GEU 0047 Meteorology Lecture 02 Heat Energy Heat




































- Slides: 82

GEU 0047: Meteorology Lecture 02 Heat Energy

Heat Energy Temperature is our way of quantifying matter’s internal kinetic energy. It is a macroscopic measurement of the average kinetic energy found in the random and microscopic motions (vibration, rotation, and collision) of countless atoms and molecules. Temperature is related to heat energy.

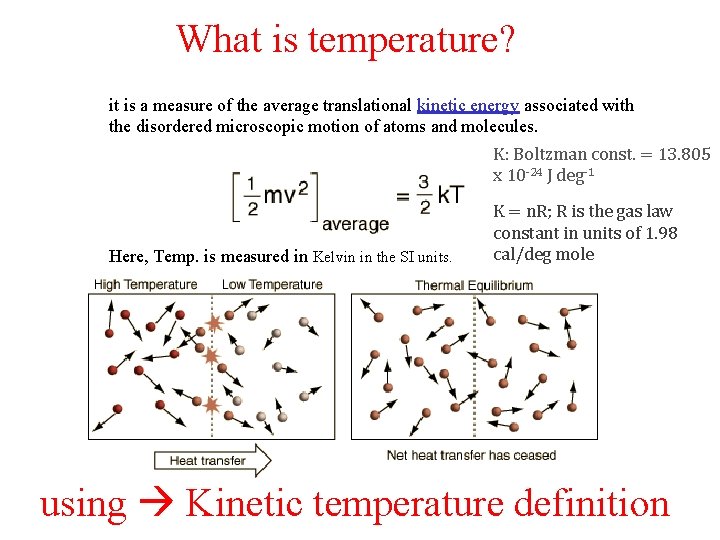
What is temperature? it is a measure of the average translational kinetic energy associated with the disordered microscopic motion of atoms and molecules. K: Boltzman const. = 13. 805 x 10 -24 J deg-1 Here, Temp. is measured in Kelvin in the SI units. K = n. R; R is the gas law constant in units of 1. 98 cal/deg mole using Kinetic temperature definition

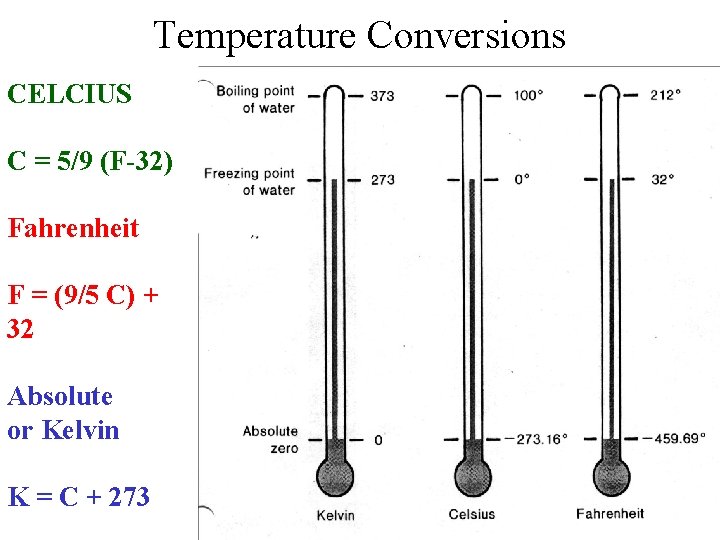
Temperature Conversions CELCIUS C = 5/9 (F-32) Fahrenheit F = (9/5 C) + 32 Absolute or Kelvin K = C + 273

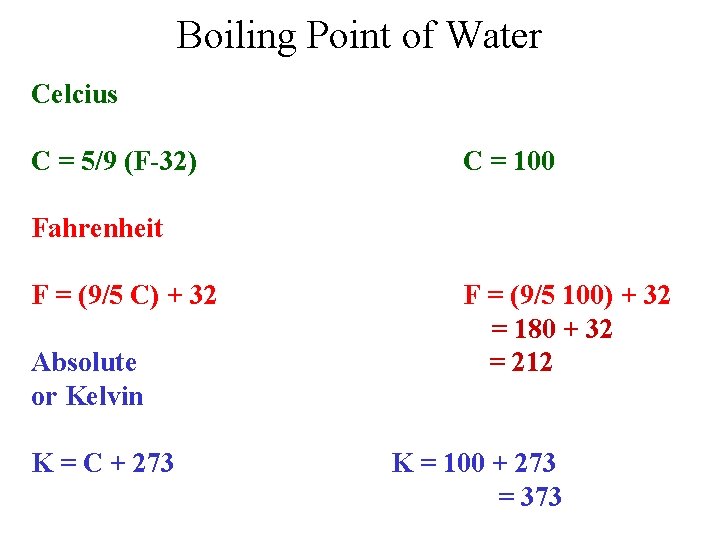
Boiling Point of Water Celcius C = 5/9 (F-32) C = 100 Fahrenheit F = (9/5 C) + 32 Absolute or Kelvin K = C + 273 F = (9/5 100) + 32 = 180 + 32 = 212 K = 100 + 273 = 373

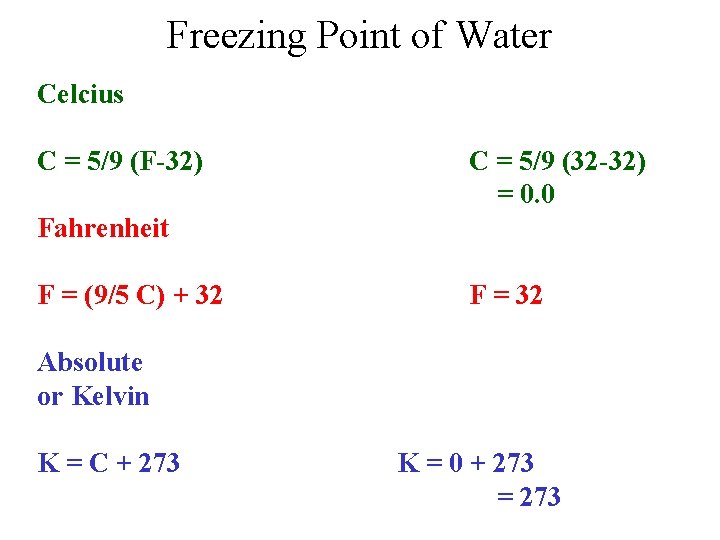
Freezing Point of Water Celcius C = 5/9 (F-32) C = 5/9 (32 -32) = 0. 0 Fahrenheit F = (9/5 C) + 32 F = 32 Absolute or Kelvin K = C + 273 K = 0 + 273 = 273

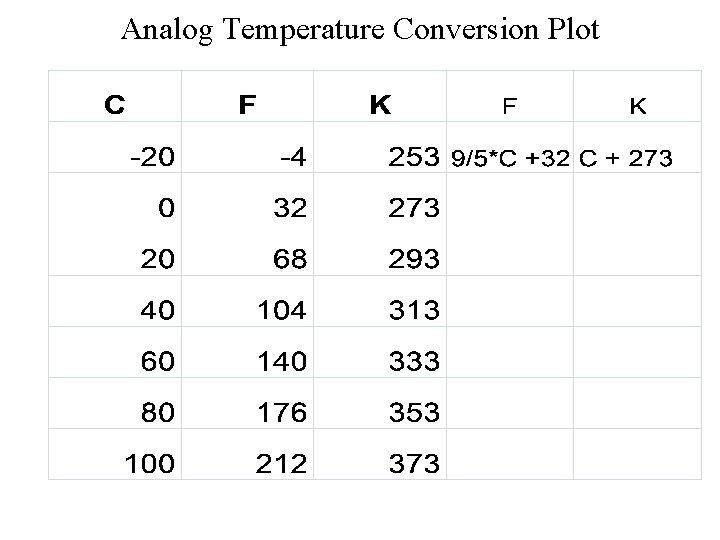
Analog Temperature Conversion Plot

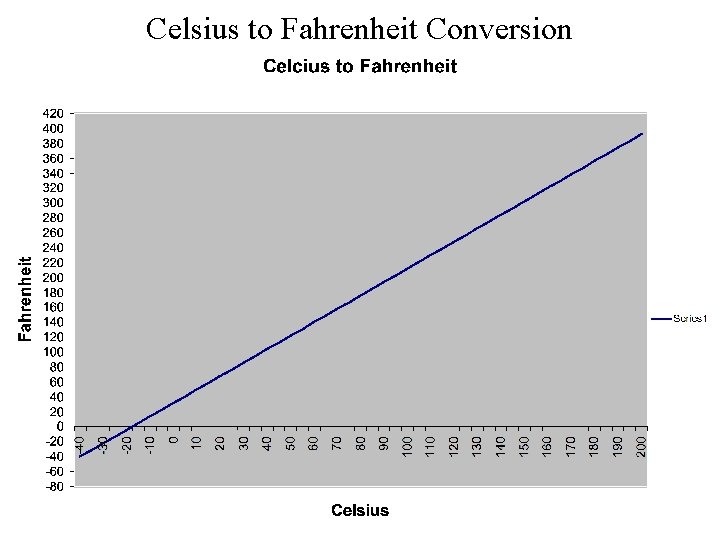
Celsius to Fahrenheit Conversion

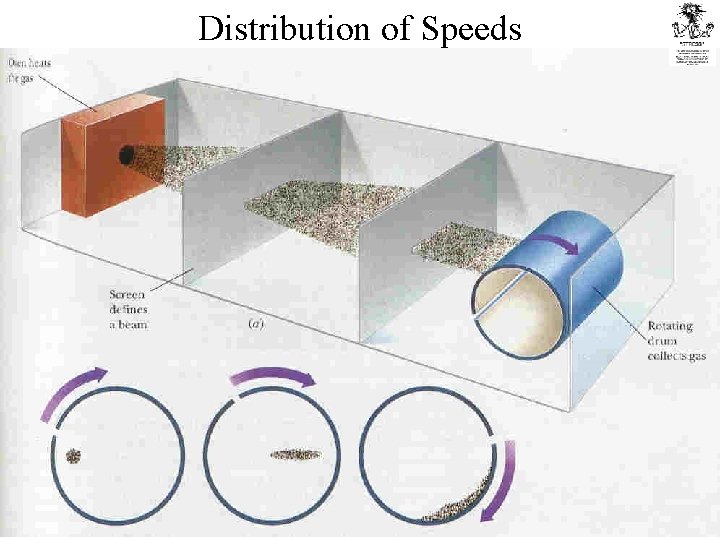
Distribution of Speeds

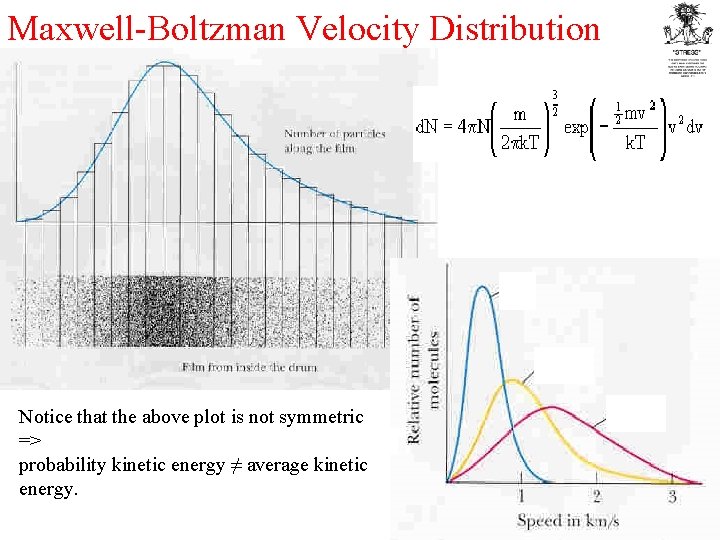
Maxwell-Boltzman Velocity Distribution E E Notice that the above plot is not symmetric => probability kinetic energy ≠ average kinetic energy. E

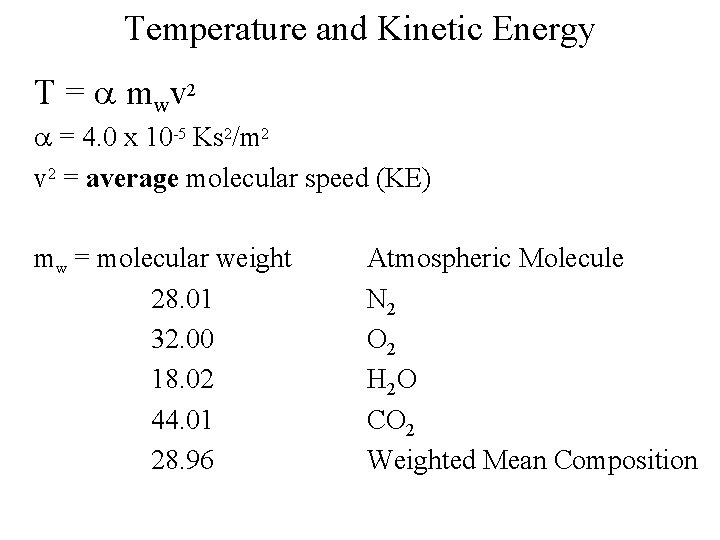
Temperature and Kinetic Energy T = a mwv 2 a = 4. 0 x 10 -5 Ks 2/m 2 v 2 = average molecular speed (KE) mw = molecular weight 28. 01 32. 00 18. 02 44. 01 28. 96 Atmospheric Molecule N 2 O 2 H 2 O CO 2 Weighted Mean Composition

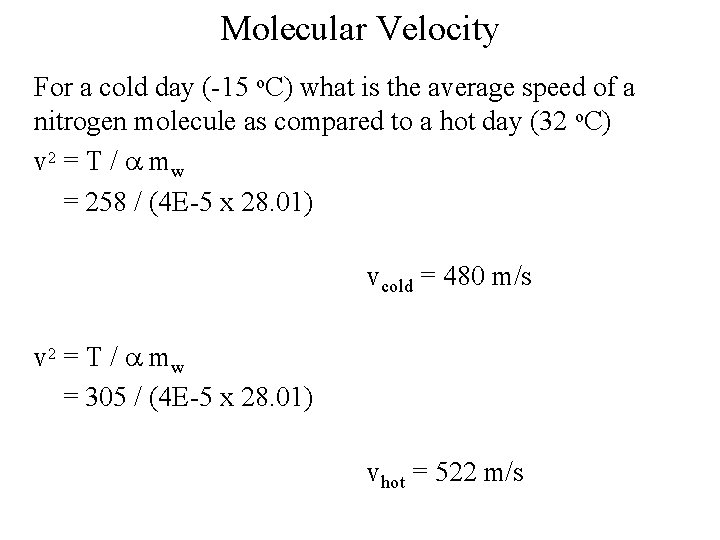
Molecular Velocity For a cold day (-15 o. C) what is the average speed of a nitrogen molecule as compared to a hot day (32 o. C) v 2 = T / a mw = 258 / (4 E-5 x 28. 01) vcold = 480 m/s v 2 = T / a mw = 305 / (4 E-5 x 28. 01) vhot = 522 m/s

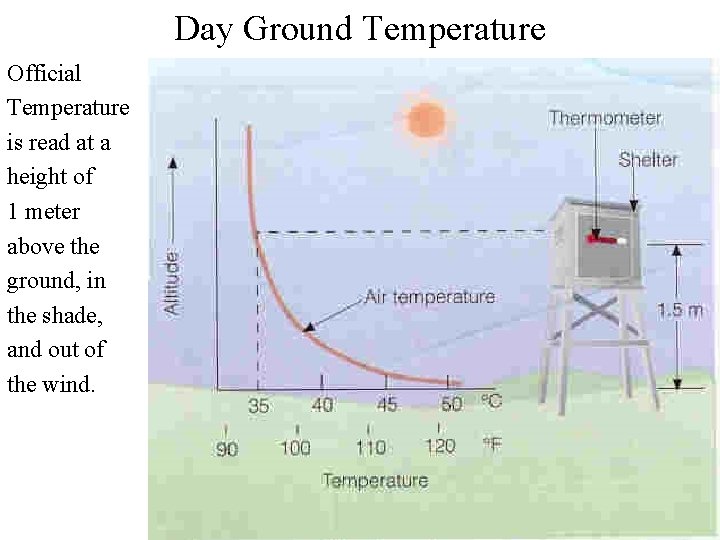
Day Ground Temperature Official Temperature is read at a height of 1 meter above the ground, in the shade, and out of the wind.

Night Ground Temperature • The ground radiates away the daytime heat faster than the air above it. Air is a very poor conductor.

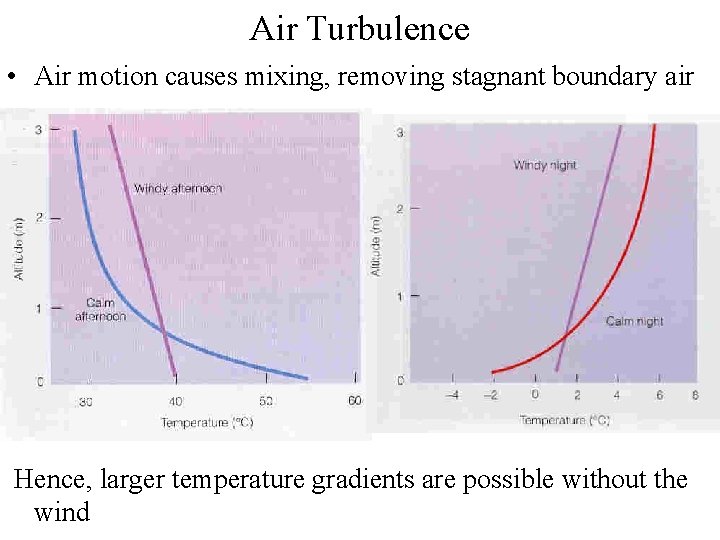
Air Turbulence • Air motion causes mixing, removing stagnant boundary air Hence, larger temperature gradients are possible without the wind

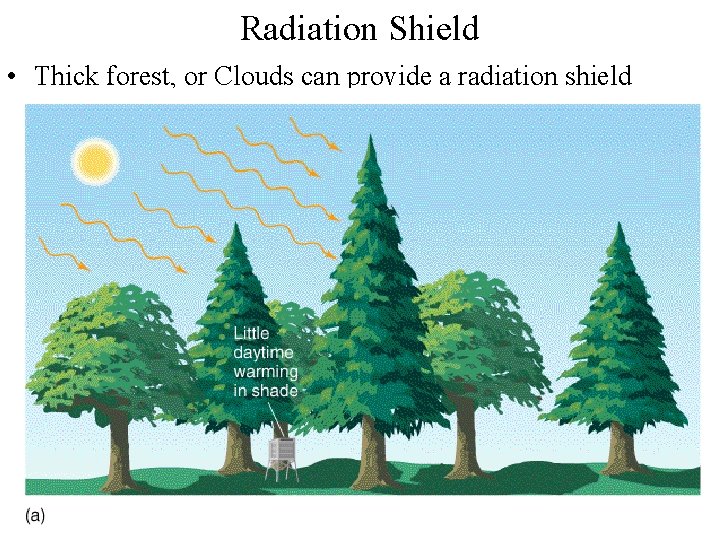
Radiation Shield • Thick forest, or Clouds can provide a radiation shield

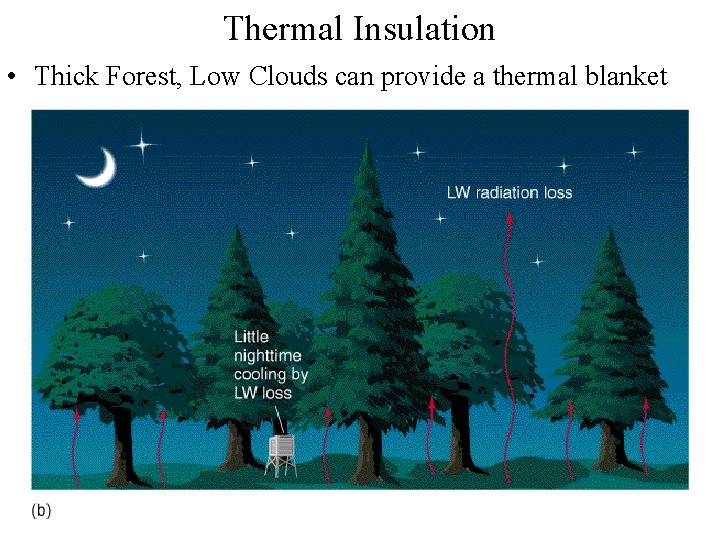
Thermal Insulation • Thick Forest, Low Clouds can provide a thermal blanket

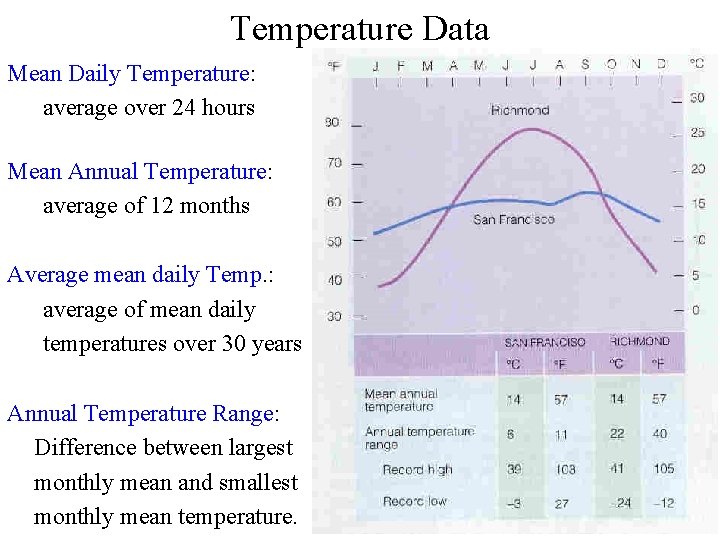
Temperature Data Mean Daily Temperature: average over 24 hours Mean Annual Temperature: average of 12 months Average mean daily Temp. : average of mean daily temperatures over 30 years Annual Temperature Range: Difference between largest monthly mean and smallest monthly mean temperature.

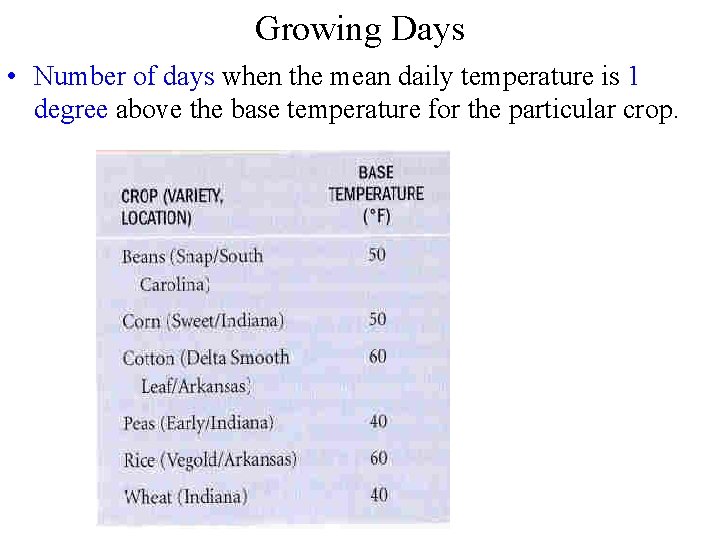
Growing Days • Number of days when the mean daily temperature is 1 degree above the base temperature for the particular crop.

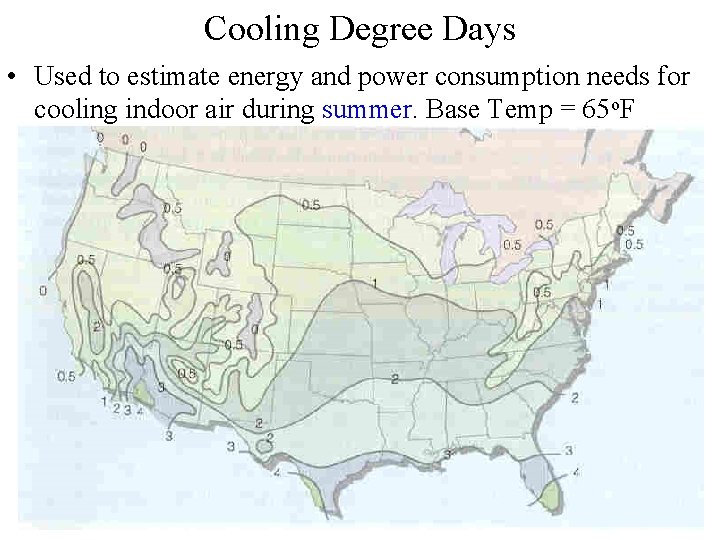
Cooling Degree Days • Used to estimate energy and power consumption needs for cooling indoor air during summer. Base Temp = 65 o. F

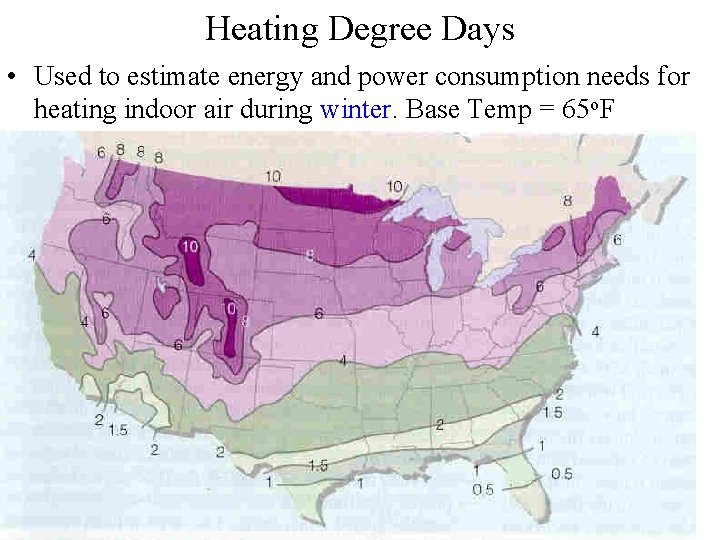
Heating Degree Days • Used to estimate energy and power consumption needs for heating indoor air during winter. Base Temp = 65 o. F

Controls of Temperature (important) • Solar Insolation – Date & Time – Latitude – Exposure (wind, humidity) • Geographic – Land – Water • Oceanic – Currents • Topography – Elevation

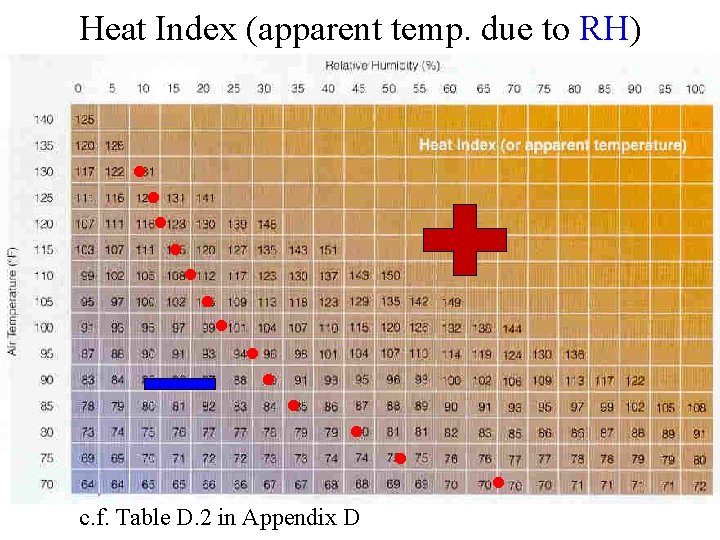
Heat Index (apparent temp. due to RH) ● ● ● c. f. Table D. 2 in Appendix D ●

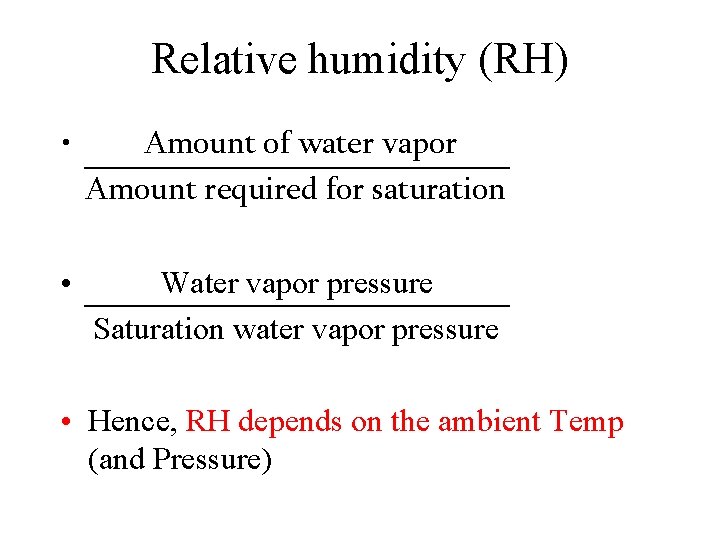
Relative humidity (RH) • Amount of water vapor Amount required for saturation • Water vapor pressure Saturation water vapor pressure • Hence, RH depends on the ambient Temp (and Pressure)

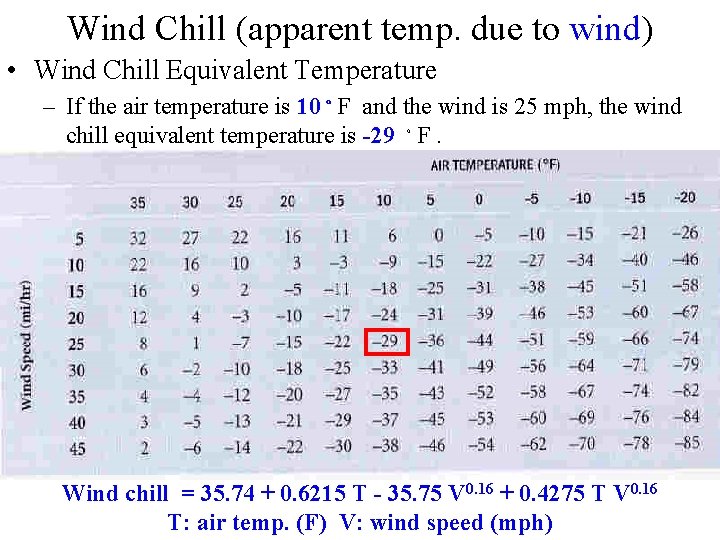
Wind Chill (apparent temp. due to wind) • Wind Chill Equivalent Temperature – If the air temperature is 10。F and the wind is 25 mph, the wind chill equivalent temperature is -29 。F. Wind chill = 35. 74 + 0. 6215 T - 35. 75 V 0. 16 + 0. 4275 T V 0. 16 T: air temp. (F) V: wind speed (mph)

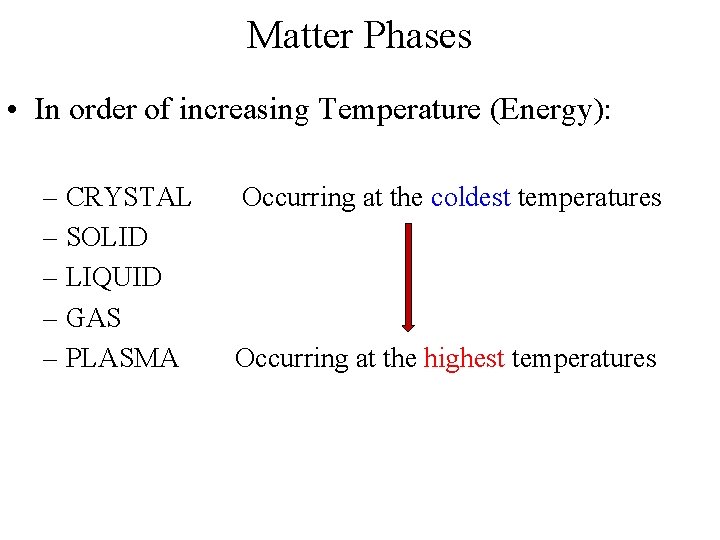
Matter Phases • In order of increasing Temperature (Energy): – CRYSTAL – SOLID – LIQUID – GAS – PLASMA Occurring at the coldest temperatures Occurring at the highest temperatures

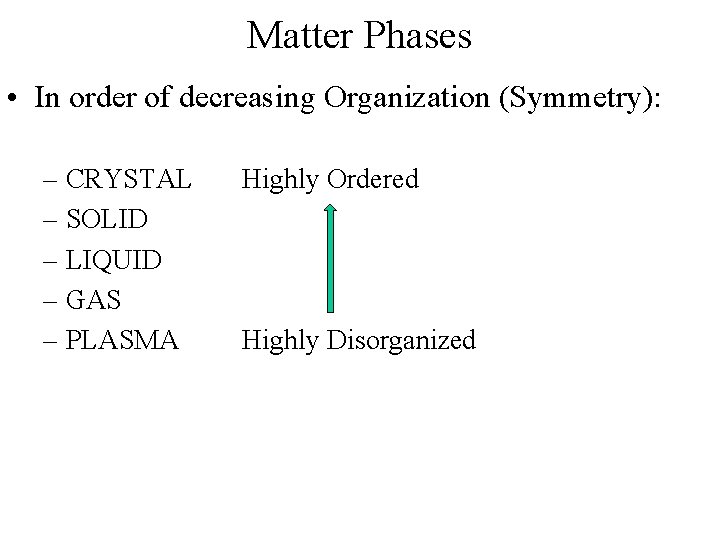
Matter Phases • In order of decreasing Organization (Symmetry): – CRYSTAL – SOLID – LIQUID – GAS – PLASMA Highly Ordered Highly Disorganized

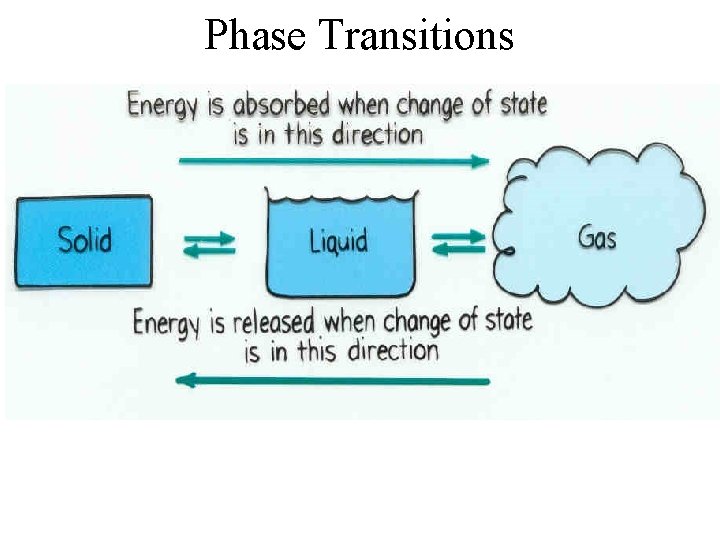
Phase Transitions

State Changes Energy increased and absorbed by substance: • SOLID to LIQUID Melting • LIQUID to GAS Boiling • SOLID to GAS Sublimation Energy decreased and released by substance: • GAS to SOLID Deposition • GAS to LIQUID Condensation • LIQUID to SOLID Freezing

Water Crystals Atomic and Molecular Structures Lead to Macroscopic Order Heat Energy must be absorbed by the solid to break the highly ordered ice crystals. Heat Energy is released by a liquid in order to crystallize.

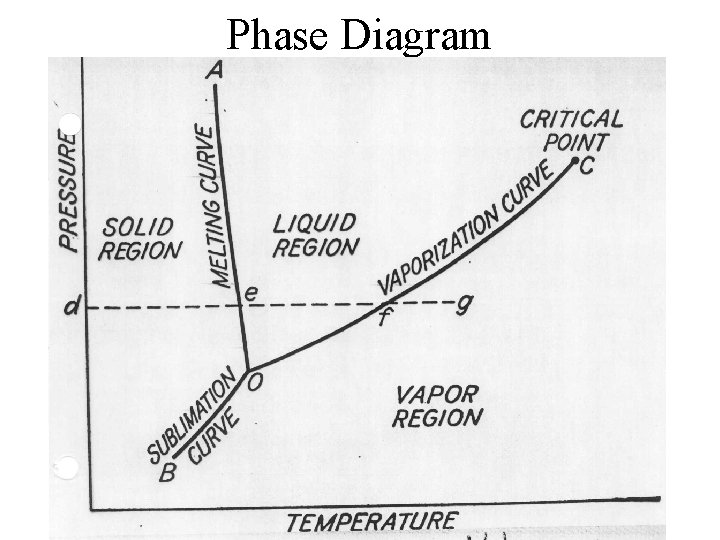
Phase Diagram

Latent Heat T = const

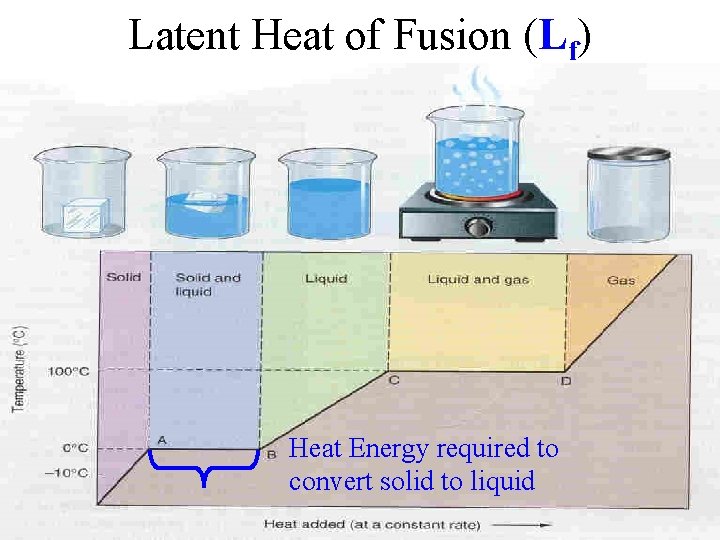
Latent Heat of Fusion (Lf) Heat Energy required to convert solid to liquid

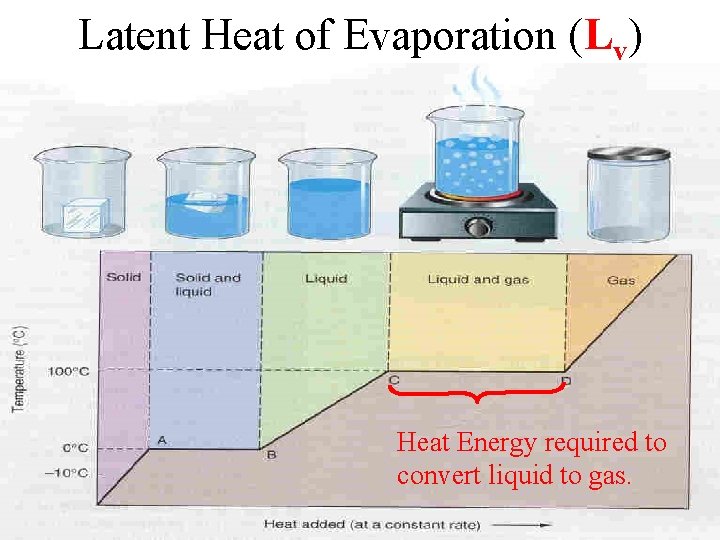
Latent Heat of Evaporation (Lv) Heat Energy required to convert liquid to gas.

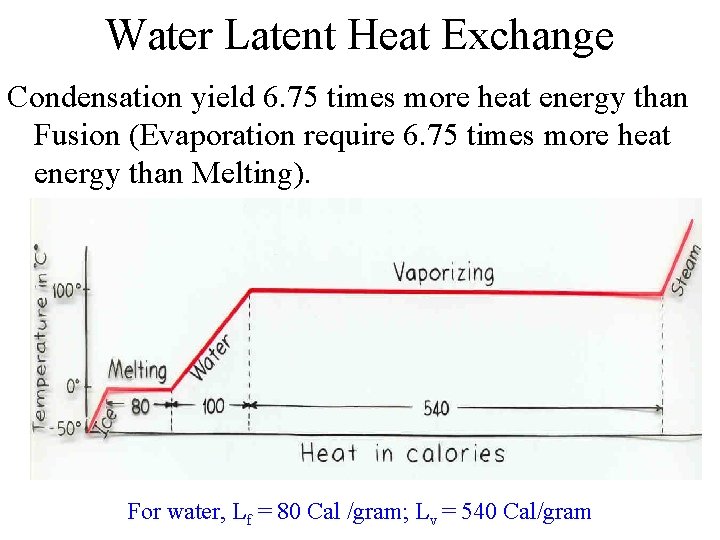
Water Latent Heat Exchange Condensation yield 6. 75 times more heat energy than Fusion (Evaporation require 6. 75 times more heat energy than Melting). For water, Lf = 80 Cal /gram; Lv = 540 Cal/gram

Heat/energy Units • Calorie: the amount of heat required to raise the temperature of 1 gram of water by 1 degree Celsius. • 1 cal. = 4. 186 Joules • (1 Food calorie = 1, 000 calories = 4186 J) (see Appendix A for more)

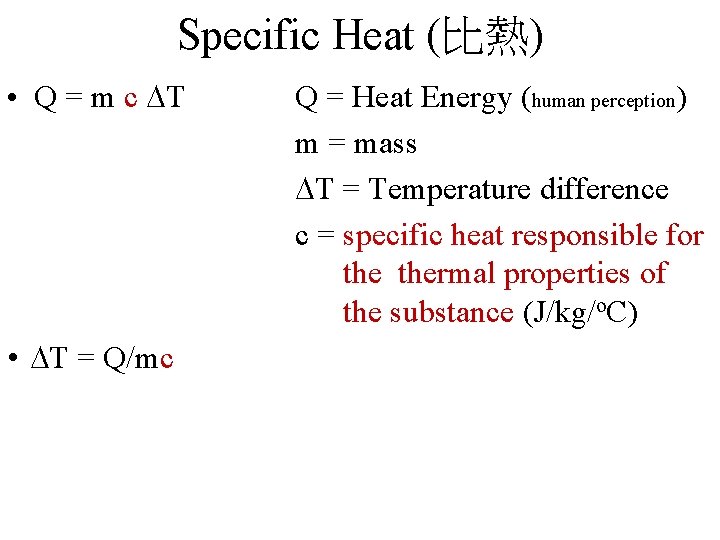
Specific Heat (比熱) • Q = m c DT • DT = Q/mc Q = Heat Energy (human perception) m = mass DT = Temperature difference c = specific heat responsible for thermal properties of the substance (J/kg/o. C)

Specific Heat DT = Q/mc For a given amount of heat energy, say 10, 000 Joules, what is the temperature change for 1 kg of water and 1 kg of sand? Csand = 838 J/kgo. C Cwater = 4180 J/kgo. C DTsand = 10, 000/1(838) = 11. 9 o. C DTwater = 10, 000/1(4180) = 2. 4 o. C

Northern Hemisphere Southern Hemisphere.

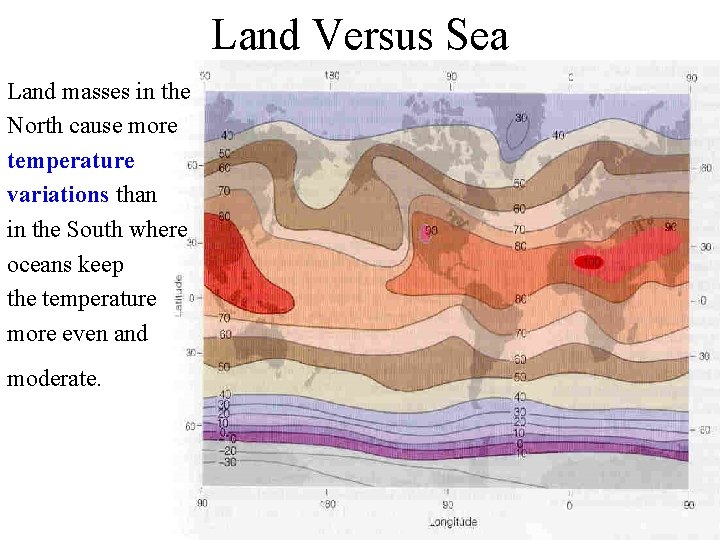
Land Versus Sea Land masses in the North cause more temperature variations than in the South where oceans keep the temperature more even and moderate.

Melting DT = Q/mc Amount of heat energy needed to bring a 0. 25 kg ice block to a temperature of 50 o. C? (Starting Temp = 0 o. C Ending Temp = 50 o. C) Q = heat needed to make transition from ice to water + heat needed to heat water from 0 to 50 o. C Q = m. Lf + mc. DT

Melting DT = Q/mc Q = heat needed to make transition from ice to water + heat needed to heat water from 0 to 50 o. C Q = (250 g) (80 Cal/g) (4. 186 J/Cal) + (0. 25 kg) (4180 J/kgo. C) (50 -0 o. C) = 83720 + 52250 = 135970 Joules Can you calculate it ? (important)

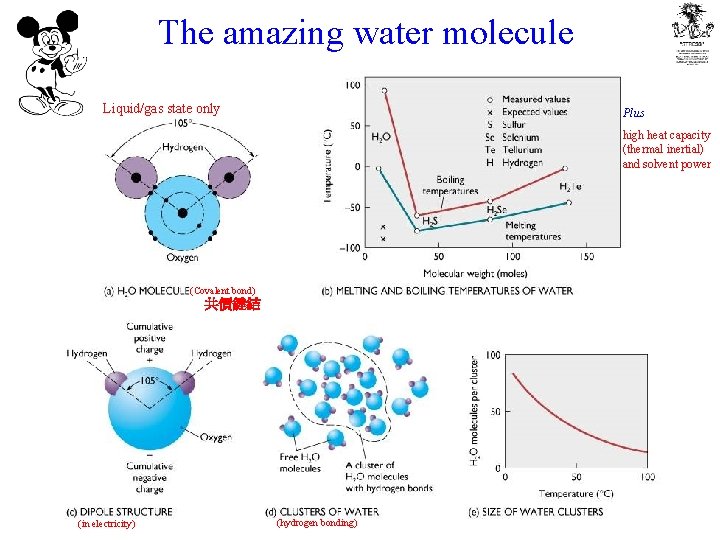
The amazing water molecule Liquid/gas state only Plus high heat capacity (thermal inertial) and solvent power (Covalent bond) 共價鍵結 (in electricity) (hydrogen bonding)

Freezing This latent heat energy is released when water droplets freeze. Water vapor that condenses also gives off latent heat. Both processes help heat the atmosphere. The opposite processes (melting or evaporation) cause heat energy to be removed from the atmosphere.

CONDENSATION • Gas to Liquid (or Freezing, Liquid to Solid) – ENERGY IS RELEASED, Gas has a higher internal energy than the liquid state. – A WARMING PROCESS

EVAPORATION • LIQUID to GAS – ENERGY IS REMOVED, Liquid has a lower internal energy than the gaseous state. – A COOLING PROCESS

Radiation Energy transport via electromagnetic waves

Convection Energy transport by mass motion

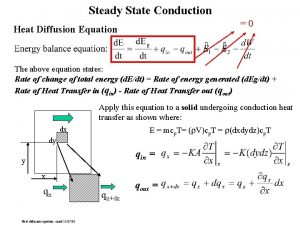
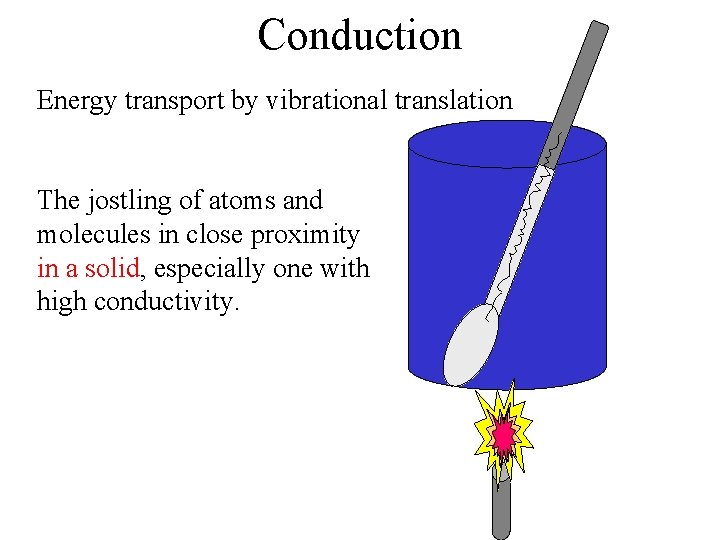
Conduction Energy transport by vibrational translation The jostling of atoms and molecules in close proximity in a solid, especially one with high conductivity.














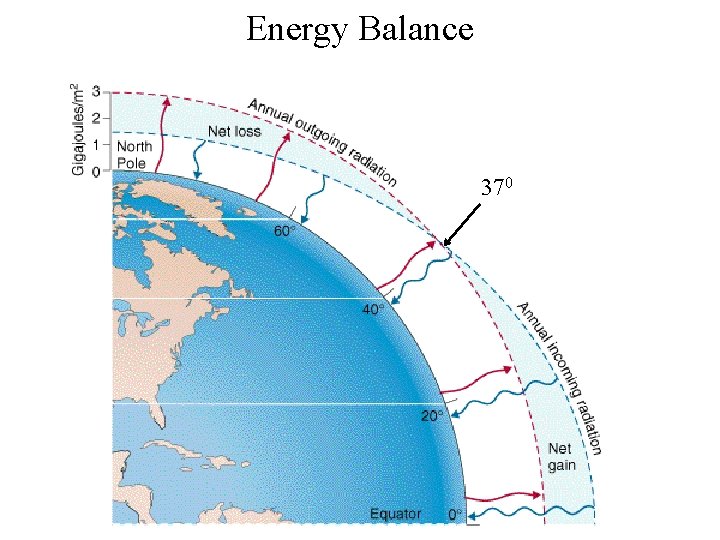
Energy Balance 370

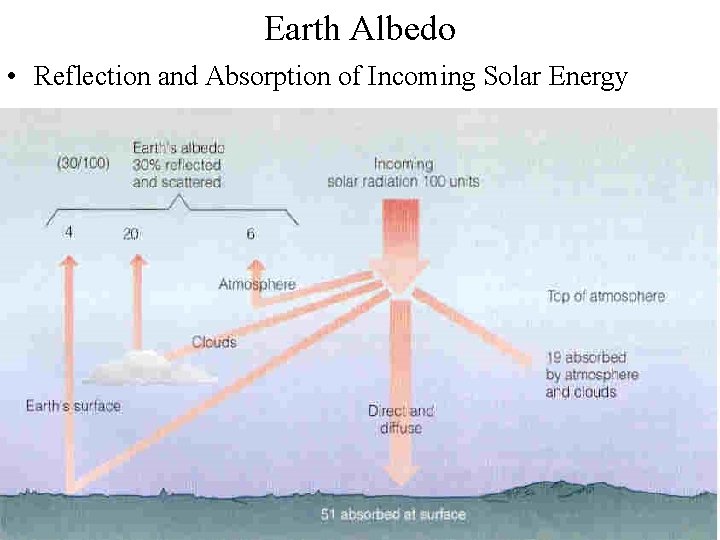
Earth Albedo • Reflection and Absorption of Incoming Solar Energy

Energy Budget • Sunbeam (1365 -1372 W/m 2) = 100 % at the top of atmosphere – 30 % reflected and scattered back out to space (411 W/m 2) • Earth’s surface (4%) • Clouds (20%) • Atmosphere (6%) – 19 % absorbed by Earth’s atmosphere (260. 3 W/m 2) – 51 % heats Earth’s surface directly (698. 7 W/m 2)



A Green House Glass is transparent to visible wavelengths (SW) but opaque to infrared wavelengths (LW).

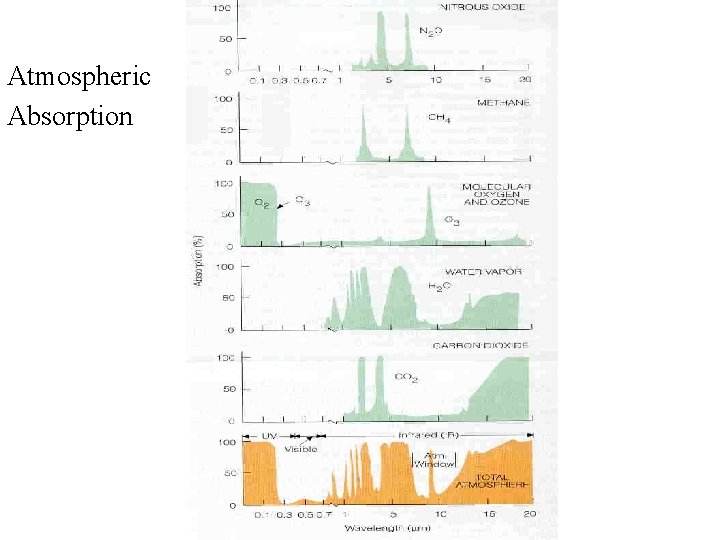
Atmosphere Absorption Atmospheric Absorption

Energy Transformations • Radiant Solar Energy (1367 W/m 2) • • Heat Energy (Gas Kinetic Energy Increased) Water Evaporation (Latent Heat) Air Convection (Potential Energy Increased) Water Condensation (Latent Heat Released) Precipitation (Converting Potential to Kinetic Energy) Erosion (Kinetic Energy causes Erosion Deformation) Reservoirs (Potential Energy of a Dam) Hydroelectric Power (Conversion of PE to KE to Electrical Energy)

Aurora

Magnetic Field

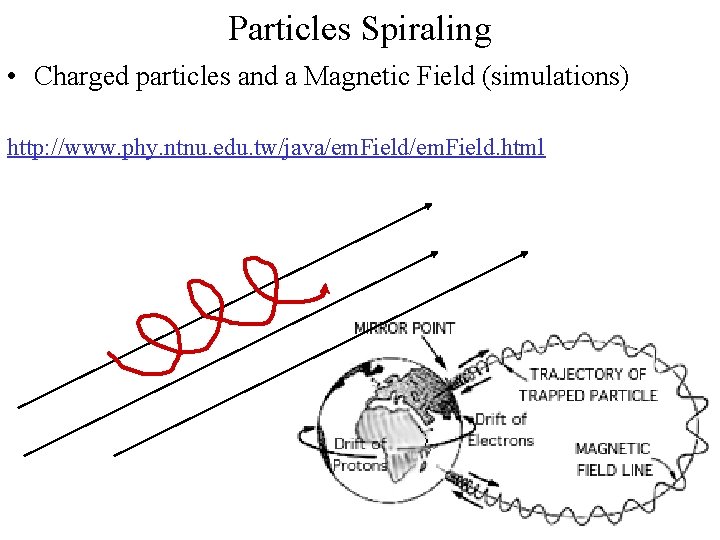
Particles Spiraling • Charged particles and a Magnetic Field (simulations) http: //www. phy. ntnu. edu. tw/java/em. Field. html

Magnetosphere • Solar Wind (charged particles) and Earth’s Magnetic Field

閃焰 S. -P. Weng 76


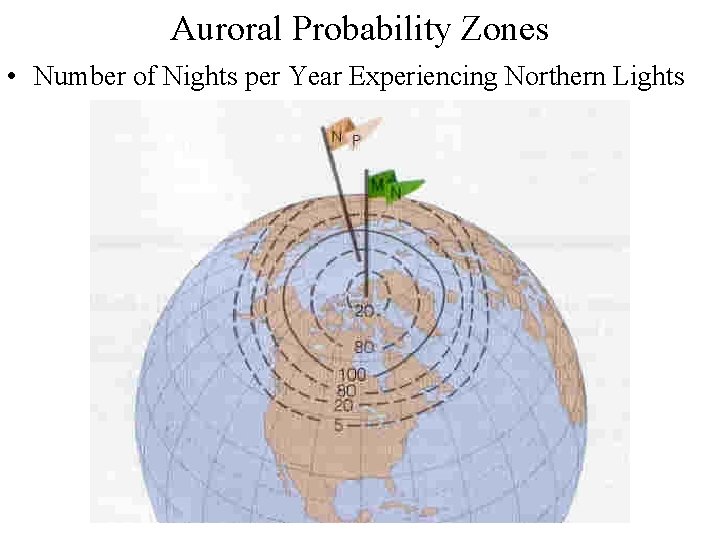
Auroral Probability Zones • Number of Nights per Year Experiencing Northern Lights

Auroral Ionization Ring http: //www. pfrr. alaska. edu/~pfrr/AURORA/

Arkansas Aurora 2003 -11 -20

Arkansas Aurora 2003 -11 -20

Arkansas Aurora 2003 -11 -20

Summary • Temperature and Kinetic Energy • Temperature Scales – Kelvin – Celsius – Fahrenheit • Physical Changes of State – Latent Heat – Specific Heat • Energy Balance and Heating – Radiation, Convection, Conduction – Albedo – Greenhouse Effect • Aurora
 Knots si unit
Knots si unit 01:640:244 lecture notes - lecture 15: plat, idah, farad
01:640:244 lecture notes - lecture 15: plat, idah, farad How are thermal energy and temperature different
How are thermal energy and temperature different Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Heat treatment of steel lecture notes
Heat treatment of steel lecture notes Utilities and energy lecture
Utilities and energy lecture How to draw a weather map
How to draw a weather map Cisk meteorology
Cisk meteorology National institute of meteorology
National institute of meteorology Penn state department of meteorology
Penn state department of meteorology The study of weather
The study of weather Metar practice
Metar practice Meteorology definition
Meteorology definition Meteorology hydrology and water management
Meteorology hydrology and water management Equation of state for dry air
Equation of state for dry air Fnmoc meteorology products
Fnmoc meteorology products Delta meteorology
Delta meteorology Bureau of meteorology
Bureau of meteorology Cin meteorology
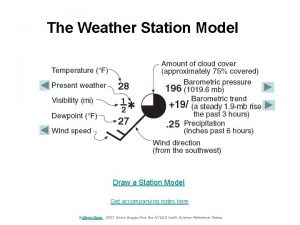
Cin meteorology Weather station model
Weather station model Us navy meteorology
Us navy meteorology Synoptic meteorology definition
Synoptic meteorology definition Meteorology jeopardy
Meteorology jeopardy Meteorology
Meteorology Latvian environment geology and meteorology centre
Latvian environment geology and meteorology centre Introduction to tropical meteorology
Introduction to tropical meteorology Elements of meteorology
Elements of meteorology Types of visibility in meteorology
Types of visibility in meteorology Doppler effect meteorology
Doppler effect meteorology Caribbean weather satellite
Caribbean weather satellite Yorcon
Yorcon Www.meted.com
Www.meted.com Math in meteorology
Math in meteorology Meteorology
Meteorology Department of meteorology maldives
Department of meteorology maldives Department of meteorology maldives
Department of meteorology maldives Meteorology
Meteorology What is vorticity in meteorology
What is vorticity in meteorology Astro meteorology
Astro meteorology Onda eletromagnética
Onda eletromagnética Meteorology
Meteorology Intemerismo
Intemerismo Meteorology today
Meteorology today Meteorology today
Meteorology today Nchm bhutan
Nchm bhutan Confluence meteorology
Confluence meteorology Meteorology
Meteorology Meteorology 101
Meteorology 101 Anders persson meteorology
Anders persson meteorology Vanuatu meteo cyclone warning
Vanuatu meteo cyclone warning Caribbean institute for meteorology and hydrology
Caribbean institute for meteorology and hydrology Introduction to tropical meteorology
Introduction to tropical meteorology Specific heat capacity
Specific heat capacity Define specific latent heat
Define specific latent heat Principle of cooking
Principle of cooking Difference between heat and thermal energy
Difference between heat and thermal energy How is thermal energy transferred?
How is thermal energy transferred? Difference between heat and thermal energy
Difference between heat and thermal energy Difference between heat and thermal energy
Difference between heat and thermal energy Heat vs thermal energy vs temperature
Heat vs thermal energy vs temperature Flannel shirt conductor or insulator
Flannel shirt conductor or insulator Heat vs thermal energy
Heat vs thermal energy Imbalances in earth’s heat energy help to create weather.
Imbalances in earth’s heat energy help to create weather. Sound energy examples
Sound energy examples Example of heat energy
Example of heat energy Solar energy is radiant light and heat from the sun
Solar energy is radiant light and heat from the sun Heat thermal energy and temperature
Heat thermal energy and temperature Potential energy diagram heat of reaction
Potential energy diagram heat of reaction Conduction and convection venn diagram
Conduction and convection venn diagram Example of conduction
Example of conduction Conduction convection radiation examples
Conduction convection radiation examples Sources of heat
Sources of heat Heat flow
Heat flow How does sound travel
How does sound travel Meaning of heat energy
Meaning of heat energy Formula formula
Formula formula Heat vs thermal energy vs temperature
Heat vs thermal energy vs temperature Potential energy diagram heat of reaction
Potential energy diagram heat of reaction Relationship between heat and temperature
Relationship between heat and temperature Thermal energy
Thermal energy Thermal kinetic energy
Thermal kinetic energy Transfer analysis
Transfer analysis