IMPAACT 2014 Overview of todays session Followup from










































- Slides: 45

IMPAACT 2014 Overview of today’s session • Follow-up from last webinar: pill size • Clinical considerations • Expedited Adverse Event (EAE) reporting

Follow-up from last webinar: pill size Doravirine (100 mg) tablet 19 mm x 9. 5 mm

3 IMPAACT 2014 CLINICAL CONSIDERATION S

Overview 4 Clinical Section 1. 0 Medical Section 6. 7 Section 6. 8 aspects of doravirine and Medication Histories Physical Examinations Source Section Expedited Section Documentation and e. CRF Requirements Adverse Event (EAE) Reporting 6& Section 7. 2 7. 3

Clinical aspects of doravirine 5 Safety data is available from Phase 1 and Phase 2 studies and 48 week data is available from two Phase 3 studies Most adverse events were mild to moderate No effect on QTc interval Can be administered with or without a meal Not expected to cause drug interactions Drug levels are affected by CYP 3 A inducers and inhibitors

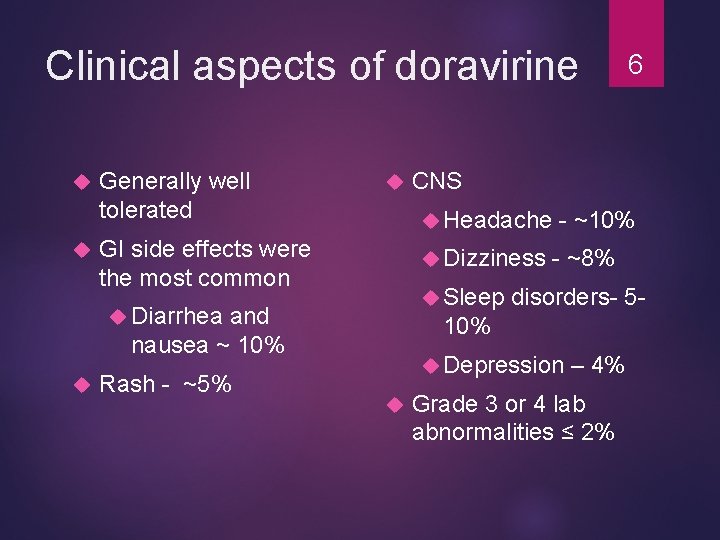
Clinical aspects of doravirine Generally well tolerated Dizziness Sleep Diarrhea and nausea ~ 10% Rash - ~5% CNS Headache GI side effects were the most common - ~10% - ~8% disorders- 5 - 10% Depression 6 – 4% Grade 3 or 4 lab abnormalities ≤ 2%

Medical and Medication History Required at each scheduled visit Baseline history at Screening and Entry Ø Establishes condition at study entry for comparison with condition during follow-up Interval (since the last visit) histories at subsequent visits (for Cohort 1, Week 2) 7

Medical and Medication History All history information may be obtained based on participant self-report or as reported by the parent or guardian but available medical records should be obtained when possible to supplement self-reported information 8

Baseline History Assess for and Source Document 9 Enter into e. CRFs Age and other socio-demographics Yes (all) HIV diagnosis, Sexual Maturity Rating (SMR), WHO clinical staging (Cohort 2), and ARV treatment history (including all ARV use within the 30 days prior to enrollment) Yes (all) History of allergy and/or hypersensitivity (including to ARVs) Yes (all)

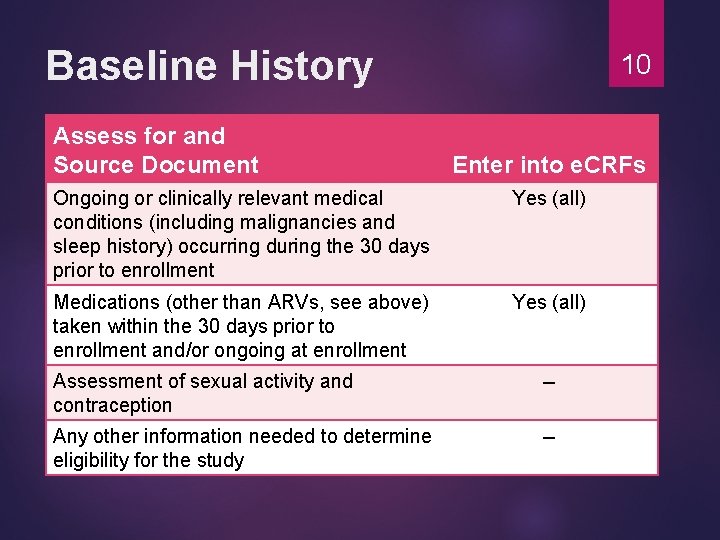
Baseline History Assess for and Source Document 10 Enter into e. CRFs Ongoing or clinically relevant medical conditions (including malignancies and sleep history) occurring during the 30 days prior to enrollment Yes (all) Medications (other than ARVs, see above) taken within the 30 days prior to enrollment and/or ongoing at enrollment Yes (all) Assessment of sexual activity and contraception -- Any other information needed to determine eligibility for the study --

Interval History Assess for and Source Document Current status of conditions that were ongoing at the previous visit Occurrence of any new conditions since the last visit 11 Enter into e. CRFs Any updates of previous entries (e. g. , resolution dates) Any newly identified adverse events that meet criteria in protocol Section 7. 2

Interval History Assess for and Source Document Current status of medications that were ongoing at the last visit 12 Enter into e. CRFs Any updates of previous entries (e. g. , stop dates)

Interval History Assess for and Source Document Use of any new medications since the last visit (see protocol Section 5. 7 for more information on concomitant medications) 13 Enter into e. CRFs • Study drug and formulation taken from time of enrollment through completion of follow-up Note: For participants in Cohort 1, DOR would be considered study drug and all other ARVs would be considered concomitant medications. continued on next slide

Interval History Assess for and Source Document Use of any new medications since the last visit (see protocol Section 5. 7 for more information on concomitant medications) 14 Enter into e. CRFs • Study drug and formulation taken from time of enrollment through completion of follow-up • Record DOR taken after enrollment on Treatment Log (TXW 10001) • Record all other ARVs taken after enrollment on Con Meds Log (CMW 10001) continued on next slide

Interval History Assess for and Source Document Use of any new medications since the last visit 15 Enter into e. CRFs • Any concomitant ARVs taken while on study drug • Any new use of concomitant medications while on study drug • All medications taken at onset of or in response to adverse events that are specified to be entered into e. CRFs per Section 7. 2 Note: e. CRFs will also capture whether traditional medications were taken during follow-up.

Interval History Assess for and Source Document Assessment of current sexual maturity, sexual activity, and contraception 16 Enter into e. CRFs --

17 What are your questions about medical and medications histories?

Physical Examinations Required For at each scheduled visit Cohort 1 Complete exams are required at the Screening and Entry Visits Symptom-directed Week 2 exam is required at 18

Complete Physical Exam Height Vital & weight signs Heart rate Temperature Blood pressure Examination of other body systems driven by other identified signs or symptoms additional assessments may be performed at the discretion of the examining clinician Examination 19 of General appearance Head, eyes, ears, nose, neck, mouth and throat Lymph notes Lungs, heart, and abdomen Musculoskeletal system Skin Neuro Sexual Maturity Rating (SMR) (Entry only)

Targeted Physical Exam Height Vital & weight signs Heart rate Temperature Blood pressure Examination 20 of body systems driven by identified signs or symptoms additional assessments may be performed at the discretion of the examining clinician

21 Safety-Related Data Collection

Pre-Existing Condition Pre-existing condition = untoward medical occurrence identified in a study participant prior to enrollment. Pre-existing conditions are not adverse events. However, if a pre-existing condition worsens in severity or frequency after enrollment, the worsened condition is an adverse event. 22

Pre-Existing Conditions Protocol Section 7. 2 Any pre-existing conditions identified among enrolled participants during the 30 days prior to initial administration of study drug that are ongoing or clinically relevant will be entered into e. CRFs 23

Pre-Existing Conditions 24

Recording Test Results on Laboratory e. CRFs All protocol-specified laboratory test results Any abnormal results of laboratory tests ordered by the site investigator to evaluate adverse events considered related to study drug 25

Participant Evaluations Histor y Exams Lab Tests Adverse Events 26

Adverse Event Protocol Section 7. 2 The definition of the term adverse event provided in Version 2. 0 of the DAIDS EAE Manual will be used in this study The same definition will be used for all participants Beginning from the time of initial study drug administration 27

The definition of adverse event provided in Version 2. 0 of the DAIDS EAE Manual will be used in this study. 28 Adverse event = any untoward medical occurrence in a patient or clinical investigation subject administered a study agent which does not necessarily have a causal relationship with this treatment. An adverse event can therefore by any unfavorable and unintended sign (including an abnormal laboratory finding, symptom, or disease) temporally associated with the use of a medicinal (investigational) agent, whether or not related to the medicinal (investigational) agent. (ICH E 2 A)

Recording on Adverse Event e. CRFs All 29 adverse events – except as specified in the IMPAACT Do Not Report List – will be entered into e. CRFs, regardless of severity grade and relationship to study drug

Recording on Adverse Event e. CRFs 30 When available, a diagnosis should be reported as the adverse event term In addition, signs and symptoms associated with the diagnosis must also be reported (per protocol Section 7. 2)

31 Example Grade 3 influenza Report diagnosis of influenza Additionally report fever Additionally report fatigue

Recording Overdose = more than twice the recommended daily dose in a calendar day All study drug overdoses should be entered into the relevant e. CRF These will be routinely reported to the pharmaceutical sponsor Overdoses are NOT anticipated among participants in Cohort 1, as their single dose of DOR should be directly observed in the clinic at Entry. 32

33 What are your questions?

Expedited Adverse Event Reporting Requirements IMPAACT 2014, PROTOCOL SECTION 7. 3

Other References and Resources 35 Manual for Expedited Reporting of Adverse Events to DAIDS (Version 2. 0) DAIDS Table for Grading the Severity of Adult and Pediatric Adverse Events, Corrected Version 2. 1, dated July 2017 Study drug investigator brochure DAERS user and reference guides DAIDS safety training resources https: //rsc. tech-res. com/clinical-research-sites/safetyreporting/daids https: //rsc. tech-res. com/clinical-research-sites/safetyreporting/safety-training-resources

Overview Terms 36 and definitions Protocol specifications for expedited adverse event reporting Relationship assessment

Expedited Adverse Events 37 The following types of adverse events must be reported in an expedited manner in IMPAACT 2014: Serious adverse events (SAEs) All Grade 4 adverse events All pregnancy complications, including intrauterine fetal demise, spontaneous abortions, or therapeutic or otherwise medically indicated abortions All malignancies Any immune reconstitution inflammatory syndrome (IRIS) events

Serious Adverse Event 38 Serious adverse event = an adverse event that… Results in death Is life-threatening* Requires inpatient hospitalization* or prolongation of existing hospitalization Results in persistent or significant disability/incapacity In a congenital anomaly/birth defect* Is an important medical event that may not be immediately lifethreatening or result in death or hospitalization but may jeopardize the participant or may require intervention to prevent one of the other outcomes listed above *See helpful clarifications of these terms in the DAIDS EAE Manual

Reporting Period For each enrolled participant: Begins: At the time of administering the first dose of study drug Ends: Through the protocol-specified end of follow-up (for Cohort 1, through Week 2) 39

SUSAR 40 Suspected = Related to use of study drug Unexpected = Nature or severity is not consistent with the study drug’s current Investigator Brochure Serious adverse reaction = SAE After the protocol-specified end of follow-up, only SUSARs will be reported if the study staff become aware of the events on a passive basis (i. e. , from publicly available information).

Related or Not Related 41 Related = There is a reasonable possibility that the event may be related to study drug. Not related = There is not a reasonable possibility that the event may be related to study drug.

Reasonable Possibility 42 Reasonable possibility is intended to convey that there are facts, evidence, or arguments to suggest a causal relationship between the event and study drug

Facts, Evidence, or Arguments to Suggest a Causal Relationship 43 A temporal relationship between the event and use of study drug A plausible biologic mechanism for study drug to cause the event Previous reports of similar events associated with study drug (or drugs of the same class) Resolution of the event after de-challenge (hold/discontinuation of study drug) Recurrence of the event after re-challenge (resumption of study drug after a hold)

Related or Not Related In addition to considering the possibility of a causal relationship with study drug, consider other potential causes of the even, e. g. , Past medical history Concurrent illness Concomitant 44 medications For assessments assessed as not related, document an alternative etiology, diagnosis, or explanation in study records

What are your questions about EAE reporting? 45
 Impaact 2014
Impaact 2014 Followup edge
Followup edge Followup:actionitems
Followup:actionitems Follow-up visits
Follow-up visits Impaact 2009
Impaact 2009 Good morning my students
Good morning my students What is todays temperature
What is todays temperature Todays objective
Todays objective Todays weather hull
Todays weather hull Todays whether
Todays whether Whats todays temperature
Whats todays temperature Todays class
Todays class Todays objective
Todays objective Todays plan
Todays plan What is a good objective for a resume
What is a good objective for a resume Title page mla
Title page mla In todays class
In todays class How to identify simile
How to identify simile Objective of cyberbullying
Objective of cyberbullying Famous people with prader willi syndrome
Famous people with prader willi syndrome Todays jeopardy
Todays jeopardy Todays objective
Todays objective Todays globl
Todays globl Conclusion of digestive system
Conclusion of digestive system Todays vision
Todays vision Welcome to sabbath school
Welcome to sabbath school Todays jeopardy
Todays jeopardy Clients often criticize public relations firms for
Clients often criticize public relations firms for For today's meeting
For today's meeting Todays agenda
Todays agenda Todays sabbath lesson
Todays sabbath lesson Todays final jeopardy
Todays final jeopardy Multiple choice comma quiz
Multiple choice comma quiz No thats not it
No thats not it Todays final jeopardy question
Todays final jeopardy question Todays final jeopardy
Todays final jeopardy Todays software
Todays software Whats todays wordlw
Whats todays wordlw Todays objective
Todays objective Todays objective
Todays objective Todays match
Todays match Todays lab
Todays lab Todays goal
Todays goal Todays price of asda shares
Todays price of asda shares Todays jeopardy
Todays jeopardy Todays worldld
Todays worldld