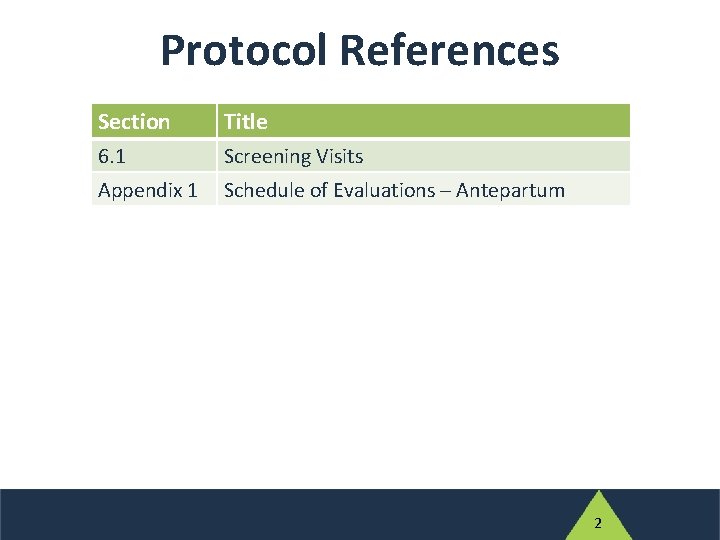
IMPAACT 2010 Screening Visits Protocol References Section Title


















- Slides: 23

IMPAACT 2010 Screening Visits

Protocol References Section Title 6. 1 Screening Visits Appendix 1 Schedule of Evaluations ─ Antepartum 2

Screening Visits Key operational reminders: • Screening may be initiated after written informed consent is obtained • Screening procedures must be performed within 14 days prior to enrollment • Screening procedures may be performed on multiple days, including on the date of enrollment • Screening procedures may be repeated, with the latest outcome used for eligibility determination 3

Repeat Screening Attempts • If the 14 -day screening period is exceeded, the screening process may be repeated, if adequate time remains to permit enrollment within 14 days of non-study ART initiation. In this case, all procedures listed in protocol Section 6. 1, including the informed consent process, must be repeated except: – – New PIDs should not be assigned Confirmatory HIV testing need not be repeated Fetal ultrasound need not be repeated Previously documented medical and medications history information should be reviewed and updated through the date of re-screening (it is not necessary to re-record history information that was previously documented) 4

5 A mother’s baseline medical history is first recorded at the Screening Visit. 1. True 2. False

6 Where in the protocol can you find the required elements of the maternal baseline medical history? Section 6. 11 Text and Table 3

7 A complete maternal physical exam is required at the Screening Visit 1. True 2. False

8 Where in the protocol can you find the required elements of the complete maternal physical exam? Section 6. 12

9 Fetal ultrasound must be performed within 14 days prior to enrolment. 1. True 2. False

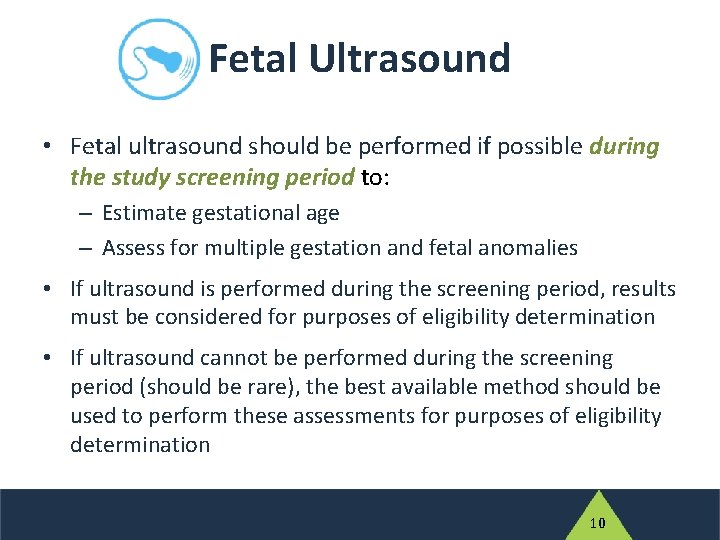
Fetal Ultrasound • Fetal ultrasound should be performed if possible during the study screening period to: – Estimate gestational age – Assess for multiple gestation and fetal anomalies • If ultrasound is performed during the screening period, results must be considered for purposes of eligibility determination • If ultrasound cannot be performed during the screening period (should be rare), the best available method should be used to perform these assessments for purposes of eligibility determination 10

Fetal Ultrasound • If a fetal ultrasound is not performed prior to entry, it must be done as soon as possible and within 14 days after study entry • Ultrasound findings will then be used in algorithms to calculate gestational age at study entry and at delivery • If a fetal ultrasound is performed after entry, enrolled participants will not be withdrawn from the study if the ultrasound identifies an exclusionary condition 11

12 AST and ALT test results must be available prior to enrollment. 1. True 2. False

13 If an exclusionary ALT result is obtained at screening, the test may be repeated, with the latest outcome used for eligibility determination. 1. True 2. False

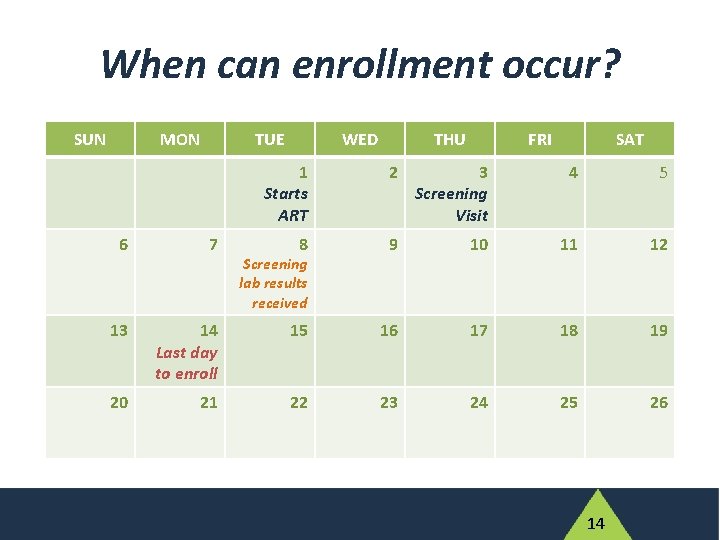
When can enrollment occur? SUN MON TUE WED THU FRI SAT 1 Starts ART 2 3 Screening Visit 4 5 8 9 10 11 12 6 7 13 14 Last day to enroll 15 16 17 18 19 20 21 22 23 24 25 26 Screening lab results received 14

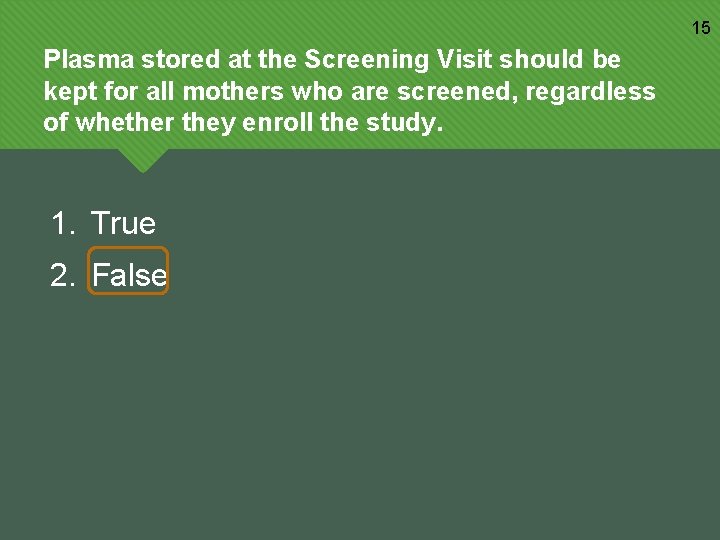
15 Plasma stored at the Screening Visit should be kept for all mothers who are screened, regardless of whether they enroll the study. 1. True 2. False

What are your questions? 16

IMPAACT 2010 Entry Visits

Protocol References Section Title 6. 2 Entry Visit Appendix 1 Schedule of Evaluations ─ Antepartum 18

Entry Visits Key operational reminders • For eligible mother-infant pairs, enrollment must occur at 14 -28 weeks gestation and within 14 days of the mother initiating ART in the current pregnancy • All Entry Visit procedures are expected to be performed on the day of enrollment • Procedures that may provide information relevant to eligibility for the study (e. g. , medical history, physical examination) should be performed first, prior to final eligibility determination. 19

Entry Visits Key operational reminders • Final eligibility determination and confirmation must precede enrollment • Enrollment must precede prescribing of study drug • Prescribing must precede dispensing of study drug • Blood collection should ideally precede enrollment and must precede ingestion of the first dose of study drug 20

21 A mother is considered enrolled in the study when … 1. She provides written informed consent 2. She and her infant are found to meet all study eligibility criteria 3. She and her infant are assigned SIDs by the Subject Enrollment System

22 An infant is considered enrolled in the study when … 1. The infant’s mother provides written informed consent 2. The mother and infant are found to meet all study eligibility criteria 3. The mother and infant are assigned SIDs by the Subject Enrollment System 4. The infant is born

What are your questions? 23