can be obtained from denaturing experiments where the protein unfolds by increasing temperature or by adding denaturing agents such as urea and guanidinium HCl (Gd. HCl). 7 Native State, one conformation

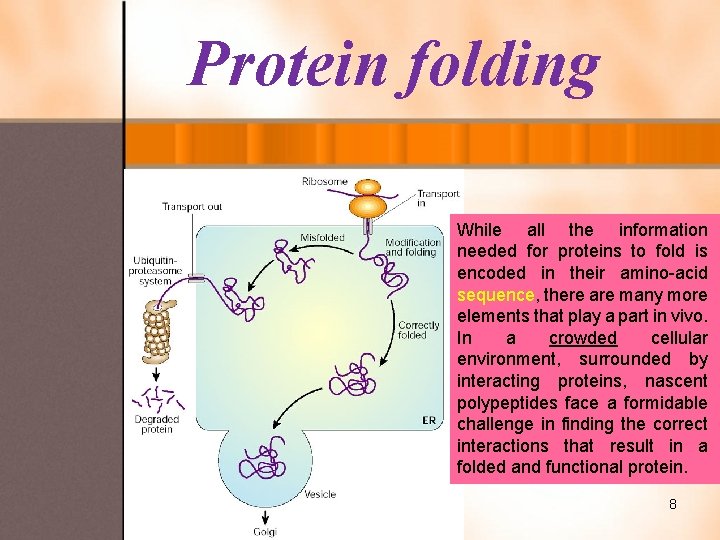
Protein folding While all the information needed for proteins to fold is encoded in their amino-acid sequence, there are many more elements that play a part in vivo. In a crowded cellular environment, surrounded by interacting proteins, nascent polypeptides face a formidable challenge in finding the correct interactions that result in a folded and functional protein. 8

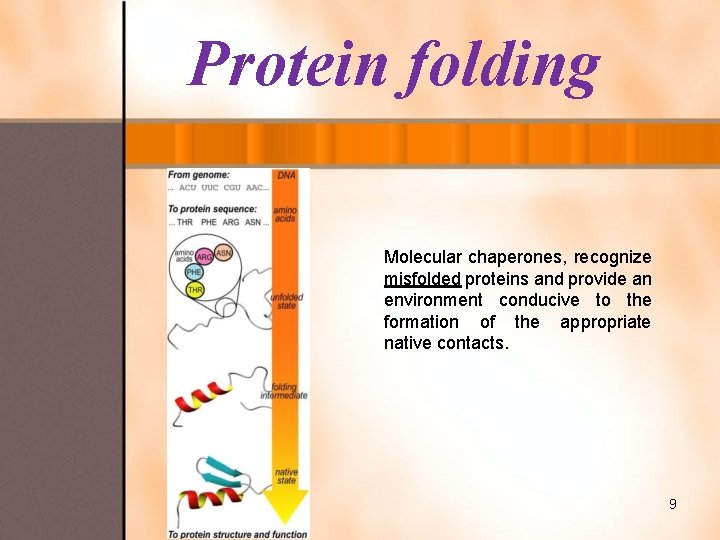
Protein folding Molecular chaperones, recognize misfolded proteins and provide an environment conducive to the formation of the appropriate native contacts. 9

Protein misfolding diseases (Alzheimer’s) Introduction Protein misfolding diseases Alzheimer’s disease Preventing amyloid aggregate formation References

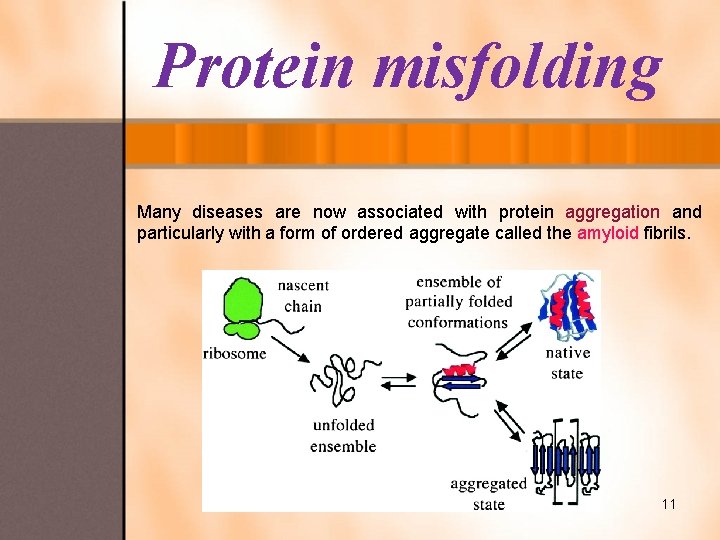
Protein misfolding Many diseases are now associated with protein aggregation and particularly with a form of ordered aggregate called the amyloid fibrils. 11

Protein folding diseases: Ø excessive quantities of wrongly folded proteins collect in the form of uncontrolled piles of molecular rubbish (amyloidoses). Ø a small error in the genetic blueprint leads to incomplete folding of a protein, which affects its function. (P 53 : the malfunctioning of central tumour suppressor could cause cancer. ) 12

Protein misfolding diseases Disease Protein Pick’s tau Alzheimer’s APP Parkinson’s alpha synuclein Prion disease (e. g. Mad Cow) prion protein Amyloid Lateral Sclerosis TDP-43 ( Lou Gehrig’s) Huntington’s Disease Huntingtin amyloidoses 13

Protein misfolding diseases (Alzheimer’s) Introduction Protein misfolding diseases Alzheimer’s disease Preventing amyloid aggregate formation References Zeinab Monday, July-5 - Mokhtari

Alzheimer Alois Alzheimer and family, 1910 Auguste D 15

Alzheimer 16

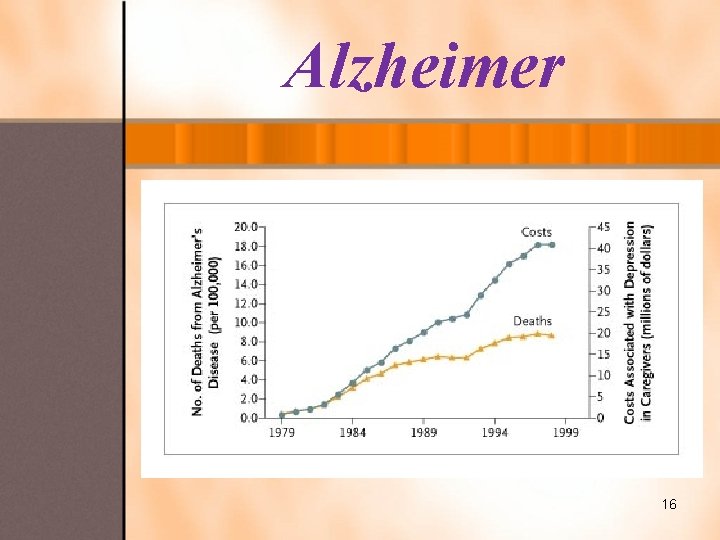
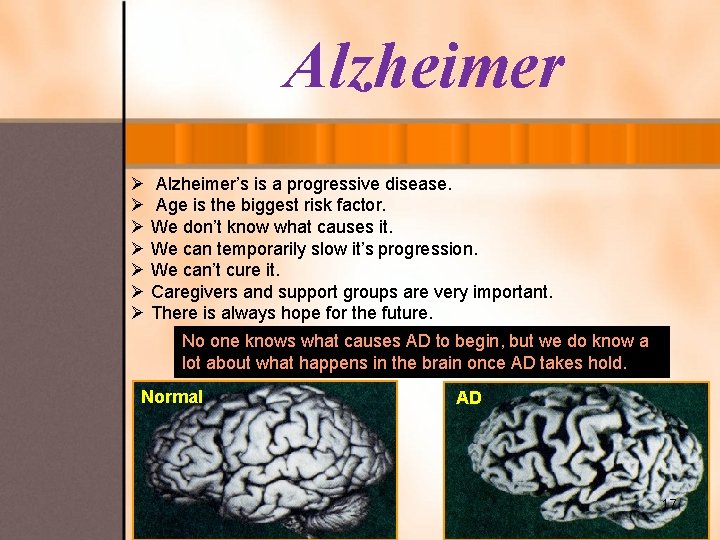
Alzheimer Ø Alzheimer’s is a progressive disease. Ø Age is the biggest risk factor. Ø We don’t know what causes it. Ø We can temporarily slow it’s progression. Ø We can’t cure it. Ø Caregivers and support groups are very important. Ø There is always hope for the future. No one knows what causes AD to begin, but we do know a lot about what happens in the brain once AD takes hold. Normal AD 17

Alzheimer 1. Recent memory loss affecting job 2. Difficulty performing familiar tasks 3. Problems with language 4. Disorientation to time or place 5. Poor or decreased judgment 6. Problems with abstract thinking 7. Misplacing things 8. Changes in mood or behavior 9. Changes in personality 10. Loss of initiative Alzheimer’s disease is an irreversible, progressive brain disease that slowly destroys memory and thinking skills. 18

Protein misfolding diseases (Alzheimer’s) Introduction Protein misfolding diseases Alzheimer’s disease Preventing amyloid aggregate formation References Zeinab Monday, July-5 Mokhtari

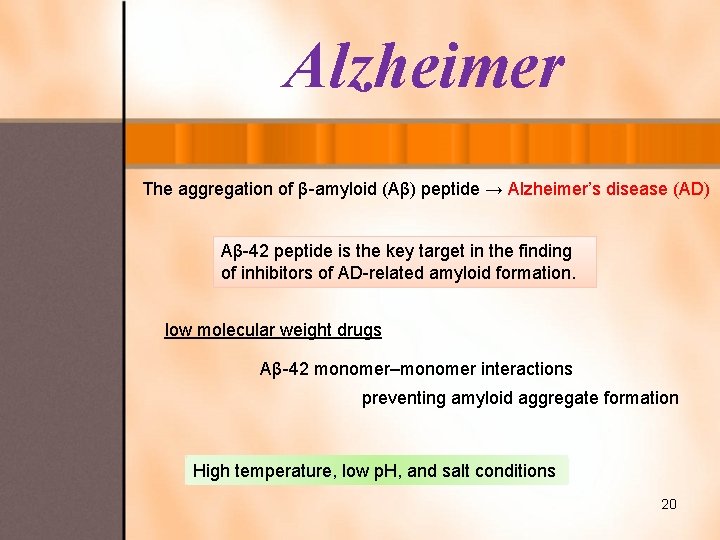
Alzheimer The aggregation of β-amyloid (Aβ) peptide → Alzheimer’s disease (AD) Aβ-42 peptide is the key target in the finding of inhibitors of AD-related amyloid formation. low molecular weight drugs Aβ-42 monomer–monomer interactions preventing amyloid aggregate formation High temperature, low p. H, and salt conditions 20

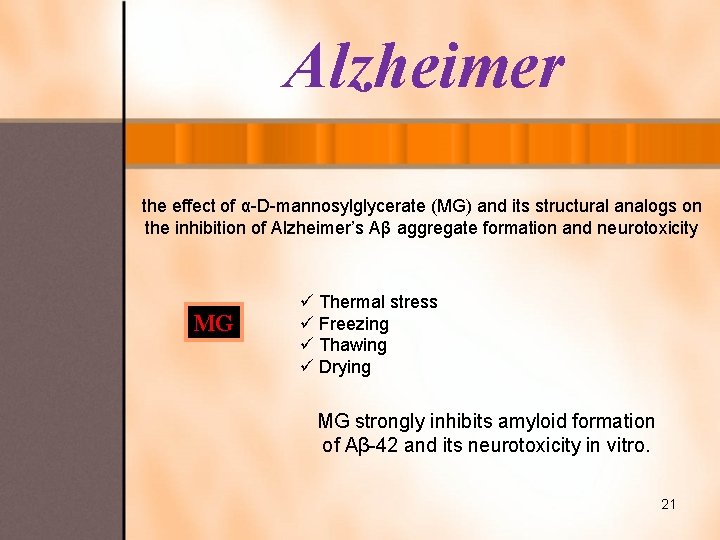
Alzheimer the effect of α-D-mannosylglycerate (MG) and its structural analogs on the inhibition of Alzheimer’s Aβ aggregate formation and neurotoxicity MG ü Thermal stress ü Freezing ü Thawing ü Drying MG strongly inhibits amyloid formation of Aβ-42 and its neurotoxicity in vitro. 21

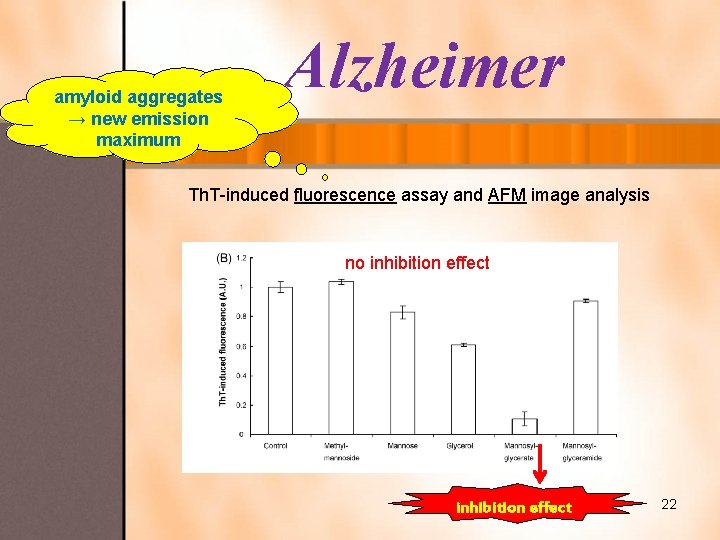
amyloid aggregates → new emission maximum Alzheimer Th. T-induced fluorescence assay and AFM image analysis no inhibition effect 22

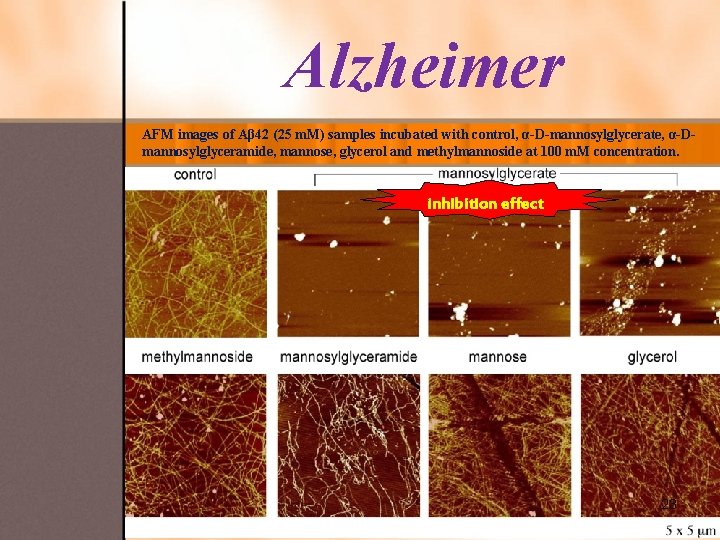
Alzheimer AFM images of Aβ 42 (25 m. M) samples incubated with control, α-D-mannosylglycerate, α-Dmannosylglyceramide, mannose, glycerol and methylmannoside at 100 m. M concentration. inhibition effect 23

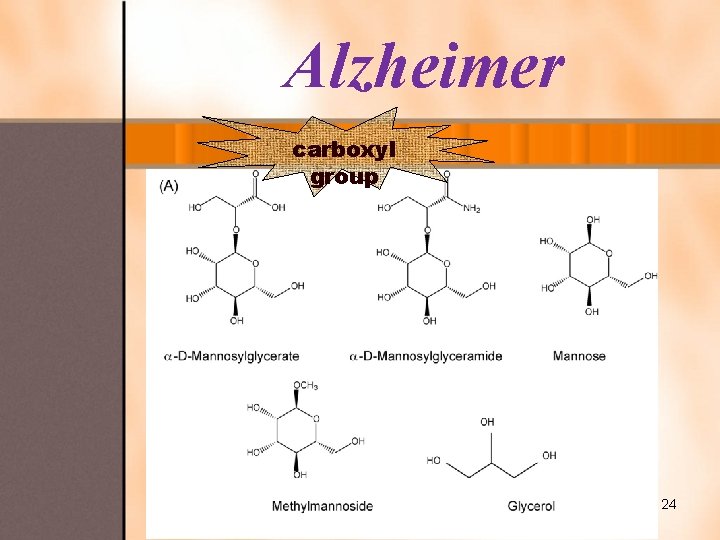
Alzheimer carboxyl group 24

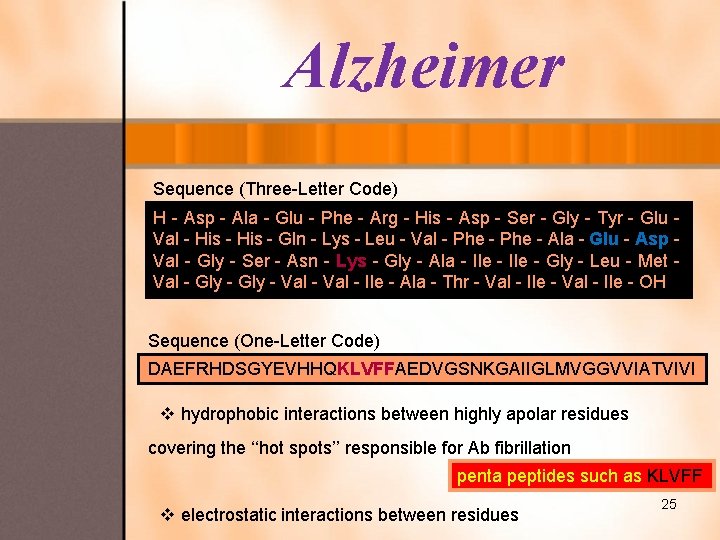
Alzheimer Sequence (Three-Letter Code) H - Asp - Ala - Glu - Phe - Arg - His - Asp - Ser - Gly - Tyr - Glu Val - His - Gln - Lys - Leu - Val - Phe - Ala - Glu - Asp Val - Gly - Ser - Asn - Lys - Gly - Ala - Ile - Gly - Leu - Met Val - Gly - Val - Ile - Ala - Thr - Val - Ile - OH Sequence (One-Letter Code) DAEFRHDSGYEVHHQKLVFFAEDVGSNKGAIIGLMVGGVVIATVIVI v hydrophobic interactions between highly apolar residues covering the ‘‘hot spots’’ responsible for Ab fibrillation penta peptides such as KLVFF v electrostatic interactions between residues 25

Some References Nanomedicine: Nanotechnology, Biology, and Medicine 1 (2005) 300– 305 Journal of Molecular Biology (2006) 362, 347– 354 Archives of Biochemistry and Biophysics 469 (2008) 4– 19 peptides 29 (2008) 578 – 584 Biochimica et Biophysica Acta 1764 (2006) 443– 451 26

Everything is okay in the end. If it's not okay, then it's not the end. 27
 Protein deficiency diseases
Protein deficiency diseases Protein pump vs protein channel
Protein pump vs protein channel Protein-protein docking
Protein-protein docking Name all the lines
Name all the lines Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Gấu đi như thế nào
Gấu đi như thế nào Tư thế worms-breton
Tư thế worms-breton Chúa sống lại
Chúa sống lại Môn thể thao bắt đầu bằng chữ f
Môn thể thao bắt đầu bằng chữ f Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Cong thức tính động năng
Cong thức tính động năng Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Mật thư anh em như thể tay chân
Mật thư anh em như thể tay chân Phép trừ bù
Phép trừ bù Phản ứng thế ankan
Phản ứng thế ankan Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Một số thể thơ truyền thống
Một số thể thơ truyền thống Cái miệng bé xinh thế chỉ nói điều hay thôi
Cái miệng bé xinh thế chỉ nói điều hay thôi Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Thế nào là sự mỏi cơ
Thế nào là sự mỏi cơ đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ Thế nào là giọng cùng tên? *
Thế nào là giọng cùng tên? *